44 Two-Stage Prepectoral Prosthetic Breast Reconstruction
Summary
Prepectoral prosthetic breast reconstruction has gained significant traction in the last several years and has become the routine form of prosthetic breast reconstruction in many practices across the country. The original subcutaneous breast reconstructions performed in the early 1970s, which were plagued with complications and aesthetic challenges have been replaced by beautiful and natural-appearing reconstructed breasts, which lack chronic pain, hyperanimation deformities, and are being performed as outpatient procedures. Two-staged prepectoral breast reconstruction allows the surgeon to slowly create an appropriate skin envelope while the patient has the opportunity to evaluate and provide input for size and aesthetics. We will focus on indications and contraindications as well as describing the technical considerations involving both first- and second-stage prepectoral breast reconstruction. It will highlight the pearls and pitfalls that will maximize reconstructive success, while providing many clinical examples of patients of various sizes and dimensions who have undergone two-stage prepectoral breast reconstruction.
Key Teaching Points
To describe the reasons prepectoral reconstruction has become popularized.
To describe the techniques used to perform prepectoral reconstruction.
To show the advantages of two-staged breast reconstruction.
To teach clinical pearls to achieve success.
To illustrate the benefits of prepectoral reconstruction in patients undergoing postmastectomy radiation therapy.
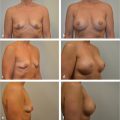
To show clinical examples of prepectoral reconstruction cases.
44.1 Introduction
Prepectoral prosthetic breast reconstruction has had a vigorous reemergence in the last half-decade. What started off in the early 1970s as placing a fixed-volume silicone implant directly below thin mastectomy skin flaps, which yielded high complication rates and failures, has evolved into a highly successful and rewarding procedure for both surgeon and patient. Using the bioengineered breast concept described by Dr. Patrick Maxwell, we have furthered the reconstructive process, using materials such as acellular dermal matrices (ADMs), form-stable silicone gel implants, and autologous fat grafting. These sophisticated materials, combined with improved mastectomy skin flaps, technology to objectively assess perfusion, and a true team approach connecting plastic surgeons, breast surgeons, medical oncologists, and radiation oncologists, have given our patients new and exciting hope regarding their aesthetic and functional outcomes following mastectomy with reconstruction. Prepectoral prosthetic reconstruction has become the workhorse of my prosthetic practice. My patients are happier, their recoveries are shorter, and their aesthetic outcomes are routinely excellent. Issues that lead us to the rebirth of prepectoral reconstruction such as hyperanimation deformities, chronic pain, and muscle spasms, as well as aesthetic considerations such as lack of cleavage, diminished projection, and breast “tightness” are no longer problems associated with breast reconstruction. By not involving the pectoralis major muscle in our reconstruction, we are eliminating significant surgical morbidity, minimizing distracting forces on the implant, which, over time, manifest as soft tissue displacement, and we are improving the functional abilities of our patients. The inherent breast parenchyma is located above the pectoralis muscle, so why would we reconstruct with an implant below the muscle? The old adage my former chairman instilled in me of replacing like with like holds much weight here. Autologous flaps are routinely placed above the pectoralis muscle, so why shouldn’t a device be placed in the same plane? Prepectoral prosthetic reconstruction, whether performed in two stages with a tissue expander, or in a single-stage direct-to-implant technique, makes sense. As a reconstructive breast surgeon, it is imperative to have this technique in your armamentarium. It may not be appropriate for every case, but it will be something you will be asked about at some point by a patient, a colleague, or your breast surgeon.
Silicone breast implants introduced in the early 1960s marked the beginning of the modern era of implant-based breast reconstruction. The first breast reconstructions, performed in the early 1960s with the introduction of the silicone implant, involved placement of the implant in a subcutaneous pocket, beneath the mastectomy skin but over the pectoralis major muscle. This subcutaneous approach was simple, quick, and preserved the integrity of the pectoralis muscle but was associated with a number of complications. Implant malposition (bottoming out), visibility, and palpability; rippling/wrinkling; implant exposure subsequent to skin breakdown; and capsular contracture were some of the most commonly reported complications. With the realization that these complications arose from insufficient soft tissue coverage, breast reconstruction technique evolved to moving the implant from the subcutaneous to submuscular position.
In submuscular placement, the implant is placed under the pectoralis major muscle without releasing the inferior origin of the muscle. Full muscle coverage of the implant is achieved by recruitment of muscle flaps (serratus anterior and rectus abdominis sheath) for lateral and inferior coverage of the implant. Full muscle coverage eliminated the soft tissue coverage limitations of the subcutaneous approach, but it resulted in unnatural-looking breasts. The inferior restrictions imposed by the submuscular pocket prevent lower pole expansion that results in poor breast projection, ptosis, and definition of the breast shape. In addition, recruitment of muscle flaps introduced donor site morbidity.
In order to address the inferior restriction of full muscle coverage, the partial muscle coverage or dual-plane technique was introduced. In partial muscle coverage, the implant is covered partially superiorly by the pectoralis major and partially inferiorly by only the mastectomy flap. The inferior subcutaneous coverage not only allows for lower pole expansion but also eliminates the need for flap recruitment for lower pole coverage. Partial muscle coverage, however, introduced a new problem. Without its inferior attachment, the pectoralis major is free to migrate superiorly, which causes “window-shading.” Moreover, subcutaneous coverage at the lower pole reintroduced the problems seen with subcutaneous implant placement.
To address the problem of lower pole coverage, ADMs were introduced in breast reconstruction in 2008. In this widely utilized modification of the partial muscle coverage technique, ADM sutured at the lower pole provides the additional support needed at the inferior pole. With lower pole ADM placement, complications associated with subcutaneous coverage are minimized without restricting lower pole expansion. Although this technique resolves some of the concerns of partial muscle coverage (without ADM), the pectoralis major muscle remains elevated and window-shading remains a problem. Window-shading can, however, be minimized by stabilizing the muscle and altering the degree of release of the muscle origins.
44.2 Rationale for Prepectoral Placement
Subpectoral prosthesis placement with partial muscle coverage, with or without ADM, is currently the standard technique for implant-based breast reconstruction. Many of the disadvantages/complications of subcutaneous implant placement (implant visibility, palpability, and exposure; and capsular contracture) are mitigated with the subpectoral technique because of the thicker coverage provided by the muscle. The elevation of the muscle, however, has introduced its own set of problems. A major concern is functional impairment of the muscle, particularly the adduction, anteversion, and internal rotation of the upper limb. In addition, animation deformities caused by muscle contraction, chest tightness, pain, and muscle spasm are other significant concerns. Furthermore, subpectoral placement creates an unnatural state as the natural breast is anterior to the muscle. Despite these concerns, patients who have had dual-plane reconstruction with ADM can have aesthetically pleasing results but with any contraction of the pectoralis major, animation deformity on the chest can immediately be noted. Fat grafting is utilized as a means to establish a natural gliding surface between the thin mastectomy flap and the pectoralis/ADM interface which decreases the pain and the visual deformities associated with the scarred mastectomy flap being pulled by the pectoralis major. This ameliorates but does not eliminate animation deformity and some degree of animation remains.
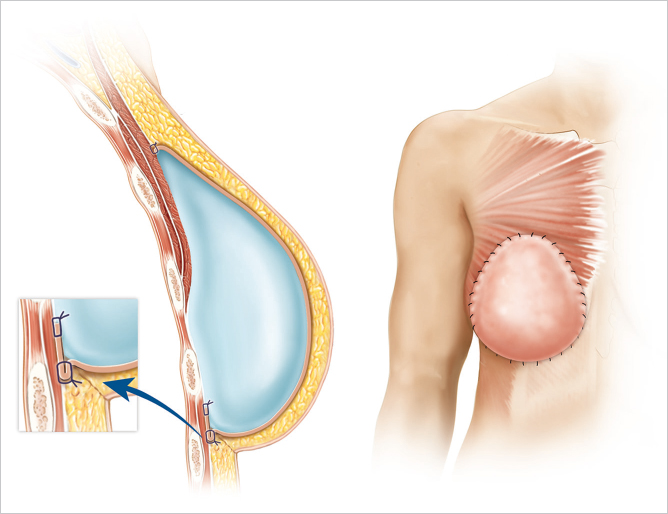
As a further means to alleviate muscle-related issues, surgeons have resorted to moving the implant from the subpectoral back to the prepectoral (subcutaneous) position in certain revision cases. Using the bioengineered breast concept has overcome the original limitations of subcutaneous placement and has greatly improved the aesthetic outcomes. Using form-stable implants and reinforcing the entire breast pocket with ADM to mimic muscle coverage and use of fat grafting to enhance the thickness and the gliding ability of the subcutaneous pocket has produced consistent outcomes with resolution of animation deformity and implant-related complications in appropriately selected patients.
44.3 Why Prepectoral Device Placement?
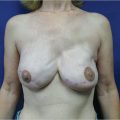
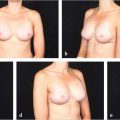
Prepectoral breast reconstruction offers the patient several short and relatively painless outpatient procedures with minimal recovery time. By not dividing and elevating the pectoralis major muscle, primary prepectoral reconstruction can be performed as an outpatient procedure, particularly with the addition of regional anesthetic nerve blockade. Patients have minimal pain postoperatively and use of opioids for analgesia has been reduced considerably. Many patients are placed on nonsteroidal anti-inflammatory medications and do quite well. Because of the relatively painless recovery, patient satisfaction is quite high (▶Fig. 44.1).
Patients who have undergone prepectoral reconstruction also do not complain of chest tightness, chronic pain, pectoralis major muscle spasms, or hyperanimation deformities. Hyperanimation deformities were challenging to correct, and patients were dissatisfied with the appearance and even the pain associated with it. These findings were often present in women who have undergone dual-plane or full muscle coverage breast reconstruction and prior to the rebirth of prepectoral breast reconstruction, these patients would be either salvaged with autologous reconstruction, or they would opt to have their devices removed and would forego any reconstruction due to persistent pain and discomfort.
With prepectoral breast reconstruction, aesthetic outcomes are, on the whole, quite impressive. Without the distracting forces of the pectoralis major muscle negatively acting on the implant, the device is truly shaping the reconstructed breast. Additionally, over long periods of time, the pectoralis major muscle is not exerting forces on the device, which historically has led to inframammary fold malposition, bottoming out, or lateral migration. The cleavage lines of the reconstructed breast are more predictable, as the medial insertion of the pectoralis major muscle on the sternum does not limit the medial extent of the device. This combined with autologous fat grafting has enabled the reconstructive breast surgeon to create beautiful and highly predictable aesthetic outcomes.
44.4 Two-Staged Reconstruction
A two-staged approach affords us the ability as reconstructive breast surgeons, to expand the skin envelope following cancer extirpation in order to achieve a soft, pliable reconstructed breast. The two-staged approach also allows us to precisely define the footprint of the reconstructed breast using biodimensional analysis and the patient’s anatomy. This systematic approach creates a natural-looking breast with long-term soft tissue stability. A two-staged reconstruction also offers the reconstructive surgeon a second chance to perfect the pocket and improve the overall outcome.
Use of tissue expanders offers patients a degree of control and allows them to provide input regarding their requests for size, shape, and aesthetic outcome. Patients can watch their reconstructed breasts evolve, and tissue expanders provide them with a glimpse of their ultimate potential outcome.
In breast reconstruction, the tissue expander functions more like an adjustable sizer and promotes communication between the plastic surgeon and the patient. In the prepectoral space, there is little to no pain for the patient during the expansion process. As the pectoralis major muscle is not being repeatedly stretched, patients maintain comfort throughout. Most patients require several office visits for postoperative fills as prepectoral reconstruction allows aggressive intraoperative volumizing provided that the mastectomy skin flaps are well-perfused. Tissue expanders are filled with either saline or air, depending on surgeon preference.
Technical Pearl
It is my preference to use air throughout the entire fill process, as it is lighter and less stressful on the mastectomy skin flaps, which reduces skin flap pressure and presumably necrosis.
Additionally, air is more comfortable for the patient and it is more efficient to inject. The mandatory bags of saline and sterile tubing are not a requirement when air is used, and thus, this technique is far less costly than saline.
Technical Pearl
The only two patient populations that still receive saline in their tissue expanders in my practice are as follows:
Patients receiving postmastectomy radiation with expanders in place.
Patients who will be traveling by air.
Radiation oncologists prefer to target their beams through saline and higher atmospheric pressures can lead to expansion of air in the expanders which could potentially lead to severe pain or even skin necrosis.
When filling the expanders with air or saline, meticulous aseptic technique is employed and ChloraPrep (chlorhexidine/alcohol) is used to prepare the skin. It is advisable to minimize the number of times the skin is penetrated in order to minimize risk for infection.
44.5 Prepectoral Considerations
When evaluating a patient for prepectoral breast reconstruction, several things must be considered. This is a technique that is not appropriate for every patient. A thorough evaluation of the patient’s previous surgical history, history of previous radiation, and pertinent medical history is paramount. It is important to have good communication with the breast surgeon in order to ensure that from an oncologic standpoint, prepectoral reconstruction is plausible. It is also standard for me to have a candid discussion with the patient regarding the history of prepectoral/subcutaneous breast reconstruction in order to quell the many misconceptions of this procedure. The following is a collection of indications and contraindications that I consider.
44.5.1 Reconstructive Indications
Although the prepectoral approach presents an alternative muscle-sparing reconstructive approach, not all patients are candidates for this approach.
Flap vascularity: Critical to this approach is the availability of adequately vascularized skin flaps. Because of the proximity of the implant/tissue expander to the skin flap, any skin-related complications can potentially compromise the reconstruction; hence, the absolute indication for adequately vascularized flaps. To ensure integrity of skin flap perfusion, close collaboration with general surgeons to minimize aggressive mastectomies is critical. In addition, utilization of tissue perfusion assessment devices (indocyanine green laser fluorescence or hyperspectral imaging) prior to prepectoral reconstruction is essential in every patient.
Risk factors: Smoking, prior radiation can compromise skin perfusion; the prepectoral approach should be contraindicated in such patients at this time.
Candidates should also have adequate fat depots for fat grafting as it is integral to the prepectoral approach.
Comorbidities: Obese and immunocompromised patients are at increased risk of complications in general and are not suitable for this approach. Obese patients (body mass index [BMI] > 35 kg/m2) with no radiation history can undergo delayed prepectoral reconstructions. These patients undergo a delayed first-stage reconstruction, which includes fat grafting under the mastectomy focusing on raising the central mastectomy scar in preparation for the second stage of prepectoral reconstruction. Elevation of the central scar helps in minimizing complications in a prepectoral placement, given the tendency for thin flaps around old scarred incisions.
44.5.2 Oncologic Guidelines and Indications
The oncologic guidelines for the prepectoral approach remain to be defined. As a multidisciplinary treatment plan is most optimal for the comprehensive care of the breast cancer patient, a combined effort is needed to balance the safest oncologic care with the most desirable aesthetic outcome. The oncologic treatment plan is frequently configured based on availability of resources and the willingness of each discipline to embrace and be able to safely adapt to changes brought about by new techniques.
Prepectoral reconstruction can be safely performed in patients undergoing skin-sparing mastectomy for breast cancer using the same oncologic parameters as would be applied for subpectoral implant-based reconstruction. As with subpectoral reconstruction, extreme caution should be exercised for large tumors, positive lymph nodes, as well as skin and chest wall involvement, especially in centers where postmastectomy radiation therapy is not utilized. When performing nipple-sparing mastectomy, identical guidelines to those used for nipple-sparing mastectomy with subpectoral reconstruction should be followed. As these guidelines are continuing to evolve, it is prudent to be cognizant of available randomized, prospective, clinical trial results. Further, it is imperative to approach patient selection from a multidisciplinary standpoint, involving the surgical team (reconstructive and oncologic) as well as medical and radiation oncology in any case where adjuvant radiotherapy is anticipated.
In addition to these general guiding principles, a number of oncologic contraindications exist. In particular, patients with late-stage breast cancer, posterior tumors lying near the pectoralis major muscle, and those with high risk of recurrence, are not candidates for prepectoral placement as the oncologic safety of prepectoral placement is not yet known. Monitoring for cancer recurrence is, thus, recommended using a protocol similar to that undertaken in autologous reconstructions. Autologous flaps are routinely placed in a prepectoral location and these flaps are regularly monitored for cancer recurrence by clinical examinations and at times by radiologic screening. Similarly, in prepectoral reconstruction with implants, clinical and monthly self-breast examinations are important, as breast cancer recurrence in the majority of the cases occurs under the mastectomy flap. Magnetic resonance imaging (MRI) studies of prepectoral reconstructions using the U.S. Food and Drug Administration criteria for implant surveillance can be initiated with and without IV contrast beginning at 3 years after reconstructive surgery and every 2 years thereafter. MRI not only monitors the implant but also helps monitor the overall chest.
44.5.3 Reconstructive Contraindications to Prepectoral Reconstruction
Poorly perfused flaps.
History of radiation.
High BMI (relative contraindication).
Immunocompromised.
HbA1c > 7.5.
Active smoker.
Lack of fat donor site (relative contraindication).
44.5.4 Oncologic Contraindications to Prepectoral Reconstruction
Large tumors (> 5 cm).
Late-stage cancer (stage III and IV).
Deep tumors.
Chest wall involvement.
Clinically palpable axillary nodes.
High risk of recurrence (based on multidisciplinary approach).
44.5.5 Patient Assessment
A complete and thorough history and physical examination is initiated. Breast surgical history, history of previous breast radiation therapy, and family history of breast cancer are important details, which may impact the reconstructive process. History of cosmetic breast augmentation, reduction mammoplasty, or mastopexy will influence the ultimate mastectomy incision.
In my practice, prepectoral reconstruction is contraindicated in patients who have received previous radiation. In our early experience, patients who received previous radiation had higher complication rates during prepectoral reconstruction. Patients who have undergone aesthetic dual-plane breast augmentation are typically converted to prepectoral expanders at the time of the mastectomy due to the improved aesthetics, lack of hyperanimation, and overall improved patient satisfaction we have seen with this technique.
On physical examination, biodimensional analysis is utilized to determine the ultimate footprint of the reconstructed breast. Measurements are taken including the sternal notch-to-nipple distance, the nipple to inframammary fold, and perhaps the most important objective assessment is the ideal breast base width (IBBW). It is the IBBW which is ultimately going to direct me regarding the final implant base width. The breasts are evaluated for symmetry, nipple position, and skin laxity. Palpation of the contralateral breast and axilla is performed to document lack of abnormal finding. Assessments are made regarding mastectomy incision, the potential (from the reconstructive standpoint) for nipple-sparing mastectomy, as well as the appropriate device to be ultimately placed.
44.5.6 Topics for Discussion between Patient and Surgeon Regarding Two-Staged Reconstruction
Tissue Expanders
Potential issues and complications of tissue expanders must be discussed. Device failure, displacement, malposition, infection, palpable folds, discomfort, rippling, asymmetry, as well as need for further operations are things that must be discussed with patients. I explain to patients that the tissue expanders may feel slightly more uncomfortable than the final implant and I express to them that the tissue expanders also do not have the same excellent aesthetic appearance as the final implants. In my practice, the tissue expanders function similar to an adjustable sizer, which both allows the patient to witness variable sizes and projections, and it also allows the surgeon to determine what the patient’s subjective wishes are for final outcome.
Hospitalization and Recovery
Women who undergo mastectomy with immediate tissue expander/ADM reconstruction either go home the same day or stay overnight in the hospital. Particularly when combined with regional anesthesia nerve blockade, patients typically are able to go home the same day. The subsequent second-stage surgery where the expanders are exchanged for final implant combined with autologous fat grafting is performed as an outpatient.
The recovery following the original procedure is 1 month. From a comfort standpoint, patients have little to no pain postoperatively and most patients take little to no narcotics. I don’t let patients use their arms without restriction for 1 month—mainly to allow integration of the ADM. The hallmark of the recovery process is truly to maximize the integration of ADM to the overlying skin flaps by reducing shearing forces. Patients will have little discomfort following surgery and will try to push themselves back to a normal routine. The surgeon must be vigilant in preventing this in order to minimize complications like nonintegration of the ADM, postoperative seromas, and wound dehiscences. Postoperative expansion is begun in the office at 2 to 3 weeks postoperatively and continues weekly until complete expansion has been achieved. I typically fill the expanders with air to the point of maximum fill based on the device. If air dissipates, patients return to the office for completion expansion prior to the second-stage procedure. I perform second-stage reconstruction with capsulotomy, tissue expander exchange for final implant, and autologous fat grafting at 3 months following the mastectomy.
For patients undergoing postmastectomy radiation therapy, it is my preference to achieve the final implant prior to the beginning of radiation therapy. Thus, if patients have completed neoadjuvant chemotherapy and radiation is set to begin 4 to 6 weeks following the mastectomy, patients come in twice a week for fills and they undergo second-stage surgery at 3 weeks. Two weeks later radiation begins. By not having the pectoralis major muscle intimately involved with the expander or implant, there is no tightening or contracting of the muscle once it has been radiated, and we are no longer seeing the inframammary fold malpositions and contractures (particularly since using ADM) that were relatively common with dual-plane or full muscle reconstructions. For patients receiving both chemotherapy and postmastectomy radiation, I typically perform second-stage reconstruction 3 weeks following completion of chemotherapy. For some patients who receive postmastectomy radiation resulting in severe skin damage, autologous reconstruction is used as a salvage procedure.
Patient Convenience
It is imperative that the patient understands the realities of a staged reconstruction, namely, there will likely be two to three separate procedures to achieve the final outcome. With prepectoral breast reconstruction, I have a candid discussion regarding rippling which occurs in some capacity, in almost all patients. Rippling is corrected with autologous fat grafting, but I explain to patients that they may need one to two rounds of fat grafting to completely correct any rippling.
Patients are also counseled that they will require several postoperative in-office fills to achieve the desired volume in their expanders. Although not used in my practice, there are tissue expanders that allow patient-directed expansion in the comfort of their own home. By simply pushing a button on a remote control, 10 cc of compressed CO is automatically injected into the expander in a controlled and gradual fashion, without the use of needles.
Unlike with subpectoral reconstruction, patients undergoing prepectoral tissue expansion experience relatively little pain from the fill process, as the muscle is not being stretched. Because intraoperative fill volumes are typically higher due to no pectoralis involvement, patients require only a few postoperative fills to achieve their desired volume.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree