Introduction
There has been a significant increase in the popularity of mammaplasty techniques being used for the reconstruction of partial mastectomy defects prior to breast irradiation for women with early-stage breast cancer. This has been fueled in part by the ever-expanding indications for breast conservation therapy (BCT), and the desire to improve outcomes from an oncologic standpoint as well as a cosmetic one. Although breast-sparing surgery has demonstrated equivalent survival rates, poor cosmetic results are not uncommon in certain patients. One such group of patients are those with large or ptotic breasts. Macromastia was initially felt to be a relative contraindication to BCT with poor cosmetic results and less effective radiation therapy. Radiation-induced fibrosis is felt to be greater in women with larger breasts, given the dosing inhomogeneity. Late-radiation fibrosis has been demonstrated 36% of the time in patients with larger breasts, compared to 3.6% for smaller breasts. Higher doses of radiation therapy are often necessary in women with larger breasts, contributing to morbidity and adversely affecting the appearance. The cosmetic results following BCT in women with large breasts are also reduced. Clarke has shown excellent results in 100% of women with A-cup breasts following BCT, compared to 50% in women with D-cup breasts. On the other hand, women with macromastia and large pendulous breasts are often overweight, and total breast reconstruction is more challenging, being associated with higher complication rates and less favorable cosmetic outcomes. The addition of reduction mammaplasty techniques was therefore welcomed by the patient, the ablative surgeon and the reconstructive surgeon. It allows women with macromastia to be candidates for breast conservation without having to accept significant deformities, allows the ablative surgeon to remove a generous amount without having to worry about a residual deformity, and it makes breast reconstruction more predictable in an otherwise difficult patient population. In addition to the many potential benefits of combining these two techniques, the disadvantages in well-selected patients are minimal. Oncoplastic reduction techniques initially became popular in Europe for reconstructing quadrantectomy defects in the lower pole. In the United States, their popularity likely evolved out of frustration in the management of breast cancer patients with macromastia, however, and is now one of the most common methods used for reconstructing partial mastectomy defects at the time of resection. As long as we continue to demonstrate high levels of patient safety and patient satisfaction, the oncoplastic reduction techniques will likely become even more popular in the future.
Indications
The indications for using oncoplastic reduction techniques are numerous. In addition to the cosmetic and oncologic reasons, the quality-of-life benefits to breast reduction surgery for women with macromastia have already been demonstrated. The two main reasons to reconstruct partial mastectomy defects are: (1) to increase the indications for BCT, making breast conservation practical in patients who otherwise might require a mastectomy; and (2) to minimize the potential for a poor aesthetic result ( Table 6.1 ). The decision is usually based on tumor characteristics (size and location) and breast characteristics (size and shape).
| Cosmetic reasons | Oncologic reasons |
|---|---|
| High tumor to breast ratio (>20%) | Concern about clear margins |
| Tumor location: central, inferior, medial | Wide excision required |
| Macromastia | Poor candidate for mastectomy and reconstruction (i.e., age, breast size) |
| Large tumor | Patient desires BCT |
| Patient desires smaller breasts | More effective radiation therapy |
| Significant ptosis, or breast asymmetry | Quality-of-life benefits |
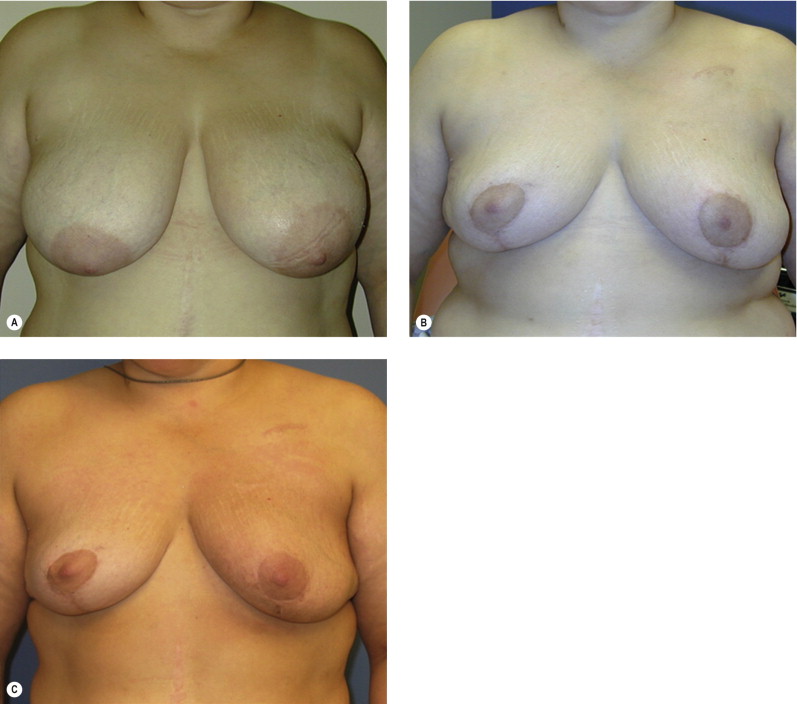
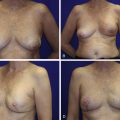
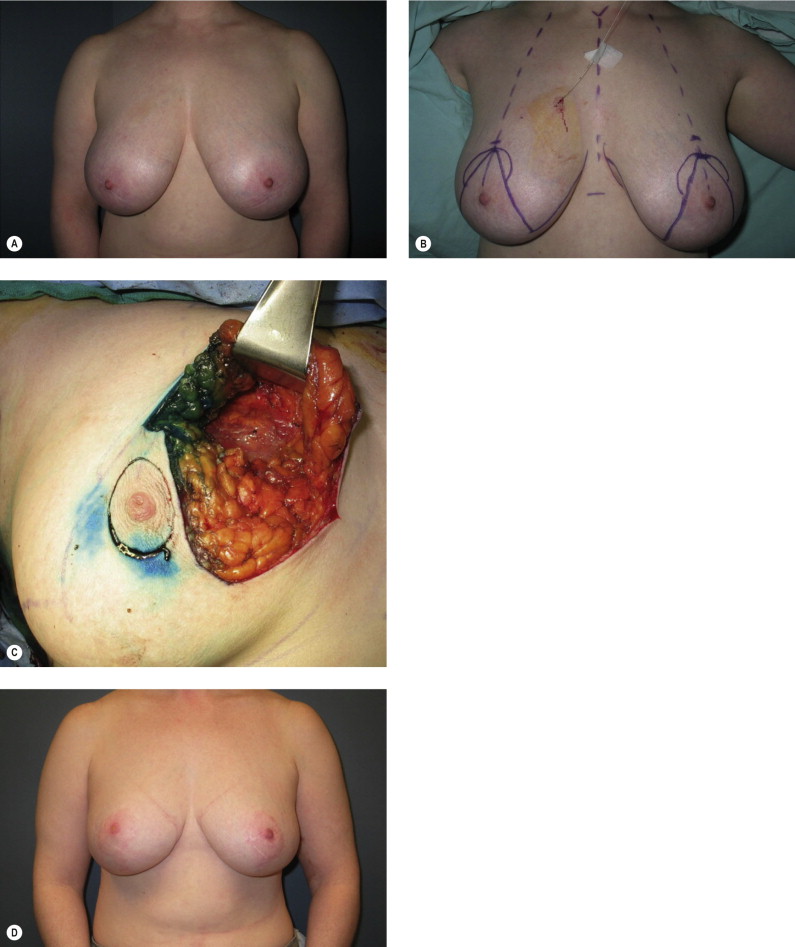
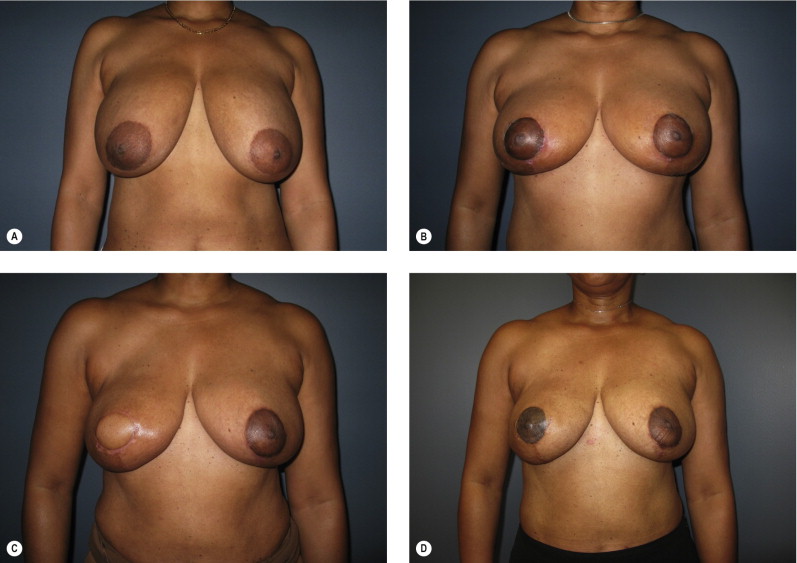
Women with large pendulous breasts who are felt by the surgeon to be poor candidates for BCT alone benefit from the oncoplastic reduction techniques, minimizing the potential for a poor cosmetic result and allowing them to be candidates for breast conservation ( Fig. 6.1 ). The ideal patient is one whose tumor can be widely excised within the reduction specimen, and for whom a smaller breast is viewed as a positive outcome. Older women with macromastia are well suited for this approach compared to mastectomy and reconstruction ( Fig. 6.2 ). Another indication for the oncoplastic reduction technique is when the surgeon anticipates a large defect, or is concerned about being able to achieve clear margins in women with moderate to large breasts. The potential for an unfavorable result exists in this situation regardless of breast size or tumor location. Other indications are patient driven, in those women who desire breast conservation, or who desire smaller breasts due to their limitations caused by symptomatic macromastia. As we become more comfortable with these techniques, the indications will become more liberal. Essentially anyone with large breasts amenable to breast conservation is a candidate for this procedure. However, the importance of stringent patient selection criteria cannot be overstated, and is required to ensure maximal cosmetic outcomes as well as oncological safety.
Contraindications include patients who are not good candidates for breast conservation, a history of prior irradiation or situations when there is insufficient residual breast tissue following resection to allow reshaping. Similar selection criteria are used when deciding on elective breast reduction procedures and need to be taken into consideration. Patients with multiple medical comorbidities or active smokers are not ideal candidates for additional elective surgery, and the risks will often outweigh the benefits in these situations.
Patient selection and margin status
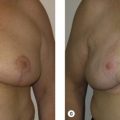
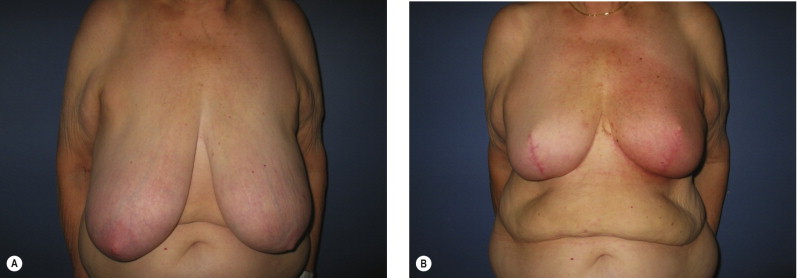
One of the most important variables in ensuring a safe oncologic outcome is patient selection and how it relates to margin status. Positive margins on final pathology are potentially complicated by altered architecture. The options for managing positive margins include re-excision or completion mastectomy and reconstruction. The extent of the disease in these situations, especially given the previous generous oncoplastic resection, will often dictate that completion mastectomy is a more appropriate treatment plan. If re-excision is performed, this needs to be done with the reconstructive surgeon. Fortunately, the incidence of positive margins using this approach is felt to be less given the more generous resections. We have demonstrated an average specimen weight over 200 g in oncoplastic resections, compared to about 50 g for non-oncoplastic procedures ( Fig. 6.3 ). The incidence of positive margins is lower in oncoplastic resections. When completion mastectomy and reconstruction are required, the disadvantages of the reduction procedure are minimal. The benefits of this approach are that (1) no reconstruction options (i.e., flaps) have been used, (2) the contralateral symmetry procedure has already been performed, (3) skin envelope has been reduced, and (4) it is now easier to reconstruct a smaller reduced breast than a large ptotic one ( Fig. 6.4 ).
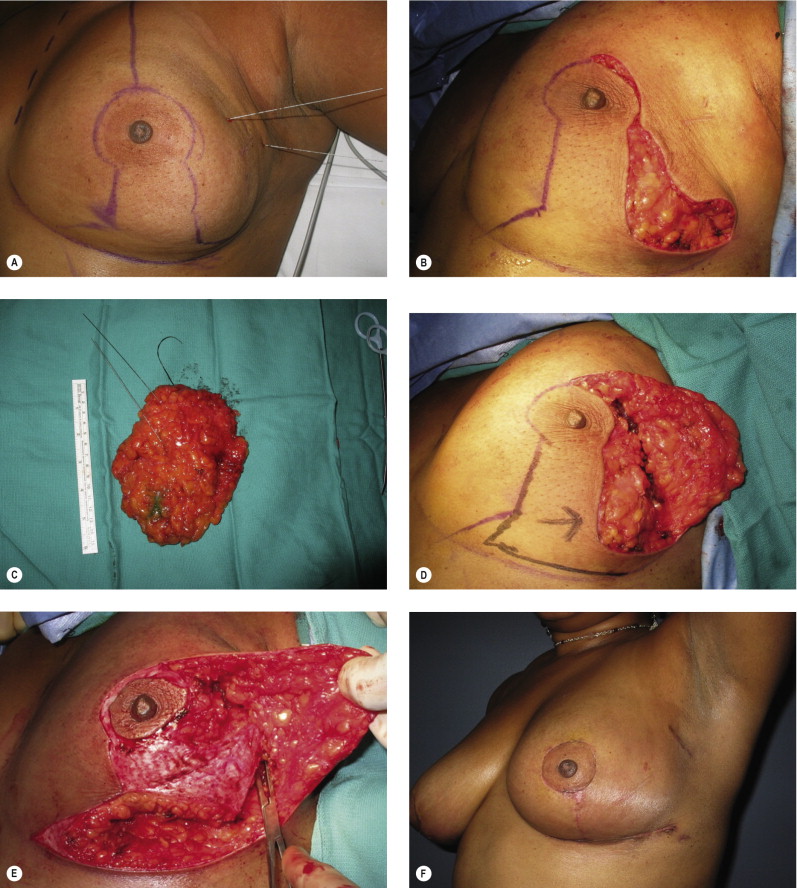
One way to avoid positive margins is to delay reconstruction a few weeks until confirmation of margin status has been obtained (delayed–immediate reconstruction). Most series report a positive margin rate of about 5–10% and, rather than perform an unnecessary second procedure 90–95% of the time, we need to minimize the incidence of positive margins. Preoperative breast imaging (i.e., magnetic resonance imaging (MRI), ultrasound or mammography) is helpful in determining the extent of the disease, guiding the necessary resection, and should be employed judiciously when indicated. An imaging study showed that tumor size was underestimated 14% by mammography and 18% by ultrasound, whereas MRI showed no difference when compared to the pathological specimen. Separate cavity margins sent at the time of lumpectomy significantly reduces the need for re-excision. Cao demonstrated that final margin status was negative in 60% of patients with positive margins on initial resection. Additional intraoperative confirmatory procedures include radiography of the specimen, and intraoperative frozen sections for invasive cancer. Patient selection is another important consideration. A recent series has demonstrated a higher rate of positive margins in women under the age of 40 years with extensive ductal carcinoma in situ (DCIS), suggesting delayed immediate reconstruction in those situations. Other patients with potentially difficult margin issues include prior chemotherapy, infiltrating lobular carcinoma, and multicentric disease. In these patients and in any other patient where there is intraoperative concern regarding margin status, the reconstruction should be delayed until margin status has been confirmed.
Operative approach
Steps
- 1.
Patient selection.
- 2.
Preoperative planning.
- 3.
Tumor resection.
- 4.
Reconstruction.
There are four steps to the operative approach. The first is patient selection , which has been discussed above. Once it has been established that the patient is a candidate for an oncoplastic reduction, the preoperative planning phase can begin. If this is being performed by a two-team approach, then it is crucial that communication exists between the teams. They should review the radiographic imaging, and discuss the anticipated defect location and defect size. This will assist with determination of the most appropriate glandular pedicle required to maintain nipple viability and reshape the mound. Always have a back-up plan, as occasionally the defect is different from that anticipated, and an alternative approach is required. The patient is marked preoperatively on both sides using relatively conservative markings. If a Wise pattern is drawn, vertical limbs are slightly longer than normal, and the angle is smaller (to ensure minimal tension on the incisions and reduce the potential for healing problems). If radiographically placed wires are being used for the lumpectomy, these should be examined and films reviewed. The combined team should discuss possible access incisions on the mound for tumor resection. Poorly placed incisions could interfere with viability of skin flaps and worsen results.
Tumor resection is then performed through or within Wise patterns if possible, with attention to blood supply and nipple viability. The specimen is weighed to assist with determination of resection goals on the contralateral side. Intraoperative margin assessment could include radiographical imaging, macroscopic assessment, frozen section, or touch cytology. Once separate cavity samples are sent to pathology, the cavity is clipped for postoperative surveillance and guidance for radiation boosts to the tumor bed if required.
Partial mastectomy reconstruction is initiated by examining the defect in terms of size and location. It is important to examine the remaining breast tissue and to determine where it is in relation to the defect, the nipple, and the breast mound.
Reconstructive goals are as follows:
- 1.
Keep the nipple alive and position it appropriately on the mound.
- 2.
Fill the dead space.
- 3.
Resect excess breast tissue when necessary.
- 4.
Reshape the breast mound using the pedicles and remaining breast tissue.
The first decision is how to keep the nipple alive . Typically the shortest pedicle will maximize nipple viability, and allow additional glandular manipulation without worrying about nipple compromise. Many options exist for nipple pedicles, and most surgeons have a favorite. For example, if the superomedial pedicle is your procedure of choice for standard breast reductions, then this technique could be employed for most oncoplastic defects if the patient is a candidate, as long as the defect location is not medial to the nipple. As a general rule, if the pedicle points to or can be rotated into the defect, it can be used. Occasionally it is not possible to preserve the nipple, either because of the size of the breasts, or the location of the tumor. Options include amputation and free nipple graft, or nipple reconstruction at a later date.
Once a decision has been made on nipple preservation, the pedicle is de-epithelialized and dissected with a cautery unit sufficient to allow rotation into the proposed nipple position. The second decision then is how to fill the dead space. At this point glandular resection has not yet been performed. If the defect is removed as part of a reduction specimen, and is adequately filled through glandular displacement with the pedicle, and/or remaining glandular tissue, then autoaugmentation is not required. If it is felt that additional glandular flaps are required to fill the dead space, as determined above, then a decision is made based on what tissue is available, and where it is in relation to the nipple pedicle. If the defect can be filled by rotating an extended portion of the original nipple pedicle, this is often the technique of choice. This single-pedicle autoaugmentation approach works well for smaller defects in women with smaller or moderate-sized breasts, or when tissue can be taken with the pedicle from a less cosmetically sensitive area and rotated to fill a defect. Another alternative in larger defects in women with large breasts is to fill the dead space with a secondary pedicle autoaugmentation approach if the primary pedicle or residual parenchyma is not sufficient. Two pedicles are often safer and will often reduce the length of each respective pedicle, subsequently minimizing the potential for fat necrosis, and maximize the ability to safely manipulate the glandular flaps. The other reason why an additional pedicle is often useful is that the primary nipple pedicle is limited in its range of motion, as the position of the nipple on the breast mound will dictate where that pedicle needs to be. Once it has been determined how to fill the dead space and reshape a breast mound, the excess dermatoglandular tissue can then be resected . The weight of the specimen is then added to this additional resection, calculating a total weight for that side. This is useful in trying to keep the ipsilateral breast larger. The breast mound shaping is then performed using the glandular pedicles and remaining breast tissue. Glandular shaping is performed using resorbable sutures where necessary, and the skin is then redraped over the mound. Drains are used if the defect is in communication with the axillary dissection.
Skin pattern
The Wise pattern markings are more versatile and allow easy access to tumor location anywhere within the breast mound. They also give more options to reconstruct the defect using glandular flaps. If it is unclear whether glandular flaps will be required to reconstruct the defect, the standard Wise flaps can be elevated about 1 inch thick up to the chest wall without resecting any additional breast tissue or skin. Numerous options will then exist for either primary or secondary pedicles to keep the nipple alive or fill the defect with the skin flaps, then redraped over the mound to complete the reconstruction. The vertical type reduction or mastopexy is useful for smaller breasts, when the defect can be easily accessed through this approach.
Contralateral breast
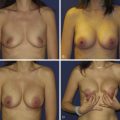
Management of the contralateral breast is typically performed using a similar technique to that used on the ipsilateral side to maximize symmetry. If an inferior pedicle was used on the involved breasts, an inferior pedicle is often chosen on the contralateral side. Since the ipsilateral side involves a volume loss procedure (partial mastectomy), glandular resection is always required on the opposite breast, even if a mastopexy technique was used for partial breast reconstruction. The contralateral side is purposely kept about 10% smaller than the ipsilateral breast to allow for anticipated radiation fibrosis ( Fig. 6.5 ). My preference is to perform the contralateral procedure at the time of resection. If minor changes in shape and size of the contralateral side are required years after radiation therapy these ‘fine-tuning’ procedures are easier and more predictable than doing the full reduction at that time, which might then require additional revisions to maximize symmetry ( Fig. 6.6 ). Other options include doing the opposite breast following breast irradiation, which then would commit everyone to a second procedure, and is often unnecessary since the contralateral revision rate when done simultaneously is only about 5–10%. It is important that the contralateral breast tissue be sent to pathology given the 2–5% incidence of synchronous breast cancer being diagnosed on that side in women with breast cancer.