6 Fat Grafting for Primary Augmentation
Summary
This chapter will focus on fat grafting for primary breast augmentation. Patient selection, process of graft survival, techniques of fat grafting, outcomes and complications, oncologic considerations, and postoperative breast screening will be discussed. Although some controversies still exist regarding primary augmentation with fat grafting, this technique continues to gain popularity among plastic surgeons. Patients should undergo extensive counseling before proceeding with fat grafting to the breast, including potential risks, complications, and effects on future cancer screening.
6.1 Introduction
Fat transplantation was first described by Gustav Adolf Neuber in 1893 for reconstruction of facial scars. 1 Autologous fat transfer to the breast was later described by Vincenz Czerny in 1895, after he successfully transferred a back lipoma to fill a breast defect. 2 Fat transfer procedures continued to develop until the mid-1900s, when fat grafting lost popularity because of high reported resorption rates. A 1950 study by Peer reported fat loss averaging 45% 1 year after transfer. 3 Fat grafting had a resurgence in the 1980s, when liposuction became available as a minimally invasive technique for harvest. In 1987, Bircoll reported a case of successful and long-lasting fat transfer to the breast using liposuction. 4 Later that same year, fat transplant to the breast was denounced by the American Society of Plastic and Reconstructive Surgeons (ASPRS, as the American Society of Plastic Surgeons [ASPS] was then named) Ad-Hoc Committee on New Procedures for potential interference with breast cancer detection. 5
In 2007, the ASPS Fat Graft Task Force was created to evaluate the efficacy and safety of fat grafting to the breast. A report was released by the group in 2009 suggesting a potential role for fat grafting to the breast and other sites, but inadequate data was available to make specific recommendations. Their review of the literature included reports of fat grafting to the breast to treat micromastia, postaugmentation deformities, congenital deformities such as Poland syndrome and tuberous breast deformity, and defects after surgical resection for breast cancer. No evidence of delayed detection of breast cancer after fat grafting was found, and complication rates appeared appropriate, with only rare cases of severe complications and death; the primary cause of poor results was found to be graft volume loss. The task force reported that fat grafting “can be considered a safe method of augmentation and correction of defects associated with various medical conditions.” The study concluded that although fat grafting to the breast and other sites can be considered, outcomes are surgeon- and technique-dependent. 6 Since that time, there has been a resurgence of fat grafting to the breast, with new innovations aimed to enhance fat retention rates.
6.2 Patient Selection and Preoperative Assessment
There are multiple potential uses for fat grafting in the breast, including primary breast reconstruction after mastectomy or lumpectomy, revision reconstruction, correction of congenital deformities such as Poland syndrome or tuberous breast deformity, and primary or secondary breast augmentation. Advantageous qualities of autologous fat include accessibility, simplicity of harvest, and most importantly, lack of immunogenic response. Patients should undergo extensive counseling before proceeding with fat grafting to the breast, including potential risks, complications, and effects on future cancer screening. Patients must understand the likelihood of partial graft resorption, the possible need for multiple procedures, and the possibility of unfavorable or inadequate results. Patients must also be counseled on the controversies of fat grafting.
A good candidate for fat grafting breast augmentation is a patient with appropriate expectations, desire for modest augmentation, adequate donor site adiposity, stable weight, and nonptotic breasts. Patients desiring more pronounced breast enlargement with substantial projection may be better suited for implant augmentation. Although the optimal candidate would have ample donor adiposity, low body mass index (BMI) is not an absolute contraindication. Studies have shown that satisfactory augmentation can be achieved in underweight patients (BMI ≤ 18.5). 7 Preoperative weight stability will contribute to a more predictable result, as significant weight loss or gain after fat grafting will result in similar effects at the recipient site. Breast ptosis should be addressed with mastopexy prior to fat grafting augmentation; combining these into a single procedure may decrease fat survival as a result of temporary decreased perfusion to the breast. Fat grafting alone will not address ptosis, and failure to correct ptosis will result in suboptimal outcomes.
Careful consideration is needed in patients at increased risk for breast cancer, such as those with family history, personal history, or relevant genetic mutation. Currently there are no studies that prove delayed detection of breast cancer or increased risk for breast cancer after autologous fat transfer to the breast, but these topics are still controversial. In light of these controversies, however, the ASPS has declared that fat grafting to the breast should no longer be considered an experimental practice and should instead be considered part of reconstructive surgery in patients with breast cancer. 8 Although there is not an absolute contraindication in these patients, extensive counseling is required. Patients must understand that postoperative changes to the breast could potentially lead to radiographic changes and increased likelihood of a need for biopsy.
Prior to surgery, photographs should be taken and the specific areas requiring augmentation identified. Communication with the patient is important to determine expectations and goals, including volume of augmentation; depending on the volume desired, the likelihood of multiple sessions should be reemphasized. Donor sites should be chosen preoperatively based on areas of available adiposity and patient preference. Preoperative mammograms should be performed, and the patient should be agreeable to repeat mammograms with the same radiologist 1 year after surgery to establish a new baseline.
Patients should be generally healthy with optimization of preexisting medical conditions. Preoperative risk stratification should be performed, including risk for perioperative venous thromboembolism (VTE); patients at increased risk for VTE should receive appropriate preventative measures. A full abdominal exam should be performed to rule out hernia prior to abdominal liposuction.
6.3 Process of Graft Survival
Survival of transplanted fat initially depends on diffusion of nutrients from surrounding plasma until neovascularization begins, usually within 48 hours. 9 As a result, ideal fat for transplantation has a high surface area–to-volume ratio to optimize absorption of nutrients from surrounding tissues for graft survival. Although fat deposits must be large enough to preserve cellular composition, excessively large deposits have a higher likelihood of central necrosis. Fat can ultimately be lost as a result of ischemia, apoptosis, or adipocyte dedifferentiation. Even distribution of fat during transfer is also important to maximize resorption of graft that does not survive.
In addition to adipocytes, harvested fat contains stromal cells in a portion termed the stromal vascular fraction (SVF). The SVF contains a high ratio of adipose-derived stem cells (ADSCs), which can differentiate into adipocytes or epithelial cells required for angiogenesis; these cells also secrete growth factors that promote angiogenesis and graft survival. Studies suggest that there are different zones of survival within nonvascularized transplanted fat. The peripheral zone has the highest survival of transplanted adipocytes, while the central zone has the greatest loss of both adipocytes and stromal cells. Between these layers is an intermediate zone where there is low survival of adipocytes but retention of viable adipose-derived stromal cells. This layer is thought to be the regenerating zone, with eventual replacement of lost adipocytes with new adipocytes as a result of the preserved stromal cells. This theory highlights the importance of stromal cell viability for ultimate graft retention volume. 10 The dead adipocytes undergo slow absorption and act to reserve space for eventual replacement with new adipocytes; adipogenesis is typically completed by 12 weeks. If dead adipocytes are inadequately removed by macrophages, then oil cysts, fibrosis, and calcifications can result. 11
Fat graft survival can be optimized with atraumatic methods of harvesting, processing, and injection, although such techniques are yet to be standardized. 12 Atraumatic technique maximizes the ratio of viable adipocytes and stromal cells that are critical for graft retention. Capacity for adipocyte revascularization differs among different patients; although not completely understood, older age may negatively affect this process.
6.4 Technique
6.4.1 Preparation and Anesthesia
Antiseptic skin cleanser is used at home for 3 days prior to surgery. The patient should be marked while standing in the preoperative holding area. Markings should include anatomic breast boundaries, areas of desired augmentation, and any contour irregularities to be corrected. Donor sites for fat harvest should be marked, as well as areas where liposuction should be avoided (e.g., zones of adherence).
Although fat grafting breast augmentation is typically performed under general anesthesia, it can also be performed using local anesthetic and sedation in accredited surgical facilities. Initial patient positioning depends on the site of harvest. Supine positioning may be adequate for liposuction of the abdomen, flanks, lateral and medial thighs, and knees, but harvest from the back or buttocks may require prone positioning with subsequent repositioning for injection. Sequential compression devices should be placed whenever possible to prevent VTE. All pressure points should be adequately padded. Unless contraindicated, chlorhexidine is used for sterile preparation.
Perioperative antibiotics are typically administered before the procedure. Careful volume monitoring is performed to avoid under- or overresuscitation, and fluid is administered depending on vital signs and urine output. 13 , 14
6.4.2 Fat Harvest
Donor sites for fat harvest should be chosen based on patient morphology; options include the abdomen, flanks, back, hips, thighs, and buttocks. Based on current studies, fat harvests from various sites lead to comparable results, and there is not a preferred site for harvest. 15 , 16 , 17 , 18 Entrance incisions should be made asymmetrically within or parallel to relaxed skin tension lines or previous scars. A superwet technique is commonly employed, using tumescent solutions with epinephrine and lidocaine or with epinephrine alone to reduce blood loss. When lidocaine is included, a serum concentration of 35 mg/kg is widely considered to be safe. Patients typically reach peak serum concentration of lidocaine with its active metabolite, monoethylglycinexylidide, 8 to 28 hours after infiltration. 19 Marcaine should be avoided in wetting solutions, given its longer duration of action and increased systemic risk. Current studies show similar adipocyte viability after harvest with wet or dry liposuction techniques. 20 , 21 Exposure to lidocaine with epinephrine has not been shown to affect adipocyte viability or retention when there is adequate washing prior to injection, but inadequate removal of local anesthetic can negatively impact adipocyte function. 16 , 17 , 22 , 23
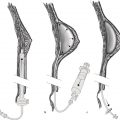
Studies suggest that use of smaller harvest cannulas, such as 2- to 4-mm cannulas, may result in less graft retention compared to larger cannulas, despite eventual use of small injection cannulas. 24 , 25 Larger harvest cannulas are thought to result in less damage to harvested fat, in part due to less shear stress. 26 Data evaluating cannula size is limited in the setting of tumescent infiltration techniques, and it is unclear whether use of wetting solutions can decrease the shear stress associated with smaller cannulas. 16 Larger cannulas, however, harvest larger fat fragments that may lead to accidental bolus injection when using smaller injection cannulas. As a result, a 3-mm cannula with multiple holes is typically used. Disposable cannulas should be used when harvesting for fat transfer (Fig. 6‑1).
Fat is most commonly harvested by traditional suction-assisted or power-assisted liposuction. Higher negative pressures should be avoided, as this can potentially decrease the final number of viable adipocytes and stromal vascular fraction cells. 27 , 28 Careful attention should be paid to preserve the superficial layer of adipose tissue and to avoid creation of contour irregularities; smaller cannulas should be used in more superficial or critical areas.
Manual fat harvest is another commonly performed technique, using a 10-mL syringe connected to a blunt liposuction cannula; the plunger is held in a partially-pulled-back position to achieve negative pressure. 29 Some authors feel that this technique minimizes damage to adipocytes compared to mechanical suction, however, currently there is no evidence that either method leads to superior results. 17 , 29 , 30 , 31 , 32 Although some studies have shown greater counts of viable adipocytes or greater enzymatic activity with fat harvested from a manual technique compared to mechanical techniques, other studies have shown that fat harvested by suction-assisted or ultrasound-assisted liposuction has similar adipocyte and ADSC viability and function. 16 , 32 , 33 , 34 , 35 In contrast, laser-assisted liposuction has been linked to decreased viability and function of stromal cells and should thus be avoided. 36 A newer method of water jet–assisted liposuction has promising potential applications in fat grafting, with studies showing greater retention of adipocytes with this method than with manual harvest. 37 , 38
Adequate fat should be harvested to account for fat lost during processing and to allow for overcorrection during fat injection. Entrance sites are closed with rapidly absorbed simple sutures.
6.4.3 Fat Processing
The most common method of fat processing over time has been centrifugation. 15 Centrifugation separates lipoaspirate into three layers: a top oily layer containing chylomicrons and triglycerides, a middle layer of fat optimal for grafting, and a bottom layer containing blood products, serum, and infiltration fluid. The bottom layer can be drained by removing the cap from the bottom of the syringe, and the top layer can be poured off, leaving behind purified fat. 30 Separation of fat by centrifugation increases the concentration of adipocytes in the graft and removes lipases, proteases, and other enzymes that lead to fat degradation. Nonviable cells are also removed, decreasing the postoperative inflammatory response. Although there is no standardized duration or revolutions per minute (rpm), Emmanuel Delay reports processing for 3 minutes at 3,200 rpm, while Sydney Coleman recommends 3 minutes at 3,000 rpm. 29 , 30 , 39 Studies show that excessive centrifugation forces can lead to increased damage to adipocytes and ADSCs in fat harvested by mechanical liposuction. 16 , 40 , 41 , 42 In contrast, lower centrifugation forces are likely to leave residual debris. Son et al found force under 4,000 rpm to be safe without affecting adipocyte or stromal cell viability. 43
Another option is decantation, in which the adipose tissue and associated fluid are separated by gravity. This is typically performed directly in syringes, although commercial devices also exist (Fig. 6‑2). Decantation is an inexpensive and easy approach to fat processing, however, it often results in inadequate removal of fluid. Remaining infiltration fluid and adipose-secreted cytokines and enzymes can lead to decreased adipocyte viability and increased resorption. 44 Separation by cotton gauze rolling is another inexpensive approach, in which the fat is gently rolled over nonadherent gauze to remove fluid. Although this is a viable option for small-volume fat grafting, it is not practical for processing large quantities of lipoaspirate.

In filtering techniques, lipoaspirate is passed through a filter to preserve larger particles while ridding it of fluid and smaller particles. Many commercial filter devices have added washing steps to help remove infiltration fluid and inflammatory molecules to improve graft survival. 18 The authors recommend using washing and filtering techniques for effective and efficient processing of lipoaspirate, using a closed system to decrease fat desiccation and contamination (Fig. 6‑3).

Although many studies have compared these methods, results are conflicting and no technique has been proven to provide superior results. 16 , 17 , 45 , 46 Some authors have attempted to increase retention of fat grafts by supplementation with various biologic products including insulin, vascular endothelial growth factor (VEGF), or platelet-rich plasma (PRP). 4 , 29 , 47 , 48 , 49 , 50 , 51 In other countries, cell-assisted lipotransfer (CAL) is gaining traction, using techniques of autologous stem cell enrichment to improve graft survival. 52
6.4.4 Fat Injection
The technique of fat injection is the most critical factor affecting fat retention and patient outcomes. 29 Fat is prepared into small syringes with small-diameter cannulas; 5-mL or 10-mL syringes and 2-mm blunt cannulas are used (Fig. 6‑4). Blunt cannulas minimize risk for intravascular injection and allow better dispersion of the fat into small aliquots. 54 Entrance sites for fat injection into the breast are placed with a 16-gauge needle, using previous scars whenever possible and avoiding the sternum to prevent hypertrophic scarring. Several entrance sites may be required to allow transfer along multiple directions and planes. To minimize risk for pneumothorax, the cannula is inserted with a slight anterior inclination of the cannula tip to avoid deep penetration into the chest wall. Furthermore, the cannula is inserted gently, without forced entry against resistance.
Multiple tunnels are first created with the cannula, and then injection is performed during cannula withdrawal; small amounts of fat, approximately 0.2 mL, are injected with each pass. Bolus injection is avoided because it is associated with increased rates of fat necrosis, liponecrotic cysts, and surgical site infection. 29 Slow injection speeds are preferred to prevent graft damage by shear stress. 54 Although the authors inject manually, automated injection devices exist to aid in controlled delivery of small fat volumes with minimal shear stress. 55 , 56 Fat is transferred from multiple directions and in multiple planes, building from deep to superficial to create a honeycomb distribution of fat.
Most surgeons recommend distributing the fat into the subcutaneous, retroglandular, or perimuscular spaces, while avoiding injection into the glandular tissue. 15 , 17 Avoiding breast parenchyma allows easier injection and theoretically decreases risk for infection. Although the effect of ADSCs on malignant and premalignant cells in the breast remains unclear, avoiding injection into breast tissue also minimizes any potential interactive effects. 17 , 57 Intramuscular injection is generally avoided because fat retention is less than in other planes, despite excellent perfusion of the muscle; this may be due to displacement of the graft during pectoralis contraction. 58 For these reasons, the authors primarily inject fat into the subcutaneous and retroglandular spaces.
The amount of fat injected is often limited by what the recipient site is able to accommodate based on skin envelope pliability and vascularity. If possible, overcorrection by 40% should be attempted to account for resorption. At the same time, grafting should be conservative so as not to exceed what the recipient site can accommodate. Excessive graft placement will result in increased interstitial pressure and graft ischemia, which will lead to increased resorption and risk for fat necrosis. The surgeon must use careful judgment when balancing these considerations. Pre-expansion devices can be considered preoperatively in patients with tight skin envelopes to prepare the recipient site to accept larger graft volumes. 59 Upon completion, entrance sites are closed with rapidly absorbed simple sutures. Studies report injecting 50–600 mL per breast each session, for a total of one to five sessions. 15 The authors typically inject 200–250 mL per breast in most women; however, larger-breasted women can often accommodate more.
The Coleman technique can also be used and has been found to be associated with favorable and long-lasting results in a systematic review. 29 This technique includes manual suction with a 10-mL syringe and a two-hole cannula, followed by processing by centrifugation. The fat is then transferred to 3-mL syringes and injected using blunt infiltration cannulas. The fat is primarily injected into the pectoralis major muscle and the perimuscular spaces, while injection into the parenchyma is limited. Breast shaping is achieved with subcutaneous injection. Arguably the most important technical point of this method is injection in small aliquots and from multiple directions. 54
6.5 Postoperative Management
Compression garments are worn over donor sites for at least 2 weeks. When small-volume liposuction is performed, patients can typically return to light duty work in 5 days, whereas patients requiring larger-volume liposuction are out of work for 7 to 10 days. Pressure to the breasts is avoided for 6 weeks, and mammograms are deferred for 6 months. Patients can generally return to exercise and regular activities 4 weeks after surgery.
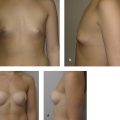
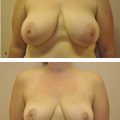
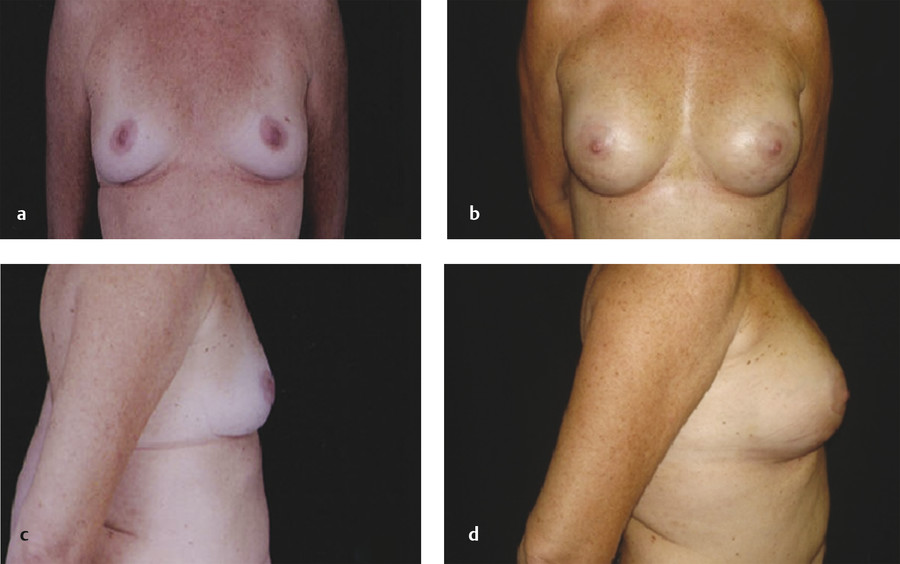
The majority of postoperative edema resolves after the first month; however, patients should be counseled that the overall volume of the breast will continue to decrease over the following months as a result of fat resorption. In a patient with stable weight, the volume typically stabilizes after 3 to 6 months postoperatively. 30 Publications vary, reporting one to five sessions of fat grafting needed to achieve desired augmentation (Fig. 6‑5). 15 , 49 Repeat fat grafting can be performed 6 months after surgery.
6.6 Outcomes
Outcomes are largely dependent on surgeon technique, which is not yet standardized. Typically 30–40% of fat is lost by resorption over 3 to 6 months; this percentage can be higher with resorption over a longer period of time when fat is inadequately purified for transfer. 30 , 53 Fat resorption is often less after subsequent sessions of fat grafting. 30 After initial resorption, volume usually remains stable in the long term, confirmed by studies using three-dimensional (3D) imaging technology. 30 One caveat is that grafted fat is affected by patient weight fluctuation, with increase or decrease in size corresponding to weight gain or loss, respectively.
Augmentation with fat grafting is often modest and limited to one cup size increase per session. Multiple procedures are often required to achieve adequate augmentation, in contrast to implant-based augmentation, which offers reproducible results in a single-stage procedure. Given that fat grafting is autologous, however, complications associated with foreign body are avoided, including implant infection, extrusion, malposition, rupture, capsular contracture, and potential need for replacement. Risk for breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) can also be avoided. Fat grafting has additional benefits, including decreased scar burden, potential to improve skin quality and stretch marks, and a more natural appearance.
A prospective study conducted by Spear et al found disproportionately high patient satisfaction after fat grafting to the breasts, despite only modest augmentation. Patients reported “feeling more attractive, sexually confident, and at ease during sexual activity.” 60
6.7 Complications
Early complications after autologous fat grafting most commonly include surgical site infection, contour irregularities, and inadequate augmentation; rarer complications include sepsis, pneumothorax from cannula penetration, or fat embolism from intravascular injection. Late complications are usually the result of fat necrosis and include fat resorption, liponecrotic cysts, and formation of calcifications. Changes in nipple sensation or lactation are not expected with fat grafting augmentation. Both early and late complications are highly associated with surgeon technique, as demonstrated by the wide variation of complication rates reported in the literature.
Localized infection can occur at the donor or recipient site and can typically be treated with suture removal, wound care, and antibiotics. Contour irregularities and inadequate augmentation can be addressed with additional rounds of fat grafting after the site has been given adequate time to heal and stabilize.
Although occurrence of pneumothorax is rare, this risk should not be overlooked, and precautions should be taken. If a pneumothorax does occur, it typically presents as respiratory compromise with oxygen desaturation, warranting an emergent chest X-ray. Pneumothorax should be treated with needle decompression, chest tube, or vascular interventional radiology (VIR) image-guided pigtail drain depending on patient stability. Typically, patients recover completely without long-term sequelae.
There is also a risk of fat embolism if fat is injected into a large vessel, classically presenting as a triad with altered mental status, respiratory compromise, and petechial rash. To minimize risk, blunt cannulas are used, and fat is injected during rapid withdrawal of the cannula while avoiding bolus injection. Studies evaluating fat embolism after gluteal fat grafting have suggested increased risk with intramuscular injection; for this reason, injection into the pectoralis major where large perforators are located is generally avoided. 61 , 62 Risk can be higher in patients with chest wall deformities, such as those with Poland syndrome who have inferiorly displaced subclavian vessels. Patients are treated with supportive care.
Clinically apparent fat necrosis occurs in about 3% of cases performed by experienced surgeons, but rates can be significantly higher depending on technique employed. 15 , 30 Fat necrosis is more likely to occur when fat is injected in large aliquots or when excessive fat is injected into the recipient site. Fat necrosis presents as a firm lump that is slightly tender to the touch. Although fat necrosis can usually be recognized on physical exam, imaging is occasionally required. Mammography should be avoided the first 6 months after surgery, and any concerning masses identified in the early postoperative period should be evaluated with ultrasound or magnetic resonance imaging (MRI).
Donor site complications can also occur, and they mirror those of general liposuction. Most commonly, contour irregularities can occur at the donor site if careful attention is not paid to liposuction technique. Other aesthetic complications include hypertrophic scarring of the entrance sites or obliteration of zones of adherence. Infection at the donor site, although rare, can occur and occasionally progress to abscess formation requiring drainage, or even more rarely, to sepsis.
A recent meta-analysis found a 12.6% complication rate for breast augmentation by fat transfer, including < 3% rate of cytosteatonecrotic lesions; most complications were acute or subacute, with very few long-term complications reported. 15 In this study, only 16 of 2,203 patients required reoperation for complication. In comparison, Hidalgo et al reported that up to 36% of patients with implant augmentation required secondary surgery over 10 years to address complications such as capsular contracture, implant malposition, and implant failure. 61
6.8 Postoperative Breast Screening
Postsurgical changes can be seen on mammogram or other imaging after any breast surgery and can lead to architectural distortion. Mammographic findings after autologous fat grafting are typically due to fat necrosis, which can appear as oil cysts, microcalcifications, coarse calcifications, focal masses, or spiculated areas of increased opacity. 62 Oil cysts are more common after bolus injection and can be identified as radiolucent round or ellipsoid lesions surrounded by a thin, smooth, calcified “egg-shell” rim. A meta-analysis found radiologic changes in 75.7% of patients after fat grafting to the breast, most commonly including benign calcifications or liponecrotic cysts. 15 Ten studies included in the meta-analysis focused exclusively on radiologic assessment after fat grafting, and none of these studies showed interference with breast cancer screening as a result of such changes. 15
A 2008 study evaluated the occurrence and character of such changes in 20 patients with history of autologous fat grafting; all mammograms evaluated were performed at least 6 months after surgery. 63 The most common finding was bilateral scattered microcalcifications, followed by oil cysts with or without associated microcalcifications. Another common finding was heterogeneity of pectoral muscle density, sometimes with microcalcifications. 85% of patients were classified as Breast Imaging Reporting and Data System (BI-RADS) 2 (“benign finding”), while 15% of patients were found to have microcalcifications in clusters, resulting in an initial BI-RADS 3 classification (“probably benign finding” with short-interval mammographic follow-up recommended). All of the BI-RADS 3 patients were reclassified to BI-RADS 2 after follow-up mammography.
Another study by Rubin et al compared mammographic changes 1 year after fat transfer breast augmentation to those after breast reduction; all patients had normal preoperative mammograms. Postoperative mammograms performed 1 year after surgery were reviewed in a blinded fashion by breast imaging radiologists. The study found significantly fewer radiographic abnormalities, significantly lower BI-RADS scores, and fewer recommendations for biopsy in the lipotransfer group compared to the reduction mammoplasty group. 63
With trained breast radiologists, cancer screening after fat grafting breast augmentation should not be problematic. 64 Mammograms can be performed prior to surgery and 1 year after surgery to establish baseline imaging. Patients who do not have any postoperative concerns are then instructed to undergo the same breast screening as the general population.
6.9 Oncologic Safety
Concern that fat grafting can potentially increase breast cancer risk stems from the theory that adipocytes and ADSCs can promote neoplastic cell production. Proposed mechanisms include increased local estrogen production as a result of adipose-derived aromatase and increased angiogenesis as a result of ADSCs.
In a 2003 murine study evaluating effects of adipocytes on tumor cells, adipocyte-secreted cytokines were shown to promote tumorigenesis in the presence of malignant breast ductal epithelial cells. 65 The cytokines were found to induce transcription factors that increased angiogenesis as well as cell survival, proliferation, and invasive potential. Adipocyte-secreted factors were also found to stabilize pro-oncogenic factors by decreased expression of inhibitors. Another 2003 study using a rat model found promotion of estrogen receptor (ER)-positive tumor cells by mature adipocytes, hypothetically related to adipocyte-produced estrogen. 66 In contrast to mature adipocytes, premature adipocytes did not promote tumorigenesis and were associated with increased expression of tumor inhibitors.
Multiple studies have also been done to evaluate the interaction between ADSCs and breast cancer cells. While one 2009 mouse study found promotion of human breast cancer cell proliferation in the presence of human ADSCs, 67 another mouse model study reported a protective effect of stem cells against breast cancer. 68 The latter study showed an inhibition of tumor growth and metastasis when adipose-derived mesenchymal stromal cells were injected into mouse mammary tissue that had also been injected with breast cancer cells. Although multiple studies have shown an antitumor effect of stromal cells, other studies suggest no effect or the opposite effect, with variation depending on the type of stromal cells and tumor cells used. 68
As described, current studies show contradictory evidence regarding the interaction of adipocytes and ADSCs with breast cancer cells and the resulting effect on tumorigenesis. In a recent meta-analysis including 2,023 patients who underwent fat grafting augmentation with a mean follow-up of 22 months, only two (0.09%) patients were diagnosed with breast cancer during their follow-up period; no study devoted to evaluation of oncological risk after fat grafting to the breast was identified in the study. 15 A clinical study with 10-year follow-up after fat grafting to the breast for reconstruction or aesthetic augmentation found no increased risk of breast cancer recurrence or new breast cancers in fat-grafted patients. 30 Studies have also been conducted to evaluate effects of fat grafting specifically in patients with known breast cancer history. A multicenter case-cohort study by Myckatyn et al including 1,197 patients did not find a higher recurrence rate among patients who underwent fat grafting as part of their immediate reconstruction after mastectomy. 69 Similarly, a matched case-control study by Petit et al evaluating 644 patients who underwent breast conservation therapy did not find higher rates of recurrence or other primary breast cancers among those who underwent fat grafting. 70 A prior study by the same author group found increased rates of local recurrence after fat grafting in the setting of carcinoma in situ, with potentially increased risk with younger age (< 50 years old), high-grade cancer, positive Ki-67, and quadrantectomy procedures; the study also suggested higher risk with shorter periods of time between surgical resection and fat grafting reconstruction. 71 In their follow-up study, however, the authors reported that additional analysis of the same patient series after a longer period of follow-up did not show any statistically significant differences in recurrence rates between patients who had undergone fat grafting and patients who had not. 70 , 71 Although animal studies have shown potential for increased cancer risk in the presence of adipocytes and ADSCs, this is yet to be seen in human studies. 72 , 73
Further studies are needed to clarify the controversies that surround fat grafting, including large, prospective, controlled studies. Currently a phase III randomized multicenter trial titled Adipose Tissue Transfer for Moderate Breast Cancer Conservative Treatment Sequela (GRATSEC) is underway in France to evaluate effects of fat transfer on breast cancer surveillance and recurrence. 15 The Plastic Surgery Foundation has also initiated a General Registry of Autologous Fat Transfer (GRAFT) project to obtain national data in the United States. Recommendations vary among societies, but most agree that fat grafting augmentation should be avoided in patients at high risk for breast cancer and that all patients should undergo preoperative radiologic evaluation and regular postoperative screening. 15 In the United States, patients with history of fat grafting to the breast generally undergo the same screening regimen as those who have not.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree