Vascular Tumors
Margaret S. Lee MD, PhD
Marilyn G. Liang MD
Classification
Vascular tumors can be characterized by the following methods:
Clinical and demographic characteristics of the patient (e.g., age, immune status)
Gross clinical characteristics of the tumor (size, shape, color, distribution, evolution over time)
Histology
Radiologic studies to assess blood flow, circumscription, and depth of involvement (Doppler ultrasound, computed tomography [CT], magnetic resonance imaging [MRI])
In most cases, the tumors can be diagnosed using just the first two categories of information.
In this chapter, we will discuss the most common and most concerning vascular tumors: infantile hemangioma (IH), congenital hemangiomas, pyogenic granuloma (PG), kaposiform hemangioendothelioma (KHE), tufted angioma (TA), Kaposi’s sarcoma (KS), and angiosarcoma.
Tumors versus Malformations
Before we discuss tumors in detail, it is important to differentiate between tumors and malformations. This distinction is relatively new in the study of vascular anomalies. In the past, vascular lesions were not well understood and all were called hemangiomas or some derivative thereof using the suffix -oma. Mulliken and Glowacki proposed a new classification system for vascular anomalies in 1982 (revised formally in 1996 by the International Society for the Study of Vascular Anomalies) based on histologic and pathophysiologic features. Tumors are cellular masses with postnatal endothelial proliferative potential. Malformations are products of abnormal vasculogenesis during fetal development. They enlarge over time predominantly through distention of congenitally malformed vessels rather than true endothelial cell proliferation as in an infantile hemangioma.
Benign Tumors
Infantile Hemangioma
The most common vascular tumor is the benign infantile hemangioma (IH). These tumors are unique in that they eventually involute over years without treatment, sometimes leaving no trace on the skin surface.
Presentation and Characteristics
Demographics. Two percent of all newborns present with an IH. The prevalence of IH is about 10% of all Caucasian children. Less than 2% of black and Asian infants are affected. There is a clear female preponderance, in a ratio of about 3-5:1. IH are much more common in premature infants, twin/multiple gestations, infants of older mothers, and infants whose mothers underwent chorionic villus sampling.
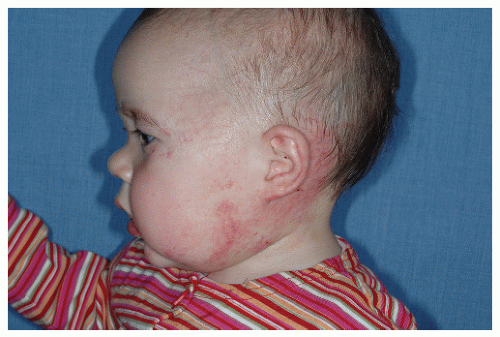
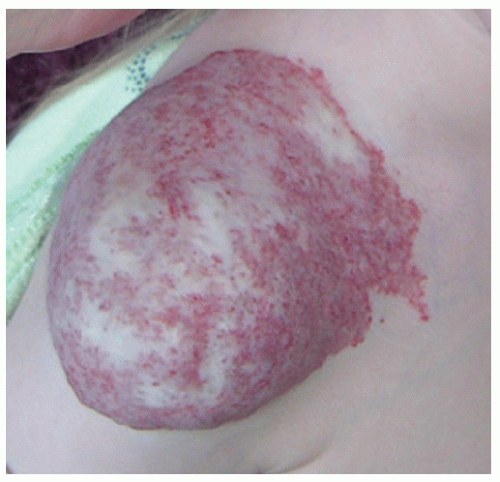
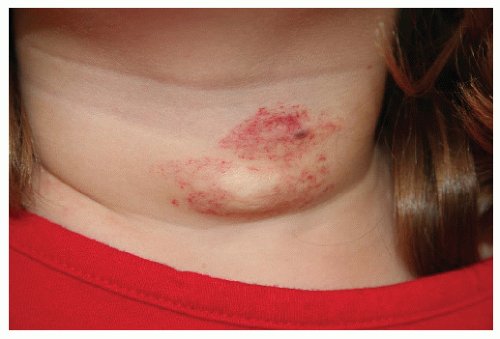
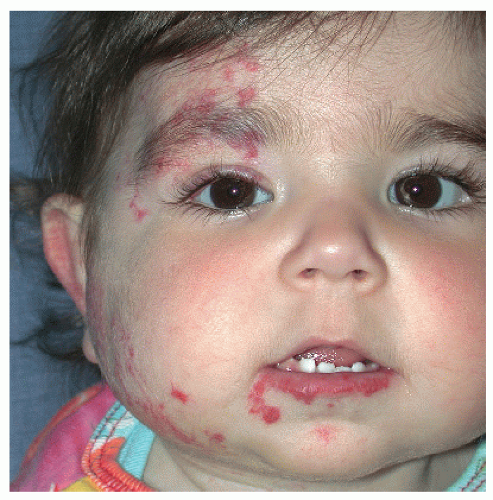
Description. Most lesions are present at birth or appear within the first week of life, often as a precursor lesion that looks like telangiectasias or a “bruise like” macule. The final size of IH ranges tremendously, from spotty macules and papules a few millimeters in diameter to extensive plaques encompassing several developmental regions. IH can be described as superficial, deep, or mixed. Superficial IH are well-demarcated papules or plaques raised above the skin surface, with a bright red color during the proliferative and early plateau phases (Fig. 30-1). Deep IH are visible through the skin surface as a bluish-purple nodule and often seem softer and more compressible than superficial plaques (Fig. 30-2). Mixed superficial and deep IH combine a bright red superficial lesion overlying a deep component. Reticular IH are thinner, more discontinuous superficial lesions than the typical superficial plaque-type IH (Fig. 30-3).
Distribution. IH are most commonly found on the head and neck (up to 85%). However, they can present on any part
of the body. A patient may have several lesions at the same time. IH can be described as focal, multifocal, or regional:
of the body. A patient may have several lesions at the same time. IH can be described as focal, multifocal, or regional:
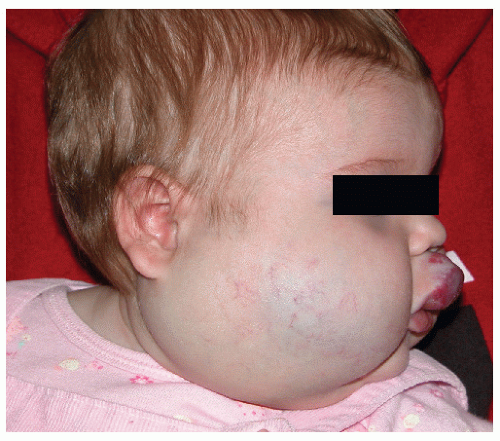 FIGURE 30-2 ▪ Deep infantile hemangioma. Also note mixed superficial and deep infantile hemangioma on the lip. |
Focal—a solitary lesion
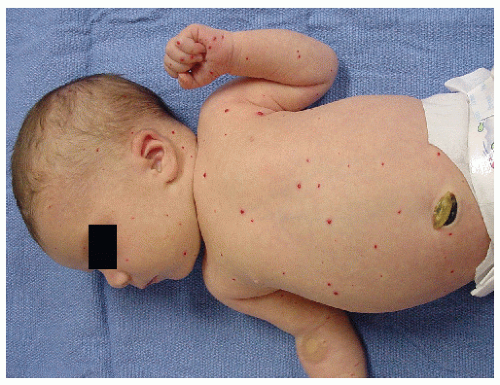
Multifocal—multiple lesions involving different anatomical regions (Fig. 30-4)
Regional—a lesion encompassing a large surface area but limited to one anatomical or developmental region (also termed segmental) (Fig. 30-5)
Course. A third of IH are present at birth, and the rest become apparent within the first few weeks of life. The clinical course of IH includes a proliferative, plateau, and involution phase. Superficial IH quickly become bright red over the first month of life. Deep IH may not become evident until 2 to 4 months of life. IH become softer and grayer or paler in color as they plateau in size, then involute (Fig. 30-6). At the end of involution, the lesions, if visible at all, range from scattered telangiectasias to pale, fibrofatty, baggy outpouchings or
soft, irregular plaques (Fig. 30-7). Reticulate IH involute very nicely, leaving behind only telangiectasias.
soft, irregular plaques (Fig. 30-7). Reticulate IH involute very nicely, leaving behind only telangiectasias.
The proliferative phase typically lasts for 6 to 10 months, although very small IH may not proliferate much and can involute away within the first year or two of life. The plateau phase varies from 1 to 4 months. Involution time tends to be proportionate to the size of the lesion; larger and thicker lesions require more time to involute. A general rule of thumb in counseling parents is that 50% of all IH stop involuting by the time a child is 5 years old. IH may continue to involute until age 10.
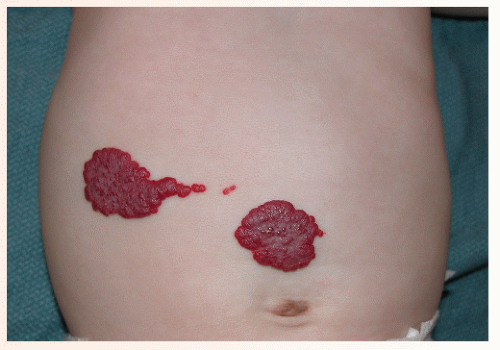
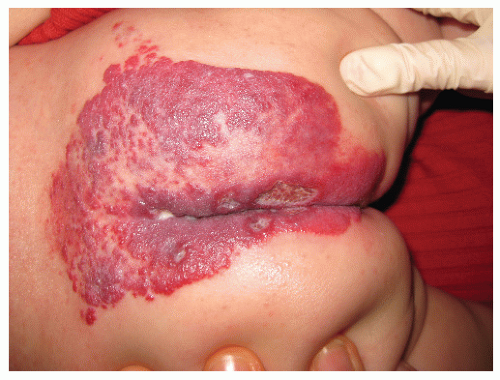
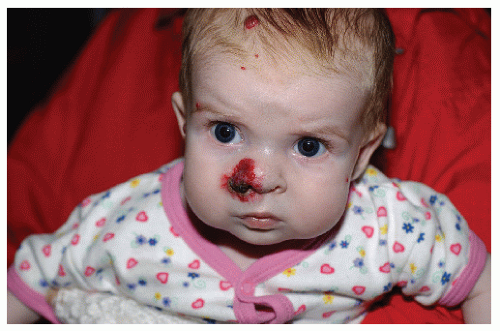
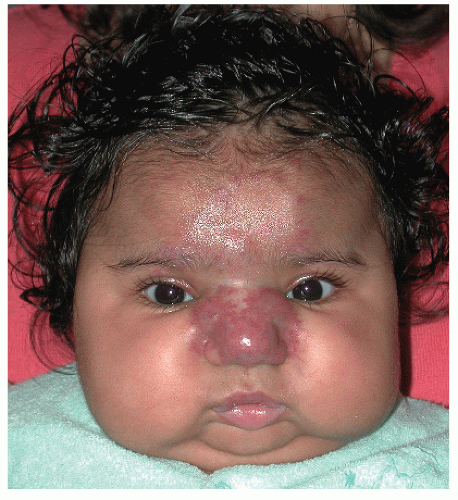
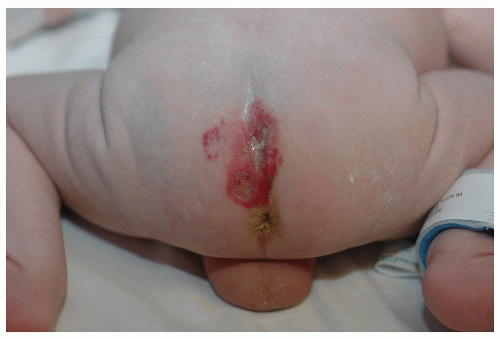
The most common complication is ulceration. This can occur due to rapid tumor proliferation, friction/trauma, or infection. Hence, the most common sites for ulceration are intertriginous sites, especially the perineum (Fig. 30-8). Other complications include impingement upon vital structures, such as the airway or visual axis, and deformation of anatomical structures such as the nose (Fig. 30-9) and lips. IH in the “beard” distribution correlate with risk of airway involvement and respiratory distress; the larger the IH in this location, the greater the risk of airway obstruction (Fig. 30-10). If an IH is large enough (e.g., in the liver), it can overburden the circulatory system, causing high-output cardiac overload.
Cause. We do not yet fully understand what causes some children to be born with IH. Recent evidence supports the existence of an IH endothelial stem cell that drives the tremendous proliferative potential of these tumors. The source of these stem cells is unknown. One possibility is that they are derived from the embolization of placental cells to the developing fetus; support for this phenomenon lies in the increased incidence of IH in babies whose mothers underwent chorionic villus sampling during pregnancy. In the fetal or postnatal tissue environment, these stem cells may proliferate due to an angiogenic milieu as compared to that in placental tissues.
Histology
On hematoxylin and eosin (H&E) preparations, proliferating IH are composed of a dense collection of endothelial cells and pericytes forming many capillary vascular channels
arranged in lobules. These lobules are fed and drained by larger vessels. Naturally, larger tumors have larger feeding arteries and draining veins. IH are characterized by positive erythrocyte type glucose transporter-1 (GLUT-1) staining, which is also expressed by placental endothelial cells. Sometimes normal appendageal structures, such as hair follicles and nerves, may be found within the capillary lobules. During involution there is a steady increase in fibrofatty tissue replacing the endothelial and stromal cells and vascular channels. Persistence of some of the afferent and efferent vessels probably correlates with grossly visible remnant telangiectasias.
arranged in lobules. These lobules are fed and drained by larger vessels. Naturally, larger tumors have larger feeding arteries and draining veins. IH are characterized by positive erythrocyte type glucose transporter-1 (GLUT-1) staining, which is also expressed by placental endothelial cells. Sometimes normal appendageal structures, such as hair follicles and nerves, may be found within the capillary lobules. During involution there is a steady increase in fibrofatty tissue replacing the endothelial and stromal cells and vascular channels. Persistence of some of the afferent and efferent vessels probably correlates with grossly visible remnant telangiectasias.
Radiologic Studies
Imaging is usually not needed to diagnose or assess an IH. Doppler ultrasound can be very helpful in differentiating a bluish, deep IH from a venous malformation based on blood flow characteristics. IH are fast flow lesions whereas venous malformations and other tumors are slow flow lesions. Standard ultrasound can be used to screen for spinal dysraphism occurring with a sacral IH or for liver IH in a patient with multifocal cutaneous hemangiomas. MRI is necessary when vital structures are compromised or to identify intracranial abnormalities associated with regional facial IH.
Associations to Consider
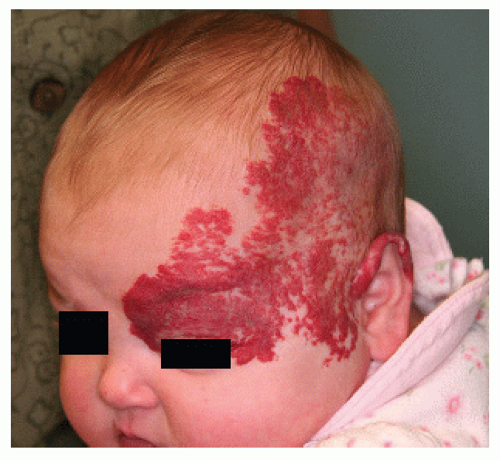
PHACES Association (Posterior fossa malformations, Hemangioma, Arterial anomalies, Coarctation of the aorta and Cardiac anomalies, Eye anomalies, Sternal clefting). This condition is much more common in females than in males. Patients with an IH encompassing one or more developmental regions on the head and neck should have a workup for the above defects. It is not necessary to have more than one or two of the additional associations to carry a diagnosis of PHACES (Fig. 30-11).
Reticular Infantile Hemangioma of Perineum and/or Limb. These IH can be associated with chronic ulceration and ventralcaudal anomalies such as omphalocele or genital anatomical defects such as fistulae or ambiguous genitalia. Renal anomalies, imperforate anus, and high-output cardiac overload has also been described.
Tethered Cord or Other Spinal Abnormalities. An IH, reticular or plaque type, overlying the lower spine should be investigated radiologically to rule out tethered cord (Fig. 30-12), occult spinal dysraphism, lipomeningocele, or, very rarely, extension of IH into the spine.
Multifocal with Visceral Infantile Hemangiomas. Patients who present with five or more superficial IH scattered over the body should be evaluated for visceral IH, anemia, and congestive heart failure. One should perform ultrasonography or MRI of the liver to rule out liver lesions, the most common visceral site of involvement. Large liver IH can cause profound hypothyroidism because IH produce type 3 iodothyronine deiodinase. This deiodinase inactivates thyroxine and triiodothyronine. The deiodinase has also been found in cutaneous IH, but there are no reports of clinical hypothyroidism associated with cutaneous IH. Hepatomegaly from liver IH can potentially lead to cardiac output overload and death. In other cases, the mass effect from large liver IH can cause abdominal compartment syndrome, leading to respiratory and renal failure.
Differential Diagnosis
IH are relatively easy to diagnose clinically by the first month of life. If there is ambiguity for any reason (e.g., deep IH), a simple and important feature to keep in mind is that IH are fast-flow lesions. Doppler investigation detects an arterial signal in fast-flow lesions.
Precursor lesion DDx:
Ecchymosis/birth trauma: Observation will easily clarify the difference since true ecchymoses will resolve within a week.
Kaposiform hemangioendothelioma
Tufted angioma
Superficial hemangiomas DDx:
Capillary malformation (CM): A very early superficial IH might theoretically be macular and confused with a CM. However, observation will show relatively rapid evolution from patch to papule or plaque. Usually an IH changes rapidly enough that a diagnosis of capillary malformation is not considered. CM are slow-flow lesions.
Deep hemangioma DDx:
Cyst or cyst-like lesions (e.g., dermoid cyst, pilomatrixoma): Ultrasonography is indicated if there is insufficient or inconsistent information supporting a clinical diagnosis of IH.
Congenital hemangiomas: These are fully formed at birth and either involute much more rapidly than IH or do not involute.
Soft tissue sarcomas (fibrosarcoma, rhabdomyosarcoma)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree