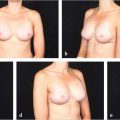
50 Anatomy of the TRAM Flap and Abdominal Donor Site
Summary
This chapter describes the anatomical basis of the transverse rectus abdominis musculocutaneous flap and its use in breast reconstruction. The anatomy of the individual components of the flap and the abdominal wall are discussed in detail, outlining important technical tips, which may help with flap survival.
Key teaching Points
Zone I is the most well-perfused area of the TRAM flap.
A superior epigastric artery should be carefully dissected and preserved superiorly.
Donor site closure must respect the anatomical layers of the abdominal wall.
Intraoperative imaging aids in deciding which portions of the flap are most well perfused.
Preoperative vascular delay can improve flap blood supply in higher-risk patients.
Observations
John Bostwick III ■ 2000
With the introduction of the TRAM flap in 1982 by Scheflan, Hartrampf, and Black, the art of breast reconstruction entered an exciting new realm. Now a woman’s breasts could be restored with her own sometimes abundant abdominal tissues, and she would receive the added benefit of an abdominoplasty. Lower abdominal skin replaced large portions of missing breast skin, while autologous lower abdominal fat was substituted for the missing breast parenchyma, obviating the need for implants with their associated potential problems and concerns. The TRAM flap was heralded as the answer to many reconstructive problems.
My initial reaction to this procedure was mixed: it was an honor to witness its development firsthand, and naturally I was excited about its potential for converting an often objectionable donor site into an aesthetic bonus. However, I was also skeptical about the magnitude of the surgery and its long-term effects. After all, at that time the latissimus dorsi flap was a reliable source of flap tissue for breast reconstruction and did not involve such major surgical intervention. Granted, it had its problems, but it also produced dependable aesthetic results. Time and experience with the TRAM flap, however, have ensured its rightful place among the operative options for breast restoration. It has proved to be a lasting and reliable technique that is capable of creating a warm, mobile, natural-feeling breast that often develops some sensory return over time. Its applications have also expanded with advances in oncologic and reconstructive procedures. It has proved to be particularly useful for immediate breast reconstruction after skin-sparing mastectomy, permitting breast restoration with healthy tissue and reduced breast scars. Greater knowledge of the anatomy of the rectus abdominis muscle has permitted the development of flap delay procedures that further enhance its flow and reliability. Microsurgical techniques have also extended the applications of the TRAM flap to a wider range of women seeking breast reconstruction with their own natural tissues.
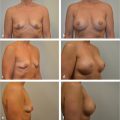
Although many of the breast reconstructions performed today still rely on implants and expanders, the use of the TRAM flap in patients requiring additional tissue for breast reconstruction is increasing. Currently I use TRAM flaps in more than 55% of my reconstructive patients. Some patients definitely require a flap procedure for satisfactory breast reconstruction. The TRAM flap is often the procedure of choice in such cases.
Current Observations
Glyn E. Jones ■ 2010
Since its inception in 1982, the TRAM flap has evolved to incorporate not only variations on pedicle techniques but has also given rise to the free TRAM flap, the muscle-sparing type 2 (MS-2) free TRAM flap, the DIEP flap, and the SIEA flap. Although the pedicle TRAM flap remains the most commonly performed variant in the United States at this time, the number of microsurgical procedures has increased progressively to the extent that many younger surgeons have never seen, let alone performed, a pedicle TRAM flap during their training. This is unfortunate, because the pedicle TRAM flap in all of its forms can still provide tremendous benefit to a wide range of patients, with shorter operating times and potentially lower operating room costs.
The primary factors driving this change have related to perceptions regarding abdominal wall strength, fat necrosis rates, and flap-shaping options as well as higher reimbursements from insurance companies for free tissue transfer. As a microsurgeon who performs a wide range of both free flaps and pedicle flaps, I still feel that there is a major place for the pedicle TRAM flap, and this chapter will focus on this procedure and its benefits, together with a realistic appraisal of potential complications. Microsurgical options are dealt with comprehensively in Chapters 52-57.
In my current practice, reconstruction with autologous abdominal flaps, whether pedicle or free, constitutes approximately 55% of all reconstructions performed. My fascination with this procedure centers on the unique advantages of providing an entirely autologous reconstruction without the need for implants and more important, the tremendous benefits achieved by having a reconstruction that matures and ages with the patient in terms of both shape and weight. Once healed, the reconstructed breast requires no maintenance.
50.1 Glyn Jones Observations 2019
It is interesting to review the past and realize its impact on the present. Before his untimely death nearly 20 years ago, John Bostwick’s original observations reflect the paradigm shift in thinking from expander-based reconstruction to a heavy focus on autologous reconstruction. This was due in large part to the poor technology and outcomes associated with our implant-based reconstructions from that era. While capsular contracture, rippling, asymmetry, infection, and extrusion plagued the outcomes from implant-based reconstruction, autologous abdominal reconstruction heralded the promise of better symmetry, more natural shapes and softer breast reconstructions requiring no maintenance. The reality of fat necrosis, bulges, hernias, and loss of abdominal strength tempered enthusiasm with the transverse rectus abdominis musculocutaneous (TRAM) flap leading to the quest for less invasive alternatives. This led to the development of the muscle-sparing free TRAM flap, the deep inferior epigastric perforator (DIEP) flap, and the superficial inferior epigastric artery (SIEA) flap as abdominal donor site alternatives. While each of these led to some reduction in abdominal wall complications, they did not eliminate them (apart from the SIEA flap) and fat necrosis rates have been a problem in some DIEP flap series. The need for microsurgical acumen and a team approach prompted others to continue utilizing pedicled flaps. Some microsurgeons familiar with the TRAM flap, myself included, have felt that to dispense entirely with this workhorse flap is to “throw the baby out with the bath water,” as it is still an excellent operation when performed well and when care is taken with abdominal closure. In addition, changes in implant technology and biomaterials have made implant reconstruction much more appealing with vastly better aesthetic and functional outcomes achievable as we enter the era of prepectoral reconstruction, acellular dermal matrix (ADM) usage, and better understanding of biofilm formation. It is clear that implant-based reconstruction has always been and probably always will be the most common form of breast reconstruction performed (at the time of writing six times more common than all autologous procedures combined in the United States).
Many surgeons find the time, effort, and stress involved in performing microsurgical reconstruction impractical in their busy schedules but find the pedicled TRAM flap to be a reasonable compromise offering their patients a safe, cost-effective alternative when well performed in carefully selected patients. While microsurgical reconstruction is assuming a much larger role in autologous breast reconstruction as reflected in this revised edition of Bostwick’s original text, the pedicled TRAM flap remains a useful procedure for many surgeons and their patients.
50.2 Origins of the Pedicle TRAM Flap
Millard described the use of a tubed lower abdominal pedicle flap for reconstructing the radical mastectomy defect in 1976. The flap was sequentially waltzed onto the chest by way of the forearm to achieve a highly successful autologous tissue reconstruction for the time. In 1979, Robbins used a vertical rectus abdominis flap for breast reconstruction. Independently, Drever, Dinner, and Sakai all refined variations on the use of vertical rectus abdominis myocutaneous flaps for breast reconstruction. Hartrampf and colleagues took the bold step of changing the skin island orientation to a transverse one across the mid-abdomen, making a more sizeable volume of tissue available for breast reconstruction with a cosmetically desirable donor site. Scheflan and colleagues confirmed the dominant inferior epigastric arterial supply to the lower abdominal skin and fat. Blood supply was most robust directly over the muscle belly where perforators were most abundant, whereas the periphery of the flap relied on the superficial epigastric and circumflex iliac terminal branches. Milloy and McAfee had documented the blood supply of the rectus muscles in 1960, and these findings, together with Scheflan’s dissections, found their culmination in the lead oxide injection studies of Taylor, Moon, and Palmer. Their publication of the angiosome concept was an extension of Michel Salmon’s classic anatomical studies. From these humble beginnings, the TRAM flap was destined to become the benchmark procedure for breast reconstruction, albeit superceded now in many centers by free flap alternatives.
50.3 Pertinent Abdominal Anatomy
50.3.1 Musculature
The anterior abdominal wall musculature consists of the following:
Central paired rectus abdominis muscles on either side of the linea alba.
A lateral layered group of three flat muscles: the external oblique, internal oblique, and transversus abdominis.
Rectus Abdominis
Morphology: A long strap muscle that is broader above, extends along the entire central anterior abdominal wall.
Origin: Central crest of the superior pubic ramus and symphysis pubis.
Insertion: Anteromedial aspects of the fifth, sixth, and seventh costal cartilages, with slips to the anterior aspect of the xyphoid cartilage.
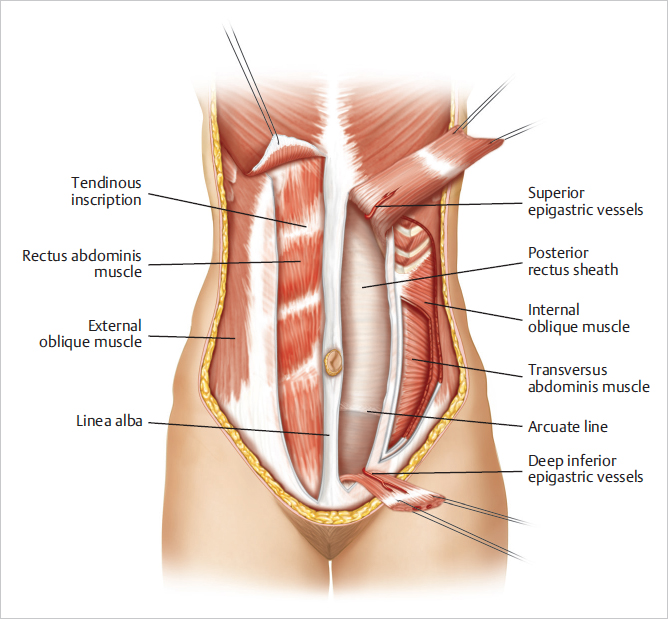
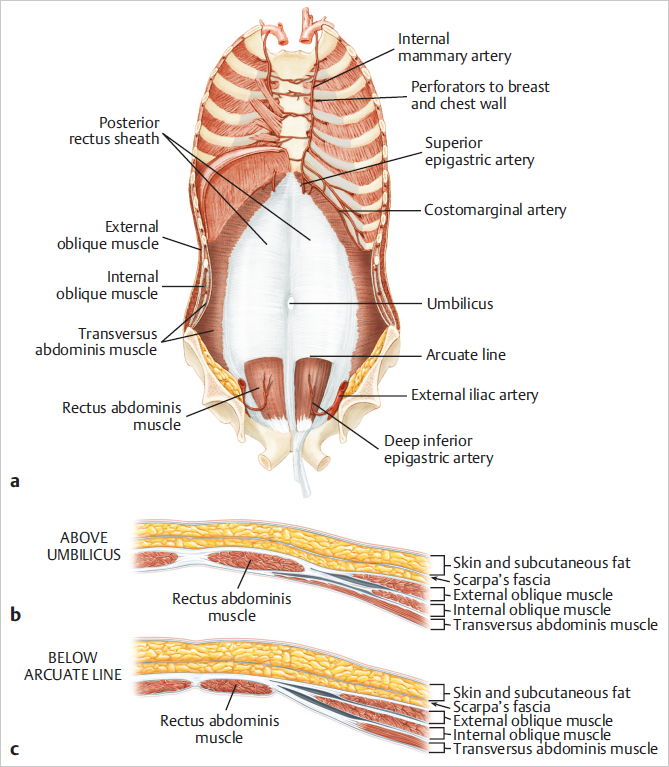
Tendinous inscriptions: The supraumbilical portion of the rectus abdominis muscle has three tendinous inscriptions that attach firmly to the anterior rectus sheath. The rectus abdominis muscle is contained within a fibrous sheath composed of contributions from the broad, flat aponeurosis of the lateral musculature.
Rectus sheath: The rectus sheath consists of a complex fusion of the fascial insertions of the lateral oblique muscles of the abdominal wall at the linear semilunaris:
Anterior rectus sheath composed of the fusion between the external and internal oblique fascia.
Posterior rectus sheath arising from a fusion of the internal oblique and transversus abdominis.
The anterior sheath extends unbroken from the anterior aspect of the lowermost ribs to the pubis.
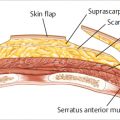
The posterior sheath begins at the lower costal arch and extends to a midpoint between the pubis and umbilicus. Here, at the arcuate line, the posterior sheath disappears completely, leaving only peritoneum and transversalis fascia covering the bowel and the posterior aspect of the distal rectus abdominis muscle (▶Fig. 50.1).
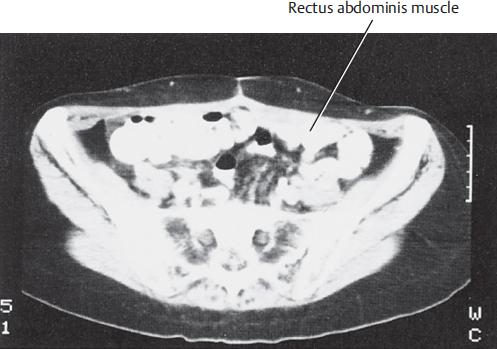
A computed tomography (CT) scan of the upper abdomen at the level of the umbilicus illustrates the rectus muscles, the external oblique muscles, and the overlying subcutaneous tissue. The relative width of the rectus muscles and their contribution to the anterior abdominal wall are shown in ▶Fig. 50.2.
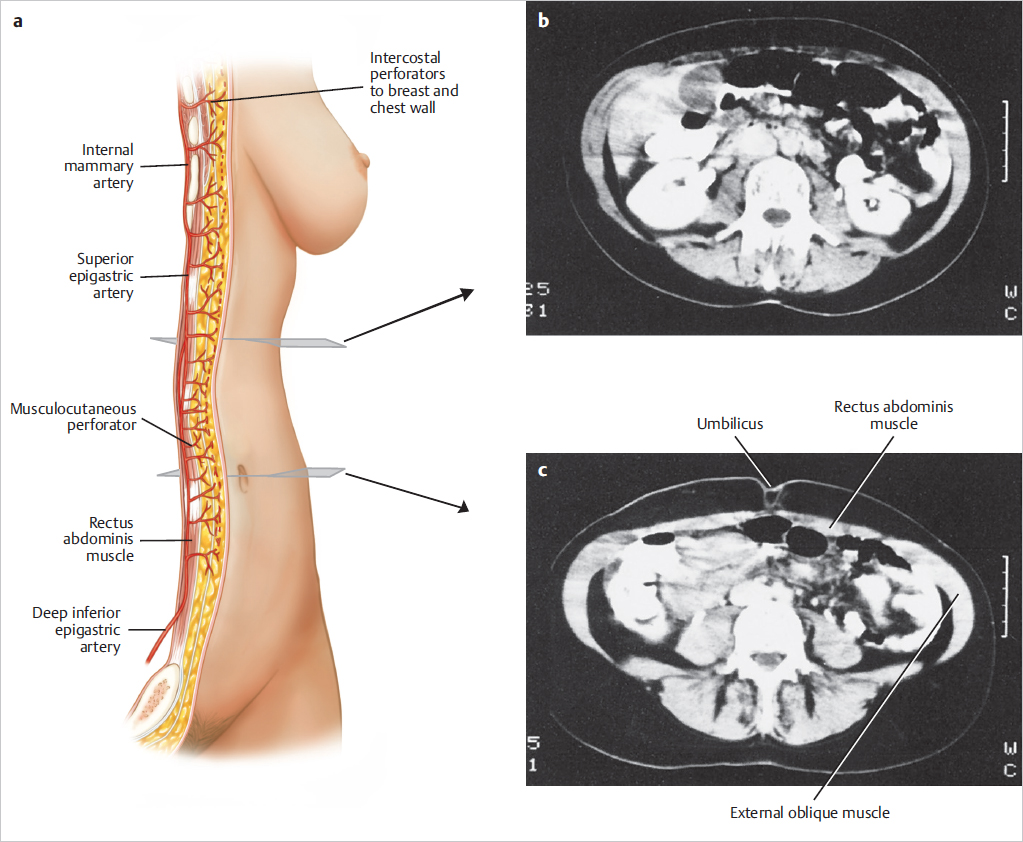
A lower abdominal CT scan at the level of the anterior superior iliac spine illustrates the width of the rectus muscles and the overlying subcutaneous tissue used for the TRAM flap (▶Fig. 50.3).
Above the arcuate line, the internal oblique aponeurosis separates at the lateral edge of the rectus and its laminae encircle the rectus abdominis muscle. Below the arcuate line, the internal oblique muscle aponeurosis as well as that of the transversus abdominis extend anterior to the rectus muscles and to the lamina in the midline above to form the linea alba, extending from the xyphoid process to the pubic bone (▶Fig. 50.4).
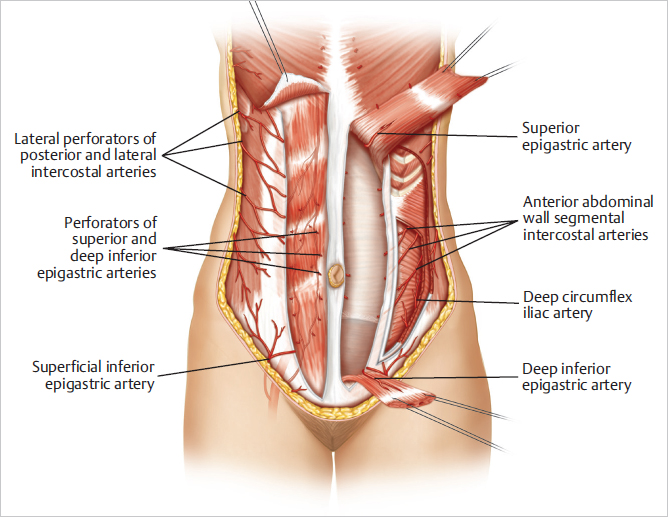
The abdominal wall muscles function primarily as support for the abdominal viscera, containing them and reinforcing them against the force of gravity. Intra-abdominal pressure is increased when these muscles contract, assisting in expiration and bowel and bladder emptying. These muscles are also essential for emesis and coughing, and they forcibly contract during a Valsalva maneuver. The rectus abdominis muscles supplement the function of the abdominal wall musculature, assisting in the initiation of trunk flexion from a supine position. They also work in concert with the oblique muscles to assist with external rotation. When one or both rectus abdominis muscles are missing, the strength of the abdominal wall and its major functions are definitely reduced but still intact as long as the strength and continuity of the rectus and abdominal wall fascia are preserved. This has been extensively evaluated in vivo by Blondeel, Nahabedian, Lejour, and Weiler-Mitthof and will be discussed later.
50.3.2 Blood Supply
The vertical intramuscular epigastric vascular system provides the major source of blood supply to the rectus abdominis muscle and the overlying musculocutaneous perforator territories of the anterior abdominal wall.
Superior epigastric artery: A terminal branch of the internal mammary (thoracic) artery, nourishes the superiorly based pedicle TRAM flap.
Deep inferior epigastric artery: Supplies the free TRAM flap and DIEP flap.
Superficial inferior epigastric vessels supply contributions to the skin and fat of the lower abdominal pannus and afford additional venous drainage to the skin island.
Taylor and colleagues postulated that the superficial inferior epigastric vein may play an important role in venous drainage of the TRAM/DIEP flap skin island, which may explain why some flaps develop venous congestion despite intact deep venous anastomoses.
Technical Pearl
This highlights the importance of harvesting the SIEV with a free flap whenever possible as a second venous outflow tract if required to decompress venous congestion.
Internal Mammary Artery
Origin: First part of the subclavian artery.
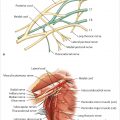
Course: The internal mammary artery enters the thorax and descends behind the upper six costal cartilages about 1 cm lateral to the lateral border of the sternum. The pectoralis major muscle, the breast, and the overlying breast skin are supplied by anterior perforating branches in each intercostal space. These medial intercostal perforators are major components of breast blood supply. The internal mammary artery also communicates with the segmental intercostal arteries in each intercostal space and across the posterior midline of the sternum.
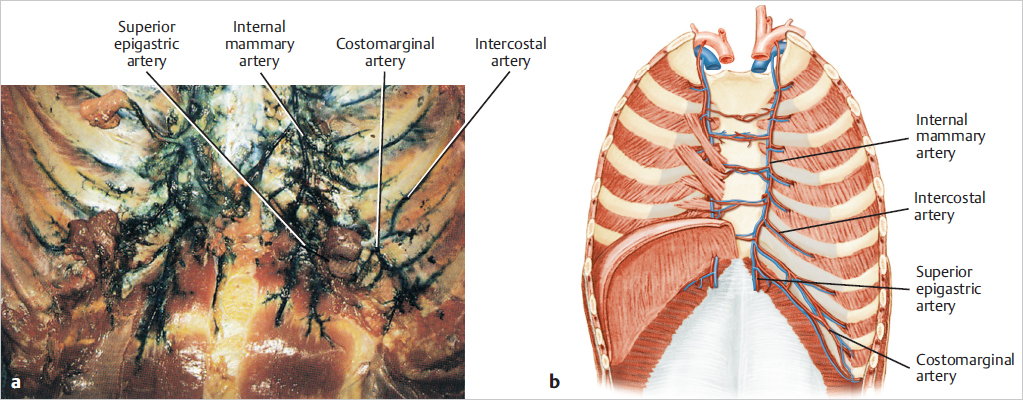
Connections with the intercostal arteries provide a strong collateral flow and communicate posteriorly with the aorta. This accounts for potentially adequate blood flow to the upper TRAM flap after upper internal mammary artery occlusion or injury. The internal mammary artery bifurcates behind the sixth intercostal space into a musculophrenic branch to the diaphragm and anterior chest wall and the superior epigastric artery (▶Fig. 50.5).
This posterior anatomical dissection after injection of the internal mammary artery highlights the collateralization between the internal mammary and the intercostal arteries as well as across the midline. It also demonstrates the costomarginal artery and the entrance of the superior epigastric artery into the upper rectus abdominis muscle (▶Fig. 50.6).
The internal mammary and superior epigastric vessels vary in size and flow in each patient. In younger patients, these vessels are subject to arterial spasm; in older patients, reduced flow, vasoconstriction, and atherosclerosis may be evident. Women sometimes have relatively smaller internal mammary vessels than do men. Mediastinal radiation can further compromise flow in these vessels, shifting additional inflow from the superior to the inferior epigastric artery and the lower intercostal arteries into the epigastric–rectus system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree