Childhood Psoriasis
Psoriasis is a relatively common immune-mediated disorder that accounts for 4% of all dermatoses seen in children under 16 years of age and occurs overall in 0.5% to 0.8% of pediatric patients with a linear increase in prevalence by age from 0.2% at 2 years to 1.2% at 18 years. Psoriasis rarely is present at birth. Approximately one-third of adults with psoriasis noted the onset during the first 2 decades of life. As in the adult population, psoriasis occurs most often in Caucasian children. The severity of the condition may vary from a life-threatening neonatal pustular or exfoliative dermatosis to a mild, localized disorder that causes no distress. Psoriasis usually follows an irregularly chronic course marked by remissions and exacerbations of unpredictable onset and duration.
Both complex genetic and environmental factors participate in the risk of psoriasis. Approximately 30% of pediatric patients have an immediate family history of psoriasis, and the risk of occurrence in monozygotic twins is two to three times that of dizygotic twins. The major genetic determinant is PSOR1 (35% to 50% of patients), which is within the major histocompatibility complex on chromosome 6. Early-onset psoriasis has been linked to human leukocyte antigen (HLA) antigen Cw6, and 73.7% of patients with guttate psoriasis show HLA Cw6 antigen, in contrast to a rate of 7.4% in the general population. Autosomal dominant mutations in caspase recruitment domain family member 14 ( CARD14 ) or PSOR2 also lead to a psoriatic (or pityriasis rubra pilaris [PRP]) phenotype. At least 35 additional susceptibility loci for psoriasis have subsequently been identified at other chromosomal locations, among them genes regulating T-cell function, especially interleukin (IL)-23 function, tumor necrosis factor (TNF) activation, nuclear factor (NF)-κB signaling, T-helper (Th) 2 cytokines, interferon (IFN)-mediated antiviral responses, and macrophage activation.
Evidence that psoriasis is an immune-mediated disorder is also based on the success of targeted therapy, such as cyclosporine and inhibitors of TNF; IL-12, -23, and -17; and their receptors. The majority of T cells in psoriatic plaques are CD45RO+ memory-effector T cells that migrate into skin exposed to an antigenic trigger. Th1 and Th17 cytokines predominate, in contrast to the largely Th2 cytokine response of the acute lesions of atopic dermatitis. The innate immune system plays a key role in psoriasis, initiating a cascade that involves activation of myeloid dendritic cells by TNF-α, IFN-γ, IL-6 and IL-1β. The activated dendritic cells express IL-12 and IL-23, leading to Th1 and Th17 cell expression of TNF-α/IFN-γ and of IL-17/IL-22, respectively. These cytokines stimulate the keratinocytes to produce more IL-1β and IL-6, TNF-α, chemokines, and antimicrobial peptides, further contributing to immune activation and cutaneous inflammation. Skin injury and streptococcal infections are well-known environmental triggers, but the occurrence of psoriasis has also been linked to Staphylococcus aureus infection and Kawasaki disease, suggesting a role for superantigens. Psoriasis has been triggered by administration of growth hormone therapy, IFN (e.g., for chronic hepatitis), and other drugs (particularly, lithium, β blockers, antimalarials, and sodium valproate). Flares of psoriasis have also clearly been linked to psychological and physical stress.
Clinical Manifestations
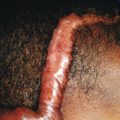
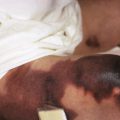
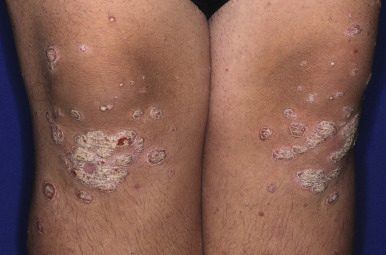
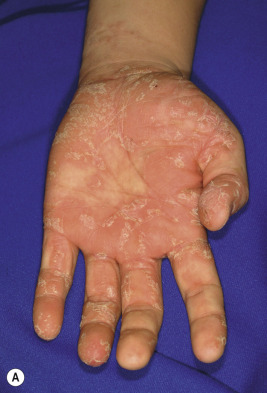
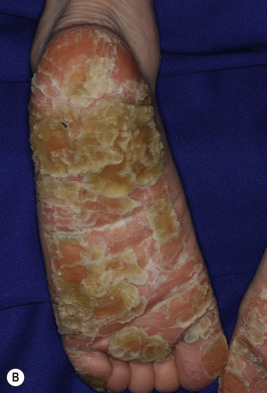
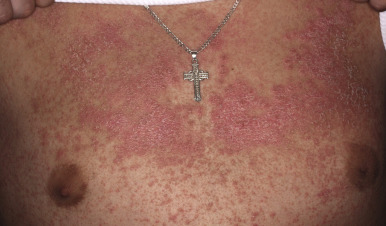
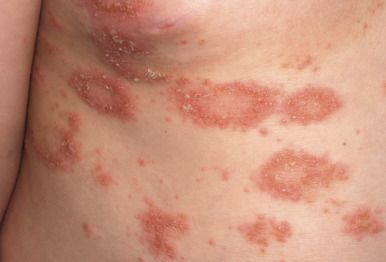
Classic lesions of psoriasis consist of round, brightly erythematous, well-marginated plaques covered by a characteristic grayish or silvery-white (mica-like, or “micaceous”) scale. Psoriatic papules coalesce to form plaques that measure 1 cm or more in diameter ( Figs. 4-1, 4-2, and 4-3 ). The disorder may present as solitary lesions or countless plaques in a generalized distribution. Lesions are usually bilaterally symmetrical with a distinct predilection for the scalp, elbows, knees, and lumbosacral and anogenital regions. However, lesions may also be found in a flexural distribution with involvement of the axillae, groin, perineum, central chest, and umbilical region. This variant, termed inverse psoriasis ( Figs. 4-4 and 4-5 ), may be seen without any extensor surface involvement in 2.8% to 6.0% of patients or in association with only regional involvement in 30% of patients. Lesions may also be limited to the palms ( Fig. 4-6 ) and soles ( Fig. 4-7, A ). A peripheral white ring is often the first sign of involution (termed a Woronoff ring ). However, when the central portions of the plaques resolve, the involuting lesions may appear nummular (small circles), annular (central clearing) ( Fig. 4-7, B ), gyrate, or arcuate (semicircular). A linear variant that courses along the Blaschko lines has also been described and may be associated with psoriatic arthritis.
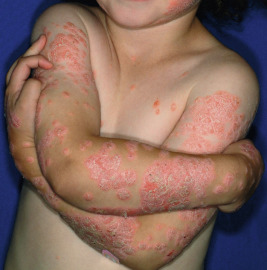
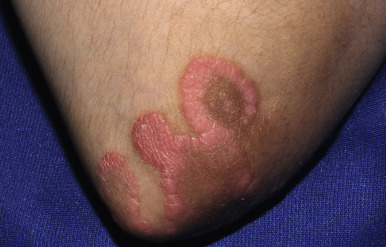
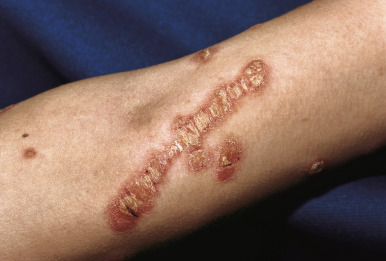
The hallmark of psoriasis is the micaceous scale that is generally attached at the center rather than the periphery of lesions. Removal of this scale results in fine punctate bleeding points. This phenomenon (termed the Auspitz sign ) is highly characteristic and relates to rupture of capillaries high in the papillary dermis of lesions. The Koebner phenomenon (an isomorphic response) is commonly seen in psoriasis ( Fig. 4-8 ) but also in verrucae, Rhus dermatitis, lichen planus, lichen nitidus, Darier disease, and PRP. This valuable diagnostic sign of psoriasis describes the occurrence of skin lesions at sites of local injury such as after irritation (a scratch or sunburn) ( Fig. 4-9 ), a surgical scar, or a preexisting disease such as seborrheic or atopic dermatitis. The Koebner phenomenon likely is responsible for psoriasis of the diaper region and around enteral feeding tubes.
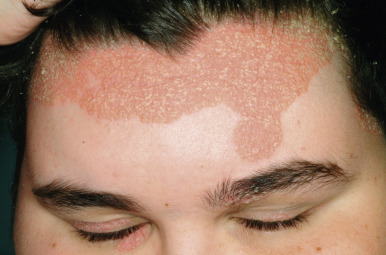
Facial Psoriasis
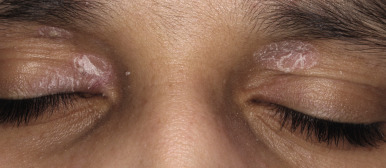
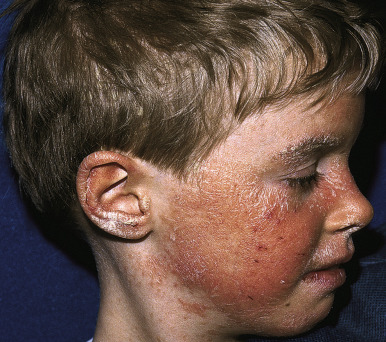
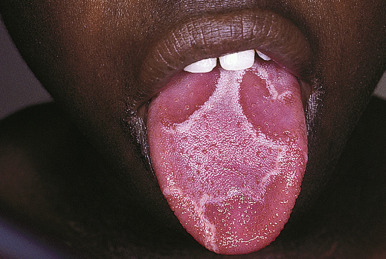
Facial psoriasis is more common in children than in adults and occurs without other involvement in 4% to 5% of patients. Involvement of the periorbital area is most typical ( Fig. 4-10 ), and lesions may be subtle, leading to confusion with atopic dermatitis ( Fig. 4-11 ). The plaques of psoriasis tend to be more clearly delineated than patches of atopic dermatitis, are less pruritic, and may show an annular configuration. It should be noted, however, that approximately 5% of pediatric patients show an eczema/psoriasis overlap, either showing typical lesions of both atopic dermatitis and psoriasis or lesions that are intermediate (e.g., nummular and psoriasiform). Almost all patients with the overlap have a family history of both atopic disease and psoriasis. Although mucosae do not tend to be affected in psoriasis, geographic tongue is an often unrecognized feature of psoriasis in many children ( Fig. 4-12 ).
Guttate Psoriasis
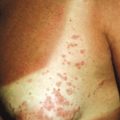
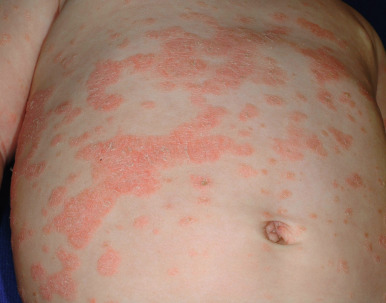
Seen in up to 44% of pediatric patients, guttate psoriasis generally occurs in children and young adults and is often the first manifestation of psoriasis. Lesions are drop-like (guttate), round or oval, measure from 2 mm to 6 mm in diameter ( Figs. 4-13 and 4-14 ), and generally occur in a symmetrical distribution over the trunk and proximal aspects of the extremities (occasionally the face, scalp, ears, and distal aspects of the extremities). Guttate psoriasis is often, but not necessarily, triggered by group A streptococcal infection of the oropharynx or perianal area. Two-thirds of patients with guttate psoriasis give a history of an upper respiratory tract infection 1 to 3 weeks before the onset of an acute flare of the disorder. Although guttate psoriasis may clear spontaneously ( Fig. 4-15 ), 40% of affected children progress to the plaque type, which may ultimately be more severe psoriasis than in children with plaque disease initially.
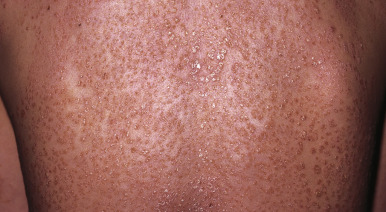
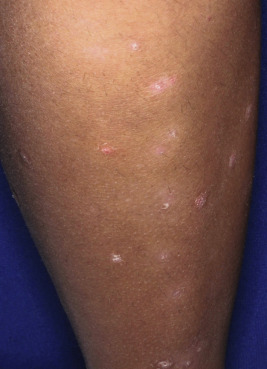
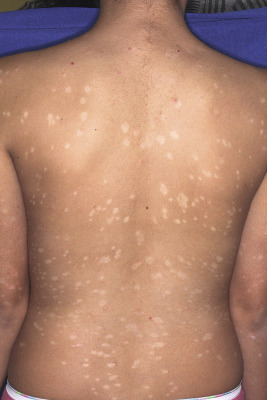
Scalp Psoriasis
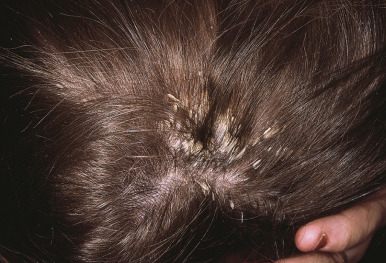
The scalp is commonly the initial site of psoriatic involvement (20% to 40%), and scalp psoriasis occurs more often in affected girls than boys, perhaps because the Koebner phenomenon associated with combing, brushing, and vigorous shampooing. Most typical are well-demarcated erythematous plaques with thick, adherent silvery scales similar in appearance to those on other parts of the body ( Fig. 4-16 ). However, psoriatic lesions of the scalp, eyebrows, and ears (the superior and postauricular folds and external auditory meatus) may instead be greasy and more salmon-colored, suggesting a diagnosis of seborrheic dermatitis. In this variant, often termed sebopsoriasis , lesions may present with features of both seborrhea and psoriasis. Whereas lesions of seborrheic dermatitis generally remain within the hairline, lesions of psoriasis often extend beyond the confines of the hairline onto the forehead (see Fig. 4-3 ), preauricular, postauricular, and nuchal regions. Rapid response to therapy further distinguishes seborrhea from psoriasis. Another scaling disorder of the scalp that may be a variant of psoriasis is pityriasis amiantacea (asbestos-like) ( Fig. 4-17 ) ; the alternative term, tinea amiantacea , should be abandoned, because the disorder is unrelated to dermatophyte infection. The disorder more commonly occurs in pediatric patients without other signs of psoriasis and is characterized by large plates of scale firmly adherent to the hair and scalp. Focal hair loss and secondary infection may be associated. Pityriasis amiantacea usually begins in school-aged children and adolescents and progresses to more typical psoriasis in 2% to 15% of pediatric patients. Pretreatment of affected scalp areas with fluocinolone 0.01% oil daily and then shampooing with a keratolytic shampoo is usually effective.
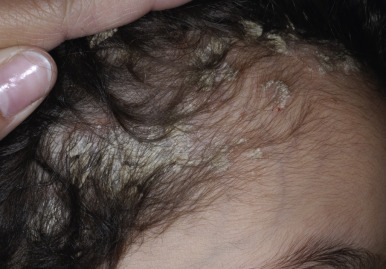
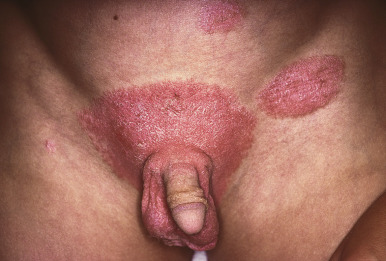
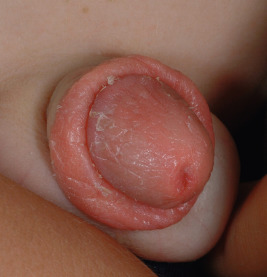
Diaper-Area Psoriasis
Psoriatic diaper rash (see Fig. 2-27 ) with or without dissemination is the presenting manifestation in 13% and 4% of patients, respectively. This form of psoriasis must be differentiated from infantile seborrheic dermatitis (see Chapter 3 ) and, when localized to the diaper area, other forms of diaper dermatitis (see Chapter 2 ). The sharply defined plaques, bright-red coloration, shininess, and larger, drier scales of psoriasis help to differentiate it from seborrheic dermatitis. Many infants with diaper-area psoriasis also show psoriasiform lesions elsewhere ( Fig. 4-18 ). Because of the increased moisture of the occluded diaper region, scale may not be visible clinically but can be revealed by scraping the area gently. The incidence of psoriasis in the diaper area during infancy probably reflects the Koebner phenomenon, triggered by trauma from exposure to stool and urine, and resolves when toilet trained. Nevertheless, boys and girls out of diapers may also show genital area involvement. Of prepubertal girls with a genital region complaint, 17% had psoriasis particularly involving the vulva, perineum, and natal cleft.
Nail Involvement
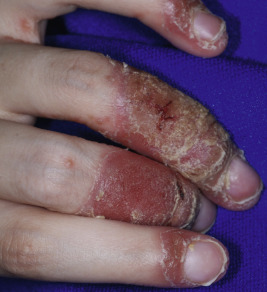
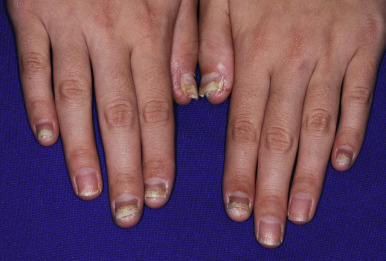
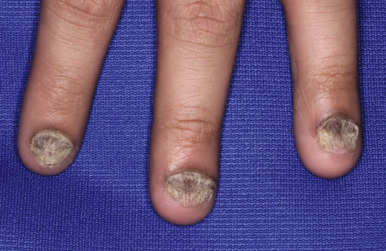
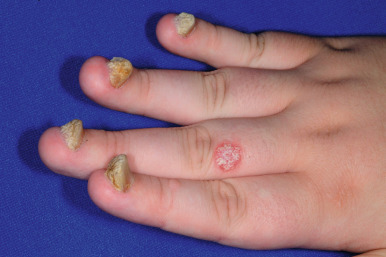
Although statistics vary, the nails appear to be affected in 25% to 50% of pediatric patients with psoriasis, more commonly during the second decade of life and in males ( Figs. 4-19, 4-20, and 4-21 ). Pitting is most characteristic, manifesting as small, irregularly spaced depressions measuring less than 1 mm in diameter. Larger depressions or punched-out areas of the nail plate may also be noted. These pits are thought to represent small intermittent psoriatic lesions in the nail matrix region that forms the superficial layers of the nail plate. Psoriatic pitting may be indistinguishable from nail pitting seen in alopecia areata (see Chapter 7 ) and atopic dermatitis (see Chapter 3 ), although other features assist in differentiating these disorders. Discoloration, onycholysis (separation of the distal and lateral nail plate edges), and subungual hyperkeratosis (lifting of the nail plate with nail thickening) (see Fig. 4-21 ) are also commonly seen. Secondary bacterial, candidal, and occasionally dermatophyte infections occur with increased incidence.
Other Forms of Psoriasis
Pustular psoriasis and erythrodermic (exfoliative) psoriasis are the most severe variants of childhood psoriasis but occur in only approximately 1% of pediatric patients with the disease. Pustular psoriasis has been described as early as the first week of life. It usually occurs as generalized pustular psoriasis, but can be limited to the palms and soles (pustulosis palmaris et plantaris) or to fold areas.
On previously quiescent psoriatic plaques or normal skin, erythematous halos develop and rapidly become studded with superficial pinpoint-sized up to 2- or 3-mm pustules ( Fig. 4-22 ). The lesions of generalized pustular psoriasis in children show an annular morphology in 60% of patients ( Fig. 4-23 ). Sheets of erythema and pustulation can involve the flexures, genital regions, webs of the fingers, and periungual areas. The cutaneous inflammation typically progresses in an explosive manner from discrete sterile pustules to crusts and ultimately to generalized exfoliative dermatitis. More than 90% of the skin shows intense erythema, massive exfoliation, and associated abnormalities of temperature and cardiovascular regulation. The nails often become thickened or separated by subungual lakes of pus. Mucous membrane lesions in the mouth and tongue are not uncommon. Fever, malaise, and anorexia are typically associated with generalized pustular psoriasis. Affected children may show failure to thrive. Patients with extensive pustular or erythrodermic psoriasis usually require hospitalization, and courses are not uncommonly complicated by cutaneous infection and bacterial septicemia. The disease is cyclic and associated with complete clearance of the pustular phase and unexplained exacerbations that span decades. Relapses are common and become progressively more severe, often with a poor prognosis. In contrast to the occurrence of pustular psoriasis in adults known to have psoriasis, pustular psoriasis is often the first manifestation of psoriasis in affected infants and children. The predominant histologic feature on biopsy is the large intraepidermal unilocular pustule containing polymorphonuclear leukocytes (the spongiform pustules of Kogoj) with little if any surrounding spongiosis or inflammation. Although staphylococcal infection may at times occur as a secondary complication, lesions are usually sterile.
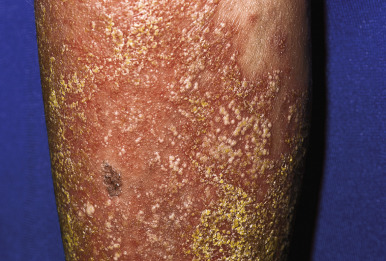
Pustular psoriasis with its onset during infancy may be localized to the neck fold. The disorder is often misdiagnosed as dermatitis caused by bacterial or candidal infection ( Fig. 4-24 ). Biopsy facilitates making the diagnosis, particularly given that many lesions clinically appear more papular than pustular. Other fold areas may be affected, and dissemination to generalized pustular psoriasis is not uncommon.
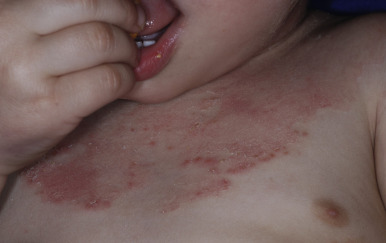
Pustulosis palmaris et plantaris (pustulosis of the palms and soles) is a bilaterally symmetric, chronic pustular eruption on the palms and soles characterized by deep-seated 2- to 4-mm sterile pustules that develop within areas of erythema and scaling. Plaque or pustular psoriasis may be seen elsewhere on the body. Within several days the pustules resolve and leave a yellow-brown scale that is generally shed within 1 or 2 weeks. Phases of quiescence and exacerbation are characteristic, and exfoliating crusted lesions may be seen concurrently with newly developing pustules.
Psoriasis-like pustules during the neonatal period or in infancy, often in association with sterile multifocal osteomyelitis or periostitis, should raise the possibility of inappropriate activation of IL-1 family cytokines (IL-1 and IL-36) from biallelic mutations in IL-1 or IL-36 receptor antagonists. Pustules may be widespread or grouped and more localized. Deficiency of IL-1 receptor antagonist (DIRA) also may feature joint swelling and pain, oral stomatitis, and pyoderma gangrenosum, especially during childhood. Most neonates with DIRA are born prematurely. Deficiency of IL-36 receptor antagonist (DITRA) is the major cause of generalized pustular psoriasis in patients without common psoriasis. DITRA has been described in babies and is characterized by irritability, tender skin, diarrhea, dysphagia, and failure to thrive in association with their pustular psoriasis and/or exfoliative erythroderma. Neutrophilia and thrombocytosis are common, but other organs are not involved. The early onset, and poor response to standard therapy are clues to diagnosis. Skin biopsies in DIRA and DITRA show features similar to those seen in pustular psoriasis. Pediatric patients often respond rapidly to subcutaneous administrations of anakinra 2 to 4 mg/kg per day.
Pustules overlying erythroderma can also be a feature of patients with mutations in CARD14, which typically has its onset during infancy or early childhood. More commonly, however, patients with CARD14 mutations often show signs of recalcitrant plaque-type psoriasis or PRP (see Pityriasis Rubra Pilaris section) and respond rapidly to ustekinumab.
Pustular psoriasis may also present after infancy in association with sterile lytic lesions of bone (sometimes called chronic recurrent multifocal osteomyelitis [ CRMO ]) as part of several syndromes. CRMO in association with congenital dyserythropoietic anemia and neutrophilic dermatoses comprises Majeed syndrome, an autosomal recessive disorder caused by mutations in LPIN2 . Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome (see Chapter 8 ) describes the constellation of synovitis, acne, palmoplantar pustulosis and psoriasis, hyperostosis, and osteitis and usually affects the bones of the lower limb, pelvis, and clavicle. The bone and joint lesions can severely impact quality of life but may respond to immunosuppressive medications such as methotrexate and aggressive intervention for the acne with isotretinoin.
Comorbidities
The most common comorbidity of pediatric psoriasis, as in affected adults, is obesity, which tends to precede the onset of psoriasis by at least 2 years in the majority of affected children. Several studies have confirmed the association of pediatric psoriasis and obesity. Being overweight (“excess adiposity,” between the 85th and 95th percentiles in body mass index [BMI]) or obese (95th percentile or greater BMI) occurs in pediatric psoriasis with an odds ratio (OR) of 2.65 (95% confidence interval [CI], 1.70 to 4.15) globally and OR of 4.22 (CI, 2.05 to 8.67) in the United States, and the association with excess adiposity is seen regardless of psoriasis severity. However, waist circumference percentile and waist-to-height ratio, which are both better indicators of metabolic risk than BMI percentile, are only increased in children with moderate to severe psoriasis. Early evidence suggests that aggressive therapy for psoriasis does not lead to weight or waist circumference reductions.
Increasing evidence suggests an association with early cardiovascular disease in affected children, as has been noted in adult psoriasis. Pediatric psoriasis is associated with approximately two to four times the rate of comorbidity from hyperlipidemia, hypertension, and diabetes than unaffected children and adolescents. In a study of 20 US children with psoriasis, metabolic syndrome (as defined by having at least three of the following: high triglycerides, high density lipoprotein cholesterol, fasting blood glucose, waist circumference percentile, and blood pressure) occurred in 30% with psoriasis but 5% of the control group (p < .05). These data suggest that early intervention with lifestyle modification and possibly systemic anti-inflammatory therapy may decrease the long-term metabolic risk for these children. Although results have been controversial, a recent study showed significant improvement in the psoriasis and quality of life in overweight adult psoriasis patients on a low-calorie weight loss diet for 8 weeks.
Joint pain has been described in approximately 10% of US children with moderate to severe psoriasis, suggesting that inquiry about pain, joint swelling, or limping, as well as examination of the joints for arthritis should be part of the routine evaluation. Psoriatic arthritis is now considered a form of juvenile idiopathic arthritis, and criteria have been established by the International League of Associations for Rheumatology (ILAR). These include arthritis with psoriasis or arthritis and: (1) a family history of confirmed psoriasis in a parent or sibling; (2) dactylitis ; or (3) nail pitting or onycholysis . The diagnosis is excluded if the patient has a positive rheumatoid factor titer or signs of systemic disease (daily fever, evanescent erythematous eruption, generalized adenopathy, hepatomegaly or splenomegaly, or serositis). The occurrence of pediatric psoriatic arthritis is biphasic. Younger children affected by psoriatic arthritis tend to be female with dactylitis and small-joint involvement that is more likely to progress and persist. The swelling often includes the juxtaarticular tissue, resulting in a blunt “sausage-shaped” appearance of the involved fingers or toes. Progression to polyarthritis occurs in 30%, leading to flexure deformities and severe bone destruction (osteoporosis, shortening and tapering of the involved distal phalanx) with longstanding disease. On radiologic examination, this resembles a sharpened pencil (the so-called “pencil-in-cup” or “pencil-and-goblet” deformity) at the metatarsophalangeal and metacarpophalangeal joints. Arthritis in older children is characterized by more enthesitis and axial joint disease. Psoriatic skin lesions occur in approximately 60% of children with psoriatic arthritis, are identical to those seen in patients who do not manifest joint disease, and are not related in severity to that of the joint disease. Either skin disease or arthritis may develop initially, and in most patients flares of joint and skin disease do not correlate.
Pediatric patients with moderate to severe plaque psoriasis have demonstrated significantly impaired physical, emotional, social, and school functioning in comparison to healthy children on par with children who have arthritis or asthma and to a greater extent than children with diabetes. In one study, 65% of children with psoriasis experienced stigmatization and 43% complained about fatigue. Pediatric patients with psoriasis have a higher risk of developing psychiatric disorders, especially depression and anxiety.
The risk of developing Crohn disease (but not ulcerative colitis) is also higher in pediatric psoriasis. Asymmetric anterior uveitis of psoriasis has been described in 14% to 17% of children with juvenile psoriatic arthritis. The cutaneous lesions of psoriasis may develop several years after the onset of persisting uveitis.
Diagnosis of Psoriasis
The diagnosis of psoriasis can usually be made clinically, although biopsy can be performed if the diagnosis is in question. Biopsy sections show epidermal thickening (acanthosis with elongation of the rete ridges), retention of nuclei in the stratum corneum (parakeratosis), and a mononuclear infiltrate. Focal collections of neutrophils in the stratum corneum or subcorneal layer (Munro’s microabscesses) are an additional feature in biopsies from patients with pustular psoriasis.
Course
Most patients have long-term mild to moderate disease, often with plaques confined to areas of frictional trauma such as the elbows, knees, buttocks, and scalp. The course of psoriasis, however, is unpredictable, ranging from spontaneous remissions to frequent exacerbations without an evident trigger. Although sunlight generally is beneficial and often leads to improvement during the summer, sunburns can elicit the Koebner phenomenon and lead to exacerbation. With appropriate therapy, satisfactory control of the disease is possible in a majority of patients.
Differential Diagnosis
Guttate psoriasis and plaque psoriasis are most commonly confused with dermatitis as well as other papulosquamous disorders described in this chapter, especially pityriasis rosea or pityriasis lichenoides chronica (PLC) ( Box 4-1 ). PRP is the hardest to differentiate, especially when involved areas are largely the palms, soles, elbows, and knees. The follicular accentuation, focal areas of sparing, and sometimes more salmon coloration of PRP can help to distinguish the conditions clinically; biopsy sections of PRP may show perifollicular inflammation. A plaque-type psoriasiform eruption and less often a generalized or annular pustular psoriatic eruption may follow Kawasaki disease (see Chapter 21 ). Generalized and localized forms of pustular psoriasis can be differentiated from infectious causes of pustulosis by cultures and from noninfectious conditions such as eosinophilic folliculitis or infantile acropustulosis by biopsy. Psoriasiform dermatitis may also be seen in boys with immune dysregulation, polyendocrinopathy, enteropathy, or X-linked (IPEX) syndrome (see Chapter 3 ). Atypical cases of psoriatic arthritis must be differentiated from other types of juvenile idiopathic arthritis or systemic lupus erythematosus.
Guttate and Plaque Psoriasis
Pityriasis rubra pilaris
Pityriasis rosea
Pityriasis lichenoides chronica
Psoriasiform dermatitis
Lichen planus
Drug eruptions
Widespread dermatophytosis
Facial Psoriasis
Discoid lupus erythematosus
Seborrheic dermatitis
Scalp Psoriasis
Tinea capitis
Seborrheic dermatitis
Nail Psoriasis
Trauma
Onychomycosis
Lichen planus
Diaper Area Psoriasis
Seborrheic dermatitis
Irritant dermatitis
Candidal diaper dermatitis
Pustular Psoriasis
Staphylococcal pustulosis
Candidal pustulosis
Herpes simplex infection
Acute generalized exanthematous pustulosis (viral, drug)
Extensive eosinophilic folliculitis
Interleukin-1 receptor antagonist deficiency
Palmoplantar pustular psoriasis
Candidiasis
Infantile acropustulosis
Erythrodermic Psoriasis
Extensive pityriasis rubra pilaris
Congenital ichthyosiform erythroderma
Erythrokeratodermia variabilis
Therapy of Pediatric Psoriasis
Education is a key component of psoriasis therapy. Patients and parents must understand the chronicity of the disorder and the tendency in 38% of pediatric patients for spontaneous remissions lasting for variable periods. Most patients respond well to available therapies, but the response is slower than with dermatitis. The therapeutic approach should be simplified and tailored to the individual patient to optimize compliance, because therapy is time consuming. Patients and family members of the patient should understand the rationale for treatment and any potential risks. Older children and adolescents should be empowered to maintain their own therapeutic routine with parental guidance. The concept that injury to skin may exacerbate psoriasis (Koebner phenomenon or isomorphic response) should also be explained ( Table 4-1 ). Removal of potential trigger factors, including medications and rapid intervention for streptococcal infection should be explored.
| Site of Potential Psoriasis | Preventative Behavior |
|---|---|
| Creases and folds | Minimize friction by maintaining appropriate weight Avoid irritating underarm deodorants |
| Face | Avoid irritating soaps and burning from exposure to ultraviolet light |
| Genital and perianal regions | Avoid irritation from tight garments and exposure to accumulated feces and urine |
| Hands and feet | Minimize excessive sweating and exposure to irritants such as harsh soaps Avoid tight shoes |
| Nails | Avoid long fingernails or toenails, trauma to nails in play situations, excessive use of nail polish and remover, and wearing tight shoes Hydrate nails before trimming and avoid manipulation of cuticles |
| Scalp | Avoid vigorous brushing, combing, or scratching of scalp |
Topical Therapy
The topical therapies most commonly used in children include topical corticosteroids, topical calcineurin inhibitors, vitamin D 3 analogues (calcipotriene and calcitriol), tar preparations, and anthralin (also called dithranol ; short-contact therapy) ( Table 4-2 ). Emollients are important adjunctive measures to decrease associated scaling and dryness but do not replace medications that treat inflammation. The mainstay of treatment for plaque psoriasis remains topical corticosteroids (see Chapter 3 , Table 3-2 ), which often produce dramatic resolution of lesions as monotherapy. Application up to twice daily of class II–IV (potent to midpotency) topical steroids is required to improve lesions on the trunk and extremities. Ointments tend to penetrate the psoriatic scale better and are preferred. If individual thick plaques fail to respond, a course of ultrapotent topical steroid ointment (such as clobetasol, halobetasol, or augmented betamethasone) can be initiated but should be restricted to 2 weeks of application because of the risk for developing striae (especially in the preadolescent/adolescent population) and local atrophy with continued use. “Weekend therapy” regimens combine class I steroids (used on weekend days only) and topical calcipotriene/calcitriol and should be administered by a dermatologist familiar with the use of these regimens. Keratolytic agents to enhance penetration, such as 6% salicylic acid, are often compounded into steroid ointment with or without tar. The keratolytic applied alone (e.g., Keralyt gel), occlusion, or steroid-impregnated tapes are alternative treatments for more hyperkeratotic, resistant lesions. Use of halogenated midpotent to potent steroids should be avoided in the diaper area, intertriginous areas, and on the face. Topical calcipotriene/calcitriol and tacrolimus ointment, combined or as monotherapy, are steroid-sparing alternatives. Once the acute lesions are under control, treatment can be tapered to lower potency steroids and/or emollients.
| Medication | Use in Children | Potential Side Effects and Comments |
|---|---|---|
| Emollients | Useful in mild disease; adjunct | None |
| Topical steroids | First-line therapy | Local side effects: atrophy, striae Systemic side effects: impaired growth, adrenal suppression, cataracts, tachyphylaxis |
| Tar | Thicker plaques | Irritation, staining, folliculitis |
| Salicylic acid | Thicker plaques; usually compounded with steroids ± tar | Irritation |
| Anthralin | Short contact application | Irritation; less staining than tar |
| Calcitriol or calcipotriene | Usually adjunct with steroids | Irritation |
| Tazarotene gel | Usually adjunct with steroids | Irritation |
| Tacrolimus/pimecrolimus | Face, intertriginous areas | Burning with initial applications |
Tar is a time-honored and effective adjunct to the topical treatment of psoriasis that is both anti-inflammatory and antiproliferative. Tar (in the form of 1% to 10% crude coal tar or 5% to 10% liquor carbonis detergens) can be compounded into preparations with topical steroids and/or salicylic acid and applied overnight or before ultraviolet light exposure. Tar as a single agent is available in several over-the-counter preparations (e.g., Estar gel, Fototar). Tar preparations, however, stain skin and clothing, have an odor that is often objectionable to children and adolescents, and increase the risk of developing folliculitis. Tar may also be administered in the form of a tar bath (e.g., Cutar bath oil, Doak Oil Forte, Balnetar) or foam.
An alternative to tar therapy is short-contact anthralin therapy, formulated in a temperature-sensitive vehicle that releases the active medication at skin-surface temperature. Anthralin, also called dithranol, is applied for 5 minutes initially with gradually increasing times of exposure as tolerated and needed for efficacy. Discoloration of skin or clothing is significantly less than that with tar therapy. Contact with the face, eyes, and mucous membranes should be avoided.
Calcipotriene (cream, ointment or solution) and calcitriol (ointment) are most effective when combined with topical steroids but serve as steroid-sparing agents that may be efficacious in children as monotherapy as well. These vitamin-D 3 analogues are best applied twice daily, but the onset of action is slow (often 6 to 8 weeks). Combination therapy of betamethasone (0.064%) and calcipotriene (0.005%) ointment is also available. Irritant dermatitis, particularly on the face and intertriginous areas, occurs in up to 20% of patients.
The topical retinoid tazarotene is available in 0.05% and 0.1% strength creams and gels. Tazarotene is best applied once a day in combination with once-daily application of a medium to potent topical steroid, but even with the topical steroid it is often too irritating for use in childhood psoriasis.
Calcineurin inhibitors, particularly tacrolimus ointment 0.1%, are useful with twice-daily application for 1 to 2 months for facial and intertriginous psoriasis. Although not found to be useful outside of the facial and intertriginous areas in double-blind trials in adults, topical tacrolimus ointment is sometimes useful outside of these areas for childhood psoriasis.
Treatment of Scalp Lesions
Psoriatic scalp lesions are a frustrating and sometimes recalcitrant component of psoriasis. Topical corticosteroids may be applied in the form of oils, solutions, or foams, and calcipotriene solution may be steroid-sparing. Clobetasol and fluocinolone shampoos are also available. Removal of scales can be facilitated by softening scales through application of oil-based medications. For example, fluocinolone 0.01% oil under shower cap occlusion can be applied for 1 hour to overnight to the wet, affected scalp and should be followed by washing with shampoos containing tar, steroid (0.01% fluocinolone or clobetasol), zinc, or keratolytic agents. Alternatively, a phenol and saline (Baker Cummins P&S) solution with a shower cap for occlusion can be applied overnight. In the morning, the patient can shampoo and then apply a steroid foam or solution if needed. Many patients will apply a second shampoo after the medicated shampoo and use a conditioner after shampooing.
Treatment of Nail Psoriasis
Psoriatic nails are extremely distressing to the patient, respond slowly to therapy, and are difficult to treat topically because of the failure of topical agents to penetrate the nail plate. Instillation of class-I steroid solutions into the subproximal nailfold area can be successful, especially applied under occlusive tape at night, but application nightly of flurandrenolide-impregnated tape (Cordran) to the base of the nail for approximately 6 months tends to yield better results. Injections of triamcinolone suspension (10 mg/mL) into the nailfold of the abnormal nail with a 30-gauge needle every 4 to 6 weeks is painful, even with the use of topical anesthetic creams and should be reserved for the motivated older child or adolescent who fails to respond to topical application. Nightly application of tazarotene 0.05% or 0.1% gel under occlusion, if tolerated, has been shown to cause improvement. Use of topical indigo naturalis oil extract has recently been described. Disfiguring nail psoriasis that is unresponsive to topical measures may respond to systemic intervention (generally methotrexate or a biologic medication), but the decision to start a systemic medication must be carefully weighed.
Compresses for Pustular Psoriasis
Local applications of wet dressings with Burow solution 1 : 40 or potassium permanganate 1 : 5000 (one crushed 65 mg tablet into 250 mL of water) often help relieve acute flares of the pustular aspect of palmoplantar or generalized pustular psoriasis.
Ultraviolet Light
Most patients with psoriasis benefit from exposure to sunlight and accordingly are often better during the summer months. However, sunburn precautions must be taken with sunscreens, avoidance during hours of most intense sunlight, and sun-protective clothing, because sudden overexposure may result in sufficient epidermal injury to cause exacerbation of the disorder. For those who can arrange exposure to sunlight on a regular basis, this can be an important aspect of therapy alone or in combination with topical therapies. Although natural sunlight is easier for children and less aggressive than artificial ultraviolet therapy, ultraviolet treatments under professional supervision may also be used as therapy, especially when psoriasis involves more than 10% to 20% body-surface area, involves the palms and soles, and/or is recalcitrant to topical therapy. Response to phototherapy is enhanced by preexposure application of oil or ointment. Narrow-band ultraviolet B (nbUVB) (≈311 nm) has a higher ratio of therapeutic-to-toxic wavelengths than broadband UVB light (290 to 320 nm) and is considered at least as efficacious. More than 90% of children treated with nbUVB improve by more than 75%.
The UVB light is best initiated in a light box at a dermatology office as outpatient therapy. Once patients and parents know how to increase the doses of ultraviolet light gradually, judge the effects of the daily treatment, and practice preventive eye care, home light-box therapy can be initiated. Home light boxes are ultimately less invasive to the mainstream activities of a family and may be more cost-effective than in-office therapy, although careful monitoring is required. In general, ultraviolet light therapy is started at 70% to 75% of the minimal erythema dose and increased by about 10% to 20% with each treatment as tolerated. A minimum of three treatments per week is required to clear psoriasis. Although rarely used in young children, phototherapy may be administered to young children who are accompanied by parents in the light unit. Tricks such as use of singing together or listening to a radio or compact disc (CD) player with earphones can be used to distract the child during treatment. Acutely, UVB therapy is associated with skin darkening, a chance of skin burning, and, not uncommonly, early pruritus. Although long-term data are lacking in children with psoriasis, recurrent exposure to UVB light could theoretically increase the long-term risk of the development of skin cancer and premature aging.
The excimer laser (≈308 nm) is fiber-optically targeted UVB light that can treat localized plaques of psoriasis without exposing normal skin to unnecessary radiation. Although its use in children has been limited, it is painless and offers safety advantages over nbUVB therapy. Psoralens and ultraviolet A (PUVA) light (320 to 400 nm) are used rarely in children because of the ocular toxicity, generalized photosensitivity, and the risk of later development of actinic changes and cutaneous carcinomas ; topical application of the psoriasis with UVA is a safer alternative but rarely needed. Protective eyewear must be worn for 24 hours after each PUVA exposure because of the risk of cataract development. The use of topical PUVA light in a hand/foot box has proven effective in adolescents and older children with psoriasis of the hands and feet.
Systemic Therapy
Oral medications for treating psoriasis have potentially harmful side effects and should be reserved for children with erythrodermic and pustular forms of psoriasis or for those with moderate to severe plaque-type psoriasis recalcitrant to topical therapies. In general, systemic corticosteroids should be avoided. Although occasionally effective, steroids are often ineffective or lead to flares of psoriasis when withdrawn, including triggering of pustular psoriasis. Especially in guttate psoriasis, examination for the possibility of pharyngitis or perianal cellulitis should be performed, and culture for β-hemolytic streptococci obtained as appropriate. Antibiotics should be prescribed if the culture is positive, although recurrent positive cultures may signal a carrier state. Although important for treating streptococcal infections, trials of antibiotic therapy are usually not helpful. Some pediatric patients with refractory psoriasis have been shown to improve after tonsillectomy for the treatment of chronic and recurring streptococcal infection A recent study showed that 86% of adult patients who underwent tonsillectomy showed sustained improvement (30% to 90%) 2 years after the procedure in contrast to the unchanged status of control patients. Clinical improvement correlated with reduction in circulating keratin peptide-reactive skin-homing (CLA+) T cells (which also recognize cross-reacting streptococcal M proteins), suggesting that the tonsils generate the T cells recognizing these determinants.
The various systemic medications that are available for treating moderate to severe psoriasis are shown in Table 4-3 . Methotrexate is indicated for severe unresponsive psoriasis, exfoliative erythrodermas, pustular psoriasis, nail psoriasis, and psoriatic arthritis. It is occasionally used for nail psoriasis if nonsystemic agents are ineffective. Methotrexate has antimitotic, antichemotactic, and anti-inflammatory activities. After appropriate screening tests (blood counts, hepatic testing, purified protein derivative [PPD] or QuantiFERON gold for tuberculosis) and testing for pregnancy in female patients of childbearing age, oral methotrexate is initiated at an oral dosage of 0.3 mg/kg per week, and can be increased to 0.6 mg/kg per week if needed for efficacy. The upper limit in children is usually 20 mg/week. If methotrexate is not efficacious orally, a trial of subcutaneous weekly administration can be undertaken, especially in obese children requiring higher dosing. Methotrexate assays can be performed to determine if a dosage increase is needed in children who response inadequately after 12 weeks.
| Intervention | Baseline Labs | Dosing | Follow-Up Tests | Potential Side Effects and Comments * |
|---|---|---|---|---|
| Methotrexate | CBC, LFTs; TB testing | 0.3–0.6 mg/kg/wk | Monthly CBCs, LFTs for first 6 months and then every 3 months; annual TB testing | GI toxicity, fatigue most common; bone marrow suppression and hepatotoxicity |
| Cyclosporine | CBC, LFTs, BUN, Cr, Mg ++ ; TB testing | 3–5 mg/kg/d; can go higher based on drug trough levels | BP checks; monthly CBCs, LFTs for first 6 months and then every 3 months; annual TB testing | Renal and hepatic toxicity, hypertension, hypertrichosis, immunosuppression, UVB-induced skin cancer |
| Acitretin | CBC, LFTs, fasting lipids | 0.5–1 mg/kg/d | CBC, LFTs, fasting lipid levels; pregnancy testing as appropriate | Cheilitis, hyperlipidemia, musculoskeletal pain, hair loss, skin fragility, bone toxicity if used long-term, teratogenicity |
| TNF inhibitor: etanercept | TB testing required; optional CBC, LFTs | 0.8 mg/kg sc injection weekly (max 50 mg) | Annual TB testing; other testing controversial | Increased risk of mycobacterial infection and possibly lymphoma |
| TNF inhibitor: adalimumab | TB testing required; optional CBC, LFTs | 24 mg/m 2 sc every 2 weeks | Annual TB testing; other testing controversial | Increased risk of mycobacterial infection and possibly lymphoma |
| TNF inhibitor: infliximab | TB testing required; LFTs; optional CBC | 3.3–5 mg/kg IV at 0, 2, 6, then every 7–8 wks | Annual TB testing; LFTs every 3 months | Increased risk of mycobacterial infection and possibly lymphoma; rarely used for psoriasis unless need very rapid intervention |
| Ustekinumab | TB testing required; optional CBC, LFTs | 0.75 mg/kg baseline, 4 wks, every 12 weeks; usually 45 or 90 mg | Annual TB testing; other testing controversial | Increased risk of mycobacterial/ Salmonella infection and possibly lymphoma |
* All interventions below other than phototherapy and acitretin are immunosuppressive. If suggested by history or examination, undertake baseline hepatitis A/B/C and/or HIV testing. Before starting any immunosuppressive agent, vaccinations should be up-to-date. Live and live attenuated vaccines should be avoided; among common ones are varicella, MMR, intranasal influenza. Systemic medications are all potential teratogens. See text for more details.
The most common side effects are nausea, abdominal discomfort, fatigue, headaches, and anorexia. The most significant side effect is bone marrow suppression. Administration of folic acid diminishes the risk of nausea, mucosal ulcerations, and macrocytic anemia. The dosage is 1 mg/day (may skip the day of methotrexate administration) or 5 mg/week (day after methotrexate). Switching to subcutaneous administration also reduces gastrointestinal complaints. In young children, one chewable multivitamin contains about 400 μg folic acid. Although optimal dosing of folic acid has not been determined, a common practice is to administer the folic acid on the 6 days when methotrexate is not given, because it antagonizes the efficacy of the methotrexate. Liver and bone marrow function should be monitored by blood testing, but testing should not be in the 72 hours after methotrexate administration when transaminases may be transiently elevated. Obese children may have hepatic steatosis and a higher risk of transaminase abnormalities. Liver biopsy is unnecessary in children, and overall hepatic toxicity is rare. Live vaccines such as measles, mumps, and rubella (MMR), poliovirus, and intranasal influenza vaccines may not be given to a child taking weekly methotrexate. Sulfa drugs, including trimethoprim/sulfamethoxazole (Bactrim), should be avoided while taking methotrexate. Improvement is sometimes seen by as little as 3 weeks after initiation of treatment but more commonly requires 10 weeks. Once clearing is achieved, the methotrexate should be gradually lowered (e.g., 2.5 mg/month) during the subsequent months.
Cyclosporine has been used in young patients with severe unresponsive psoriasis, exfoliative erythrodermas, or pustular psoriasis. Its mechanism of action involves inhibition of cytokine production by T lymphocytes. Cyclosporine is usually initiated orally at a dosage of 4 to 5 mg/kg per day (and 3 mg/kg per day if microemulsion) and maintained for 3 to 4 months followed by gradual downward titration and discontinuation within a year of initiation. The dosing can be increased if trough cyclosporine levels prove to be low. The potential complications are hypertension, renal and hepatic toxicity, and hypertrichosis; however, concerns about future leukemias, lymphomas, cutaneous carcinomas, and other oncogenic risks are heightened with childhood use. Live vaccines (e.g., MMR and poliovirus) cannot be used in patients receiving cyclosporine therapy. Patients must also avoid macrolide family medications (e.g., azithromycin) because they can markedly increased cyclosporine levels.
Retinoids tend to be less effective than methotrexate or cyclosporine as a single agent for treating plaque-type psoriasis but can be quite effective for exfoliative erythrodermas and for pustular psoriasis that does not respond to compresses and topical corticosteroids alone. Oral retinoids are often used more successfully in combination with topical ointments, ultraviolet light treatment, methotrexate, or cyclosporine. Acitretin normalizes epidermal differentiation and has an anti-inflammatory effect. The usual regimen is oral administration at a dosage of 0.5 to 1 mg/kg per day, although the dosage can be titrated depending on patient response and laboratory results. Complications related to retinoid usage are most commonly dryness of the skin and mucous membranes and elevation of serum triglyceride levels. The potential for skeletal toxicity (premature epiphyseal closure and hyperostosis), although rare, must be monitored clinically and if appropriate radiographically during infancy, childhood, and puberty in patients who receive retinoids long term. Screening tests include blood counts, fasting lipid profiles, and hepatic studies. Retinoids cause severe teratogenicity and should be used with caution in adolescent girls. Isotretinoin may be an alternative retinoid for female adolescents with pustular psoriasis because of its much more rapid clearance but generally it is not as effective as acitretin.
Dimethylfumarate, a fumaric acid ester, is an immunosuppressant available outside of the United States for treating moderate to severe psoriasis. In a retrospective study of 14 pediatric patients (median age at onset, 15 years), only 36% of patients were nonresponders and 36% showed complete clearance. Severe diarrhea and flushing were the causes of discontinuation in two patients, although transient mild elevation in liver function testing or leukopenia were noted in 36% of the children.
Biologic agents are highly effective, but as with all systemic immunosuppressant medications, are not currently approved by the US Food and Drug Administration (FDA) for pediatric psoriasis (although etanercept is approved for use in pediatric psoriasis in several other countries). Currently available biologic agents inhibit either TNF-α (adalimumab, etanercept, infliximab) or IL-12 /IL-23 (ustekinumab). Most of the use of biologic agents for psoriasis in pediatric patients has involved TNF inhibitors. Of the TNF inhibitors, etanercept has been used the most in children, including in infants. Etanercept is also the only agent tested in a double-blind, randomized trial in US children. In this trial of 211 pediatric patients with moderate to severe plaque type psoriasis, 57% achieved 75% improvement by 12 weeks of therapy with 0.8 mg/kg etanercept; only 11% of patients treated with the vehicle control achieved this degree of improvement. However, etanercept is given weekly, whereas adalimumab requires dosing every other week and has been shown to be more efficacious than etanercept in adults, leading to the pediatric use of adalimumab by many physicians, especially in adolescents. Ustekinumab administration has only been described in a few anecdotal reports for treating severe pediatric psoriasis but may be the optimal therapy for CARD14 -related psoriasis and PRP.
Weighted average psoriasis area and severity index (PASI)-75 scores in adults with moderate to severe psoriasis after 12 weeks of treatment in placebo-controlled studies were 78.6% for infliximab, 72.1% for ustekinumab, 70.5% for adalimumab, 48.1% for etanercept, and 21% for alefacept, suggesting greater efficacy for adalimumab and ustekinumab than etanercept. Similarly, a meta-analysis showed adalimumab and ustekinumab to have similar efficacy and also greater efficacy than etanercept, cyclosporine, methotrexate, and fumaric acid. Nevertheless, multiple factors need to be considered in making the choice of systemic agent, including patient preference, cost, tolerance, adverse effects, dosing schedule, and mode of administration. Of importance, biologic agents are also considerably more costly than methotrexate, which is a major deterrent to their use. The mean annual cost for TNF inhibitors for adults in the United States is approximately $15,000 for etanercept, $18,000 for adalimumab, and $24,000 for infliximab. Although primarily used for plaque psoriasis, TNF inhibitors have led to improvement for recalcitrant palmoplantar pustular and erythrodermic psoriasis. TNF inhibitors can show loss of efficacy with time; in adults the 4-year drug survival for etanercept or adalimumab is approximately 40%. Should a TNF inhibitor lose its efficacy, switching to an alternative TNF inhibitor (or a different class of medication) can significantly improve psoriasis severity.
The long-term risks of biologic therapy in children are unknown, although to date serious acute adverse events are rare. Patients may be at risk for mycobacterial and Salmonella infections and baseline intermediate strength purified protein derivative (PPDi) or quantiferon gold with annual reevaluation is recommended; the need to obtain baseline and follow-up levels of other laboratory tests (e.g., blood counts and liver function tests) in children is controversial. Adverse effects on the development of the immune system in young children and an increased risk of lymphoma are theoretical concerns.
Development of Psoriasis and Psoriasiform Dermatitis During Administration of Tumor Necrosis Factor Inhibitors
Just as psoriatic arthritis can develop in children with psoriasis who are administered a TNF inhibitor, children taking biologic agents for indications other than psoriasis can develop psoriasis or psoriasiform dermatitis. Occurring most often in children treated for Crohn disease, this seemingly paradoxical inflammatory cutaneous response has also been described in children with juvenile idiopathic arthritis, hidradenitis suppurativa, or Behçet syndrome. The psoriasis from TNF inhibitors favors the scalp, periorificial skin, nails, dorsal aspect of hands and feet ( Fig. 4-25 ), and often the palms and soles, where it can be pustular. Lesions are not uncommonly secondarily infected with S. aureus . The eruptions are described after a wide range of duration of treatment, ranging from one dose to up to 63 months. Its occurrence in no way affects the response to the biologic agent for the original indication, and the risk of occurrence is not reduced by concurrent administration of methotrexate. The mechanism for the development of psoriasis despite suppression by TNF inhibition remains unclear. Cytokine imbalance with increased expression of IFN, known to increase risk of psoriasis in chronic active hepatitis, has been proposed. Patients with Crohn disease who developed psoriasis on infliximab were more likely to have homozygous IL-23R polymorphisms, suggesting a genetic tendency. An infectious trigger, particularly streptococcal or staphylococcal infection, has been suggested as well.