GENITAL WARTS
Genital warts are caused by infection with human papillomavirus (HPV). They are common benign neoplasms that are significant because of their transmissibility, the association of some HPV types with the development of malignancy, and our current inability to eradicate latent HPV from infected tissue. In contrast with herpes simplex virus (HSV) where there are two viral types, HSV 1 and HSV 2, there are over 100 HPV types. These are usually divided on the basis of their oncogenic potential into low-risk and high-risk groups. Anogenital infection may occur with viruses from either of these groups.
Clinical Presentation
The data presented in a recent review regarding HPV prevalence and incidence indicate that anogenital infection due to HPV is extremely common. About 20% of women in their late teens and early twenties have polymerase chain reaction (PCR) evidence of HPV DNA infection (
1). This rate decreases with age, declining to about 5% in older women. Not surprisingly, the rates are even higher in special groups such as prisoners and patients who attend an sexually transmitted disease (STD) clinic. The few studies carried out in men show great variability but the average prevalence appears similar to that in women.
HPV infection is quite transmissible and the major risk factor for acquisition of the virus relates to sexual activity. Specifically, the risk of infection correlates best with number of past sexual partners and the acquisition of a recent, new sexual partner. Most HPV transmission occurs by way of vaginal and anal intercourse but other forms of sexual activity also result in high transmission rates. In contrast, among children with anogenital warts, most transmission occurs through nonsexual contact (
2). Incidence figures parallel prevalence data, and it is estimated that over a lifetime, 75% of all men and women will have contracted HPV infection. This observation suggests that HPV infection is the most common of all sexually transmitted infections. Condom use and male circumcision appear to decrease the risk of transmission (
3).
Most anogenital HPV infections occur at a subclinical level and only a small percentage of those infected will develop the clinical lesions in the form of genital warts. The prevalence of genital warts in Western countries varies but the rate lies between 0.1% and 1% (
4,
5).
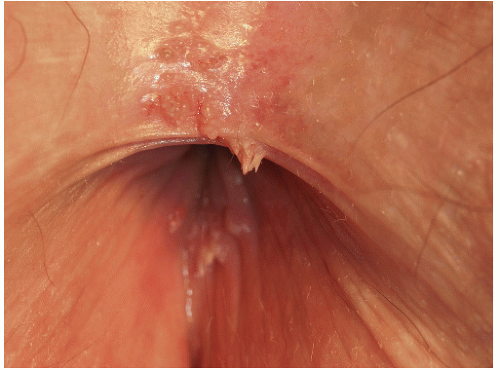
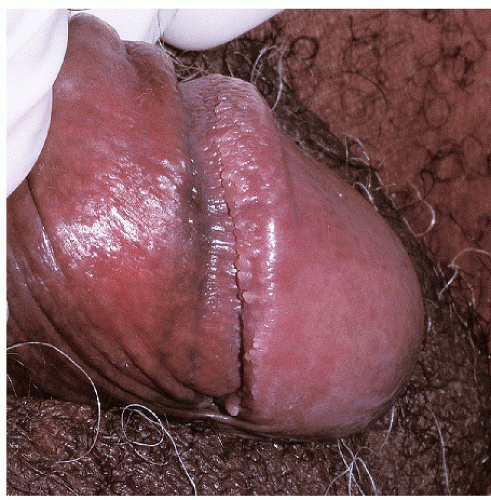
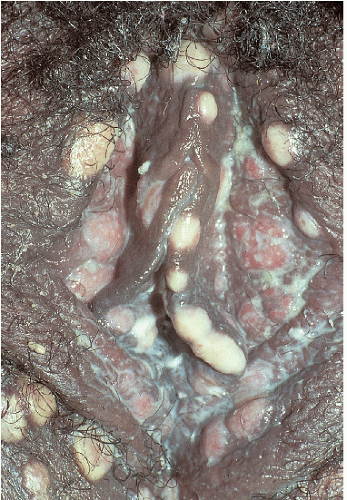
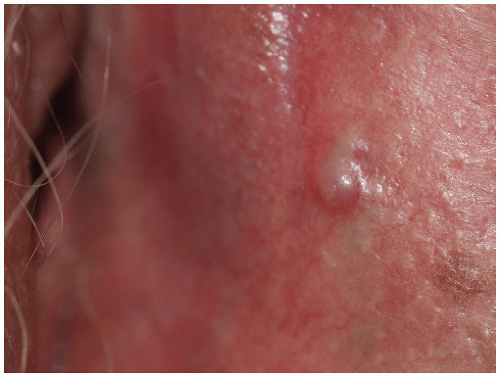
There are four major morphologic variants of genital warts. Those found in persistently moist areas tend to be skin colored, filliform (e.g., tall and narrow) with or without a brush-like tip (
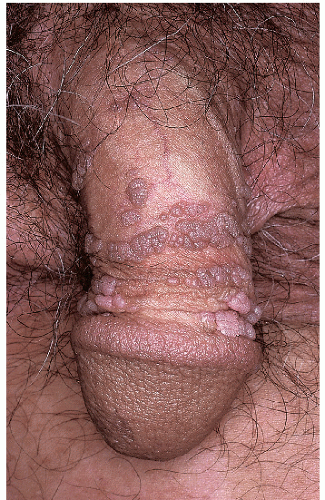
Fig. 12-1). This variant is appropriately termed “condylomata accuminata” and, strictly speaking, is the only variant for which this term should be used. The second variant occurring in the anogenital area is verruca vulgaris (common warts) (
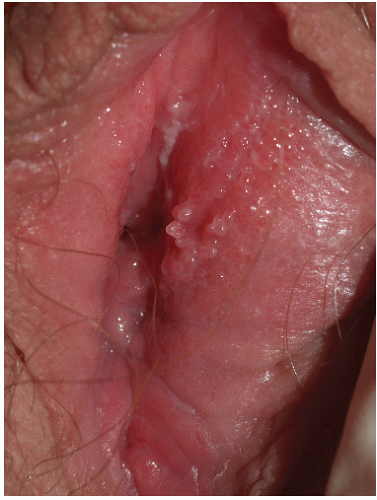
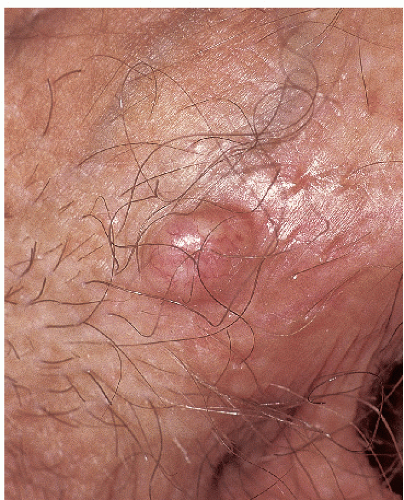
Fig. 12-2). These are skin colored and similar in appearance to hand warts. They are about as wide (5 to 10 mm) as they are tall and typically have palpable, if not visible, surface roughness due to the presence of scale. They are located on the drier aspects of the anogenital tissue. Smooth-surfaced, flat-topped papules (wider than they are tall) represent the third variant (
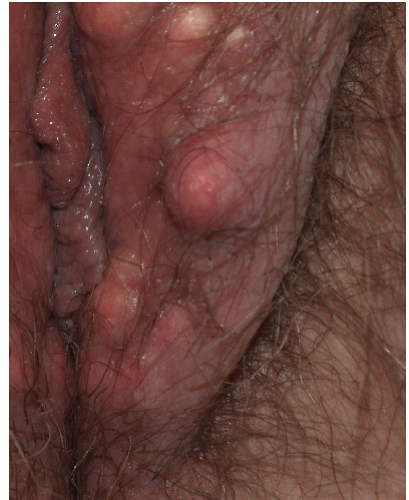
Fig. 12-3). These are usually 3 to 15 mm in diameter and are wider than they are tall. They can occur on either moist or dry surfaces. While often skin colored, these flat-topped warts may also be pink, red, brown, or black. Large globular warts, 2 to 4 cm in diameter, correspond to the fourth variant (
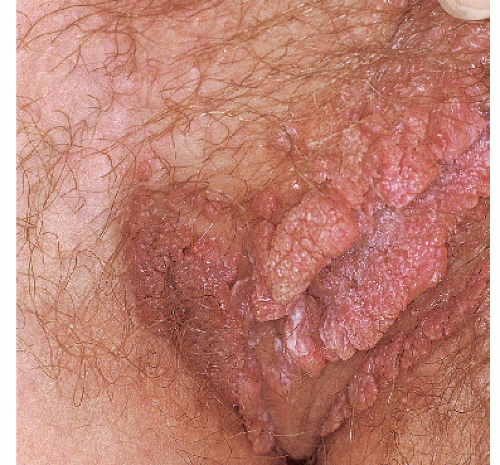
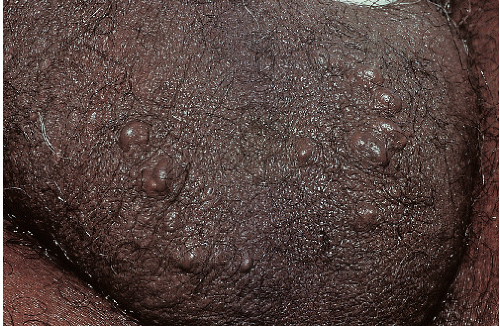
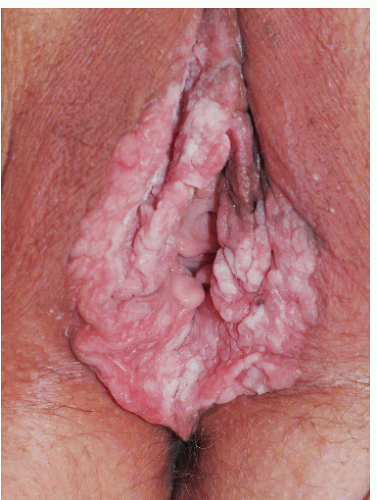
Fig. 12-4). They have a smooth, but often cauliflower-like surface and are skin colored, pink, or red in hue. This type of lesion is often termed a giant condyloma or Buschke-Lowenstein tumor.
Genital warts occur most often in individuals between the ages of 16 and 25 years. Although genital warts are usually asymptomatic, they occasionally produce itching and irritation. The number and size of lesions in an infected individual presumably depend on the immunologic resistance that the host can mount against the infecting virus. Thus, some individuals with very good immune response may have only a few small lesions that
respond readily to treatment, whereas other patients exhibit numerous small and large lesions that are very resistant to almost all attempts at therapy.
In men, most genital lesions involve the shaft of the penis and, less frequently, the glans, foreskin, scrotum, groin, and periurethral or intraurethral area. Perianal warts occur more commonly in homosexual men but also can develop in heterosexual men. In women, genital warts occur most commonly around the vaginal introitus, the vulvar vestibule, and surrounding anogenital skin. Fifty percent of women with vulvar warts have evidence of cervical HPV infection. Women may develop perianal warts and, not surprisingly, their presence at this site is especially likely in those who practice receptive anal intercourse.
Diagnosis
A diagnosis of anogenital warts is generally made on a clinical basis. However, biopsy is required for two types of warts, flat-topped, smooth-surface lesions and giant condylomata. This is so because in situ or invasive
squamous cell carcinoma may be present histologically in these types of otherwise normal-appearing warts. Some clinicians apply 5% acetic acid (common vinegar) to the anogenital area for several minutes prior to visual examination. Whitening occurs where warts are present but unfortunately neither the specificity nor sensitivity of this procedure is high enough for this technique to be recommended. Gynecologists sometimes examine the anogenital area with high magnification using a culposcope. I believe this has little usefulness and greatly increases the duration and cost of the examination. Cytologic preparations, similar to those used for cervical smears, are helpful in the diagnosis of suspected anal HPV infection and are highly recommended in the anal examination of immunosupressed patients. This is especially true for men who have sex with men (MSM) and are human immunodeficiency virus (HIV) positive.
Biopsy can confirm a clinical diagnosis of genital warts. The histologic hallmarks include variable acanthosis often with some elongation of the rete ridges. The presence of koilocytosis (vacuolated cells with dark pyknotic nuclei) is particularly helpful. True koilocytosis occurs throughout most of the epidermis. Pathologists who are not expert in cutaneous pathology often overcall this feature, failing to recognize that scattered vacuolated cells (usually without nuclear change) are normally present in anogenital tissue.
Tests for determining the HPV type are commercially available. However, this adds significant cost and rarely changes either the treatment or the follow-up of the patient. This testing is not currently recommended by the Centers for Disease Control (CDC). Viral cultures cannot be carried out as HPV does not grow in artificial media.
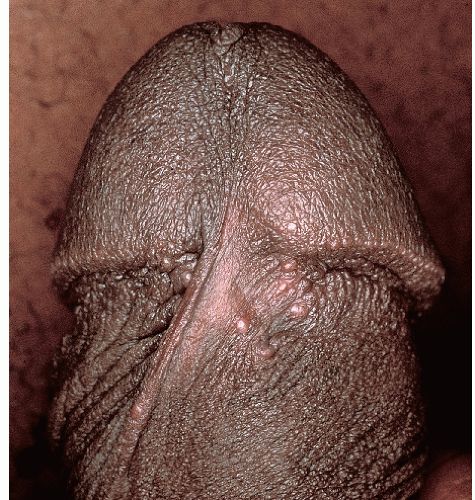
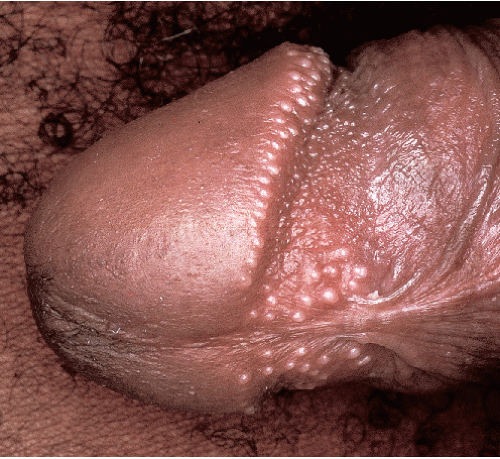
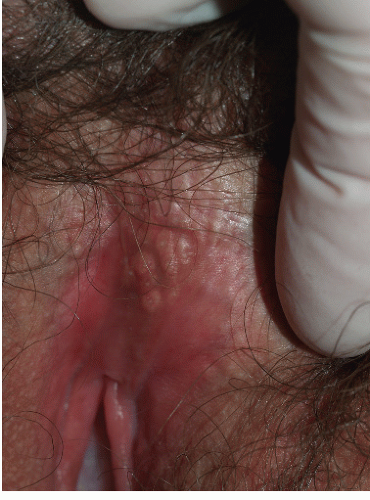
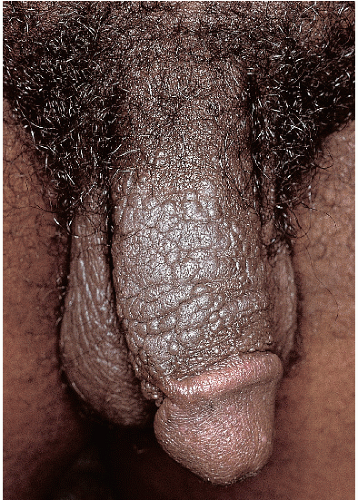
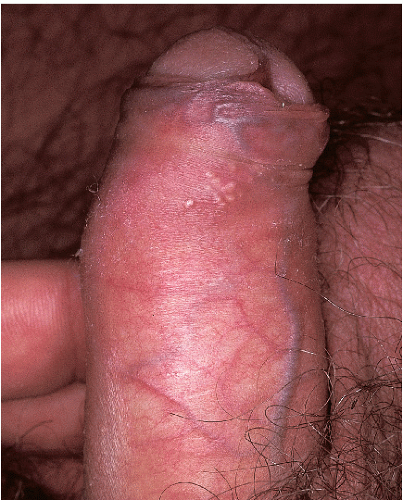
Many conditions must be considered in the list of differential diagnoses for genital warts. In women, vestibular papillomatosis can easily be confused. Patients with this condition have closely set, minute, hemispherical papules, arranged in a cobblestone-like pattern, within the vulvar vestibule (
Fig. 12-5). This is a normal physiologic variant that needs no therapy. The equivalent variant in men consists of one or two rows of pearly penile papules encircling the corona of the glans penis (
Figs. 12-6,
12-7 and
12-8). Other conditions to be considered include molluscum contagiosum, common skin tags, lymphangiectasia, and enlarged sebaceous glands (Fordyce spots) (
Figs. 12-9 and
12-10). As indicated above, both in situ and invasive squamous cell carcinoma may have an identical appearance as flat-topped or large globular warts (
Fig. 12-11).
Pathophysiology
Genital warts are caused by the alpha group of HPV. This group contains about 60 HPV types and all of these can infect both mucous membranes and keratinizing epithelium. This group can be subdivided on the basis of their oncogenic potential into approximately 20 high-risk and 40 low-risk types. About 90% of genital warts are due to four HPV types. The vast majority of infections are caused by low-risk types HPV 6 and 11 and the remainder are mostly due to high-risk types 16 and 18. It is not possible clinically to determine which of these HPV types represents the cause of genital warts. Coinfection with multiple HPV types is quite common.
GENITAL WARTS: |
Diagnosis |
• |
Sexually active individuals 15 to 30 years of age |
• |
Four clinical variants: filliform warts, verruca vulgaris, flat-topped papules/plaques, and large globular nodules |
• |
Lesions may be pink, red, brown, black, or skin colored |
• |
Biopsy to confirm diagnosis and to rule out malignancy |
• |
Look for cervical involvement in women and anal involvement in MSM |
Information on the duration of HPV infection in untreated women is restricted almost entirely to data regarding cervical infection in immunocompetent women (
6). These data indicate that for cervical infection, 50% to 90% of women will clear their infection spontaneously within 1 year. Clearance appears to take place more quickly with low-risk HPV infection than that it does with high-risk HPV infection. Men with asymptomatic HPV infection have similar or even more rapid clearance rates (
1).
Data regarding the clearance of asymptomatic genital HPV infection are not available due to the lack of simple inexpensive tools for screening patients with latent disease. Likewise, the natural history of genital warts is not well established due to the almost universal treatment of clinically expressed infection. By analogy with warts in other sites, it is likely that most anogenital warts will resolve with the passage of time and there is some evidence to suggest that half of the anogenital warts occurring in children will resolve spontaneously (
7).
Spontaneous resolution occurs when an HPV-specific immune response develops. Antibodies to HPV gradually appear and can be measured. Cell-mediated immune (CMI) response, as judged by the presence of lymphocytes in biopsies of warts that subsequently resolve, also occurs. CMI response is more important than humoral response. This is based on two observations. First, the
titer and timing of antibody response do not correlate very well with resolution of warts, and second, many patients with visible lesions are not seropositive and many patients who are seropositive do not currently have clinically detectable infection (
1).
Management
As noted above, genital warts may resolve spontaneously, especially in children and young adults. Contrarily, those occurring in immunosupressed patients are likely to remain in place indefinitely. In spite of the potential for resolution, nearly all anogenital warts should be treated to reduce contagion through sexual activity. This is particularly important because it is not possible to determine clinically which warts contain high-risk HPV types.
Before considering treatment options, clinicians should consider whether or not biopsy of one or more lesions should be undertaken. As indicated above, flat-topped warts (regardless of color) and large globular warts should be biopsied because of the possibility that in situ or invasive squamous cell carcinoma may be present. Shave biopsy is acceptable for flat-topped warts, whereas shave excision is preferred for large globular warts because of the greater risk of sampling error in these large lesions. Filliform lesions and those mimicking hand warts (see above) may be treated without biopsy due to the very low likelihood that dysplasia will be present.
Multiple approaches to treatment are available (
8,
9). Treatment needs to be individualized for each patient as no single therapy is universally preferable. One of the first priorities in the treatment process is the education of patients regarding the contagious aspects of the viral infection, the likelihood of recurrence after therapy, and, in some instances, the potential for malignant transformation. After this has been accomplished, input from the patient should be sought as to whether the treatment is to be undertaken by the patient (patient-based therapy) or by the clinician (clinician-based therapy).
There are three medications available for patient-based therapy: application of 0.5% podofilox, 5% imiquimod, or green tea polyphenon E. Podofilox (Condylox) solution or gel is applied to the warts twice daily for 3 days followed by 4 to 7 days without treatment. Imiquimod (Aldara) cream is applied once daily three times per week on alternate days. For both products, the cycle is repeated as necessary. A meta-analysis comparing these two products found that they were approximately equal in efficacy with clinical cure rates of 50% to 55% (
10). However, the severity of adverse effects was slightly greater with podofilox (
10). The Food and Drug Administration (FDA) has recently approved a green tea extract (Veregen), marketed as 15% ointment, to be applied to warts t.i.d. Data to support Veregen’s use are limited. Three randomized, double-blind, placebo-controlled studies have been carried out with complete clearance rates of 50% to 60% (
11). Adverse effects of redness, burning, pain, and erosion occur with all three of these products though possibly to a slightly lesser degree with the green tea extract.
Clinician-based therapy can be either medical or procedural. Two
medical approaches are available. With the
first, 25% podophyllin is applied to the warts once weekly or every other week. This approach is less used today than it was in the past due to nonstandardized podophyllin preparations, highly variable cure rates, and significant problems with irritation and pain. With the second, trichloroacetic acid can be applied (carefully!) in the office at 2- or 3-week intervals. Clearance rates of 70% to 80% have been reported after six applications (
9). Burning on application is troublesome for patients but it is a reasonable approach when the warts are relatively nonkeratotic and the number and size of the lesions are small (
12).
Clinician-based
procedural therapy includes cryotherapy, electrosurgical destruction, laser destruction, and surgical excision. With cryotherapy, liquid nitrogen is sprayed (or applied with cotton-tipped applicators) at 2- to 3-week intervals. Clearance rates of about 80% are achieved with three to six treatments (
9,
13). Electrosurgical destruction (electrofulguration, electrodessication) is usually carried out using the ConMed Hyfrcator under local anesthesia. Other electrosurgical approaches using loop excision or a bipolar Bovie-type apparatus can also be used. Laser therapy, generally with a CO
2 laser, can also be considered but the cost of this equipment, and thus the treatments, is very high. Surgical excision is most often accomplished with a shave or scissors-snip technique; rarely elliptical excision might be considered. All three of these ablative approaches result in clearance rates of somewhat over 80% (
9,
14). Drawbacks, however, are the requirement for local anesthesia, the possibility of secondary infection, and potentially long healing times.
The choice of which medical or procedural approach is taken depends on patient preference and on the experience level of the clinician. For most patients, I prefer either electrosurgical destruction or shave removal with very light electrosurgery to the base. These two approaches are time honored, inexpensive, and do not depend on patient compliance. Moreover, only a single clinic visit is generally necessary and the patient leaves the clinic with the knowledge that he or she is free of all of the visible lesions. Of course, if the number and size of the warts are large, staged eradication at monthly intervals may be necessary.
The recurrence rate with all forms of therapy is very high, running generally in the 25% to 45% range. This presumably is the result of latent virus left in the perilesional skin after clinical visible lesions have been successfully treated.
Anogenital warts in children present a special problem because of the concern that sexual abuse may have taken place. However, for children under the age of two, it is very unlikely that the transmission occurred through sexual contact (
7). Anogenital warts in these very young children may have arisen through normal parental contact or by way of contagion from an infected birth canal. A greater level of concern exists for those over the age of four and, in any case, inquiry by an experienced clinician or by other skilled interviewers must be carried for all children with anogenital warts.
The best approach for genital warts is to prevent them for occurring in the first place. A quadrivalent vaccine (Guardasil) directed against HPV types 6, 11, 16, and 18 has been approved by the FDA for use in 9 to 26 year old children and adults of both sexes. The possibility of using this vaccine for women over the age of 26 is under consideration by the FDA currently. A bivalent (Cervarix) vaccine directed at HPV 16 and 18 has also been approved for girls and women 10 to 25 years of age. Clinical trials and almost 4 years of experience has documented that both of these vaccines are highly efficacious, safe, and durable. In addition both vaccines have some, but limited, crossover effect against HPV types other than those toward which the vaccines are primarily directed (
15). The cost remains high and some parental objection exists. These two factors somewhat limit the use of these vaccines in many situations.
Both vaccines appear to be nearly 98% effective in preventing cervical and vulvar intraepithelial neoplasia (CIN 2 and 3) due to HPV types 16 and 18 for up to 3 years in those who were naïve to these types at the time of vaccination (
15,
16 and
17). Efficacy in older women aged 24 to 45 was slightly lower but still excellent at 90% (
18).
GENITAL WARTS: |
Management |
• |
Home therapy with imiquimod, podofilox, or green tea extract |
• |
Office therapy with liquid nitrogen or trichloroacetic acid |
• |
Office therapy with electrosurgery, excision, or laser ablation |
• |
Follow-up for recurrences and development of new warts |
• |
Check for sexual abuse in children with anogenital warts |
MOLLUSCUM CONTAGIOSUM
Infection with molluscum contagiosum virus (MCV) is common and self-limited. It can be viewed as more of a nuisance than a threat to health or well-being.
Clinical Presentation
Molluscum contagiosum is a common infection that occurs primarily in children (
19). Incidence rates can be as high as 20% of children in lesser developed countries but are lower elsewhere. In a large series reported from Greece, children with this condition accounted for 3.2% of outpatient pediatric visits over a 6-year period (
20),
and in a series from India, they accounted for 1.9% of new patients visits in a dermatology/STD clinic (
21). It has been estimated that in the United States, office visits for molluscum contagiosum account for about 0.2% of total outpatient visits (
22). Males appear to be affected somewhat more often than females. Adults account for less than 10% of all MCV infections. Transmission occurs primarily by casual skin-to-skin contact in children, whereas sexual transmission accounts for most of the cases in adults. Molluscum contagiosum occurs more commonly, and presents with more lesions, in patients with atopic dermatitis and in those who are significantly immunocompromised such as those with HIV/acquired immunodeficiency syndrome (AIDS) (
23,
24).
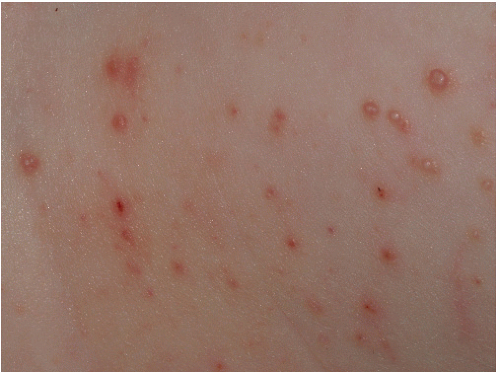
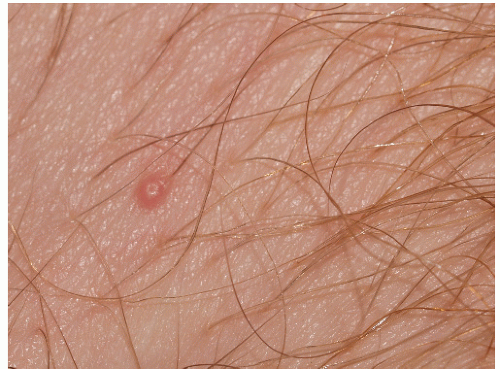
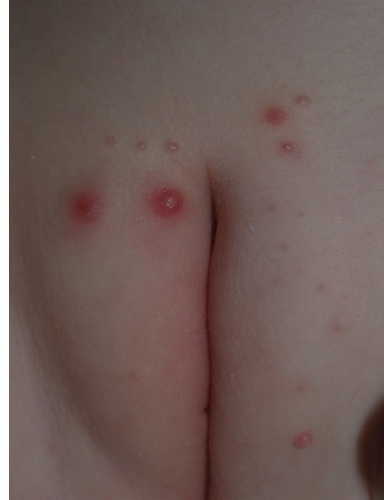
The lesions of molluscum contagiosum are skin colored, pink, white, or, occasionally, translucent hemispherical papules 3 to 10 mm in diameter (
Fig. 12-12). Rarely, and mostly in immunocompromised patients, giant lesions are encountered. Immunocompetent patients generally have 15 to 50 lesions at any one time. Most of the lesions develop on keratinizing epithelial skin but they rarely also occur on mucous membranes. The skin surrounding lesions is usually normal in appearance but redness and eczematous changes may occur circumferentially. Sometimes in the resolution stage the lesions develop brisk, red inflammation (
Fig. 12-13).
The papules of molluscum contagiosum characteristically have a central depression or umbilication. However, many lesions, especially early and small lesions, lack this feature (
Fig. 12-14). Although this umbilication may not be seen in every lesion, a careful examination usually shows at least some umbilicated lesions. Sometimes, the papules of molluscum contagiosum mimic vesicles, thus accounting for the lay term “water warts” (
Fig. 12-15). Molluscum contagiosum is typically asymptomatic but some patients may experience low-grade pruritus. Lesions in children may be found anywhere on the skin but most often the trunk is affected. Lesions in adults, because of sexual transmission, are most commonly located on the mons, the medial thighs, and the buttocks. They are less often found on the penis and on the labia.
Diagnosis
The diagnosis, especially when umbilicated lesions are present, is usually made on a clinical basis. Confirmation is possible through curettage of a lesion, which then reveals a small white “molluscum body.” A shave biopsy may be appropriate if the clinical appearance is atypical. The
histologic findings include pathognomonic features of intracytoplasmic inclusion bodies (“molluscum contagiosum bodies”) made up of globular viral proteins.
The list of differential diagnoses includes HPV infection (genital warts), scabies, lichen nitidus, and lymphangiectasia. Genital warts are not usually hemispherical and often have a rough, rather than smooth, surface. The lesions of scabies occur in a characteristic distribution pattern (web spaces, axillary folds, areolae, and penis) and small linear burrows can usually be found. The lesions in lichen nitidus are more numerous, mostly flat-topped, and more morphologically homogenous. The small papules and vesicles of lymphangiectasia contain fluid when pierced with a needle.
MOLLUSCUM CONTAGIOSUM: |
Diagnosis |
• |
Small, smooth, dome-shaped papules |
• |
White, pink, translucent, or skin colored |
• |
Umbilication at summit is characteristic in larger lesions |
• |
Bright red color may occur when undergoing resolution |
• |
Confirm by curettage for molluscum body |
Pathophysiology
Molluscum contagiosum is caused by the MCV which is a very large DNA poxvirus. There are four virus types (c.f. two for HSV) of MCV (
19). Most infections are caused by MCV 1. There are no significant differences in appearance or distribution for infections caused by the four types. As indicated above, transmission is primarily by skin-to-skin contact but a lesser role for fomites may also be likely. There is a long incubation period after inoculation of the virus. The time of this latency averages about 2 months but much longer intervals have been reported. MCV cannot be cultured using artificial media.
Resolution of the infection is associated with the development of both a humoral and a cell-mediated immune response. Antibodies to MCV are found in most, but not all, patients with molluscum contagiosum. However, the timing of their appearance and the titer levels do not closely parallel the severity or duration of the infection. The CMI response is much more important in allowing for resolution of the infection. The onset of the CMI is sometimes clinically apparent by the development of inflammation in and around individual lesions. Once such inflamed lesions are present, total resolution of the infection usually follows within a month or two.
Management
In immunocompetent patients, untreated individual lesions of molluscum contagiosum resolve spontaneously over a matter of 1 to 3 months. But, “seeding” of virus into surrounding skin or at distant sites occurs such that new lesions develop while old ones are resolving. The total duration of the infection, at least in children, averages 18 to 36 months. In immunocompromised persons, the number of lesions is larger and the variability in size is greater. Spontaneous resolution takes much longer and may not occur at all. On this basis, it seems prudent to check the HIV status in adults who develop lesions that are larger, more numerous, or last more than several months.
All of the treatments currently available are problematic in one way or another. For this reason, most clinicians advise “watchful waiting” rather than active therapy for pediatric patients. For adults and impatient patients, both medical and procedural approaches are possible. The most commonly used medical treatments include cantharidin, trichloroacetic acid, imiquimod, and potassium hydroxide. The first two are applied in the office by the clinician but the latter two can be used at home.
Cantharidin 0.7% solution is applied very carefully to prevent contact with the normal surrounding skin. Special care must be taken if cantharidin is used in intertriginous areas as the retained sweat can allow unwanted spread of the applied solution. For lesions in the anogenital area, it is useful to apply a loose bandage over the treated lesions to prevent spread of the cantharidin due to friction and retained sweat. Many clinicians advise that the cantharidin be washed off 6 to 8 hours after application but I have not found it necessary to do so. A blister develops at the cantharidin application site within 24 hours and the lesion is eventually sloughed off 4 or 5 days later when the blister roof peels away. Two to three office visits for repeat application are usually necessary. It is painlessness when initially applied, and for this reason, it is generally the treatment of choice for children. Mild pain and irritation may occur later. Clearance rates for
individually treated lesions are about 90% and patient satisfaction is quite good (
25,
26).
Trichloroacetic acid 85% solution must be applied very carefully so that the solution does not run off of the papule onto normal skin. This approach is quite effective but it is somewhat painful at the time of application (
27). Imiquimod may be used at home and the method in which it is used is identical to that described in the section above on genital warts. Home application reduces the number and expense of office visits but this approach is accompanied by considerable discomfort and the duration of treatment is long enough to hamper patient compliance. Potassium hydroxide (KOH) has only recently been described as a treatment modality. It can also be applied at home. In a recent study comparing 10% KOH solution with imiquimod, the two treatments were equally effective but KOH use was accompanied by more adverse effects (
28).
The most commonly used procedural approaches are cryotherapy and curettage. Liquid nitrogen cryotherapy is used in the same manner as is used for actinic keratoses and warts. A total freeze time of about 10 seconds is recommended and this can be obtained either in a single freeze or in a freeze-thaw-freeze cycle. It is moderately painful. Curretage, in which the lesion is scraped away with the edge of a skin curet, is also moderately painful but the fact that treated lesions are completely removed leads to good patient satisfaction. Because of discomfort, both cryotherapy and curettage are best used when the number of lesions is small. In a randomized trial, comparing curettage with cantharidin and imiquimod, curettage was found to be the most efficacious and was associated with the fewest adverse effects (
29).
MOLLUSCUM CONTAGIOSUM: |
Management |
• |
Consider observation without specific therapy in kids |
• |
Office application of cantharidin is painless and effective |
• |
Liquid nitrogen cryotherapy if lesion number is large |
• |
Curretage for removal if lesion number is small |
• |
Imiquimod or potassium hydroxide can be considered for home therapy |
CONDYLOMA LATUM
Condyloma lata is the name used for the flat-topped papules and nodules that develop in the anogenital area during the secondary stage of syphilis. These lesions are uncommonly seen in conventional office practice but are, of course, encountered with some frequency in STD clinics. The primary coverage of syphilis is located in the section on chancre in
Chapter 10 and only the clinical presentation and therapy of condyloma lata will be covered here.
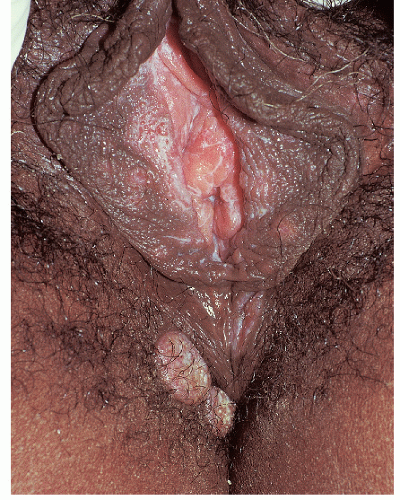
Condylomata lata are sharply demarcated, large (1- to 2-cm), flat-topped, moist, skin-colored or pink papules (
Fig. 12-16). When the lesions are located on a mucosal surface, they often exhibit a white surface because of retained moisture (
Fig. 12-17). They are most commonly found on the vulva and the perianal area but can occur elsewhere on the genitalia and perigenital skin. As opposed to the other cutaneous lesions of secondary syphilis, the lesions of condyloma lata are teeming with spirochetes and pose a high risk for transmission to others. Condylomata lata are usually associated with other signs and symptoms of secondary syphilis such as fever, malaise, and generalized lymphadenopathy. Other skin findings such as slightly scaly red papules on the trunk, brown-red palmar and plantar papules, white patches on the oral mucous membranes, and patchy hair loss may also be present.
Patients with condylomata lata almost always exhibit a positive rapid plasma regain (RPR) or Venereal Disease Research Laboratory (VDRL) serologic test for syphilis. The only exceptions are those who are severely immunocompromised (e.g., HIV/AIDS) and those with such a high antibody titer that they exhibit the so-called prozone phenomenon. The lesions of condyloma lata can be confused with flat-topped HPV-induced warts and intraepithelial neoplasia of the vulva, penis, and scrotum. If there is any question about the correct identity, a serologic test for syphilis as well as a biopsy should be obtained. The treatment for secondary syphilis as recommended by the CDC is a single 2.4 million unit IM injection of benzathine penicillin G (Bicillin L-A) (
30).
LYMPHOGRANULOMA VENEREUM
Until recently, lymphogranuloma venereum (LGV) was rarely encountered in Western countries. However, during the last 5 years, there has been a significant rise in the number of cases reported from the United Kingdom and Europe and a smaller rise in the United States. Nearly all of this increase is accounted for by infections occurring in MSM. Currently, the prevalence of LGV serovars in the rectum in UK MSM is about 1% (
31). LGV occurs very rarely in women.
Nearly all (90% to 95%) of the patients with LGV have presented with proctitis and most of the others have had inguinal adenitis (
31). Transient genital ulcers together with appreciable lymphadenopathy occur in a minority of patients (
32).
A discussion of proctitis, with or without perianal ulcers, falls outside the scope of this textbook and the rarely encountered ulcers are not well described in the literature. The most striking feature is likely to be impressive lymphadenopathy (buboes). The inguinal lymph nodes are most commonly affected though in some cases femoral nodes are also involved. When both sets of nodes are affected, Poupart ligament (ligamentum inguinale) creates a characteristic grove between the two groups of nodes. The enlarged nodes are usually bilateral and they are exquisitely tender. In untreated patients, nodes that have been present for some time undergo liquefaction with attendant fluctuance. Eventually, breakdown of the overlying skin occurs, resulting in the presence of chronic purulent draining sinuses. Massive genital edema (elephantiasis) frequently develops. Patients with untreated proctitis develop rectal stenosis, strictures, and perianal abscess formation. The latter may be mistaken for Crohn disease.
LGV is caused by the L1, L2, or L3 serovars of the bacterium, Chlamydia trachomatis. Nearly all of the newly reported cases have been with the L2 serovar. The diagnosis is usually suspected on a clinical basis but similar adenopathy can occur in chancroid. Confirmation of the diagnosis requires identification of a LGV-type serovar from the site of infection. Previously, this had been accomplished primarily with bacterial cultures, but today molecular techniques are more widely used. In situations where these tests are not available, serologic techniques can be used but there are many problems with sensitivity and specificity.
The CDC guidelines recommend treatment with oral doxycycline 100 mg twice daily for 3 weeks (
30). In an attempt to obtain better compliance, single dose or short course azithromycin has also been used but there is insufficient evidence of efficacy to support the use of this medication on a regular basis (
30). Fluctuant lymph nodes should be aspirated.
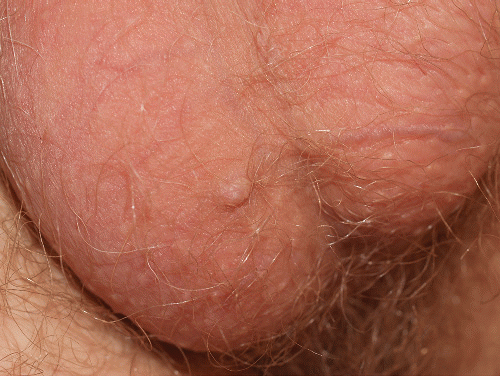
EPIDERMAL CYSTS (EPIDERMOID CYSTS)
Epidermal cysts are commonly, but erroneously, termed sebaceous cysts. They are the most common type of cysts found in the anogenital area. They occur as firm, slopeshouldered, smooth-surfaced, white, yellow-white, slightly pink or skin-colored papules or nodules (
Fig. 12-18). Very small cysts (1 to 2 mm) are called milia but these are not commonly seen in the anogenital area. The average diameter for genital lesions is 0.5 to 2.0 cm. Noninflamed
cysts are asymptomatic. Epidermal cysts are primarily found on the hair-bearing aspects of the genitalia. They are most commonly located on the scrotum in men and the labia majora in women. One or several may be present; rarely there are 10 to 20 clustered lesions (
Figs. 12-19 and
12-20). The diagnosis is usually made on the basis of clinical appearance. Confirmation, if necessary, can be obtained when white cyst contents are visualized after incising and gently squeezing the lesion. Syringomas arising on the labia majora may be confused with epidermal cysts. The lesions of molluscum contagiosum may mimic epidermal cysts but the lesions of molluscum contagiosum are more superficial and appear to sit on the surface, rather than within the skin. Enlarged sebaceous glands (Fordyce spots) on mucosal aspects of the genitalia may resemble milia.
So-called “inclusion cysts” arise when bits of epithelium are implanted in the skin during the course of a surgical procedure or as a result of trauma sufficient to break the surface of the skin. The much more common, typical epidermoid cyst found on the genitalia, develops from the remnant of an anatomically malformed pilosebaceous unit that lacks a fully adequate outlet to the surface of the skin. These cysts may have a black dot, representing a comedone (“blackhead”), at the summit of the cyst. The white material located within an epidermal cyst is keratin that is produced by keratinocytes located in the lining of the cyst wall.
Epidermal cysts are medically unimportant and cause no problems unless the cyst wall leaks or ruptures. If this occurs, cyst contents come into contact with the connective tissue surrounding the cyst where they engender a brisk foreign body inflammatory reaction. This inflammation causes the cyst contents to liquefy into pus-like material. In spite of the appearance and smell, this mixture of liquefied keratin and inflammatory cells almost always lacks pathogenic bacteria. Nevertheless, such lesions may clinically resemble a furuncle or an inflammatory pseudocyst as seen in hidradenitis suppurativa. No treatment is needed for uninflamed cysts. Inflamed cysts may be incised and drained. Most clinicians prescribe antibiotics for inflamed cysts but any beneficial effect that occurs is likely due to the anti-inflammatory, rather than the antimicrobial, properties of these antibiotics. Rarely, symptoms or patient insistence will result in a need for excisional removal of the entire cyst.