Abstract
This chapter focuses on disorders characterized by dysfunction of skin vasculature, including livedo reticularis, flushing, erythromelalgia, and nevus anemicus. In addition, vascular ectasias such as telangiectasias and venous lakes are discussed. The significance of these cutaneous vascular diseases can vary, from being an important sign of a systemic disease to simply a cosmetic concern. Distinguishing physiologic changes of the skin vasculature from pathologic disease is sometimes challenging, and it is the dermatologist who often makes this distinction.
Keywords
livedo reticularis, livedo racemosa, vasculitis, vasculopathy, erythromelalgia, flushing, telangiectasia, venous lake, nevus anemicus, angiospastic macules, Bier spots, spider telangiectasia, hereditary hemorrhagic telangiectasia, generalized essential telangiectasia, unilateral nevoid telangiectasia, angioma serpiginosum, cutaneous collagenous vasculopathy
Introduction
This chapter will cover several disorders of the skin vasculature, including livedo reticularis, flushing and erythromelalgia, as well as vascular ectasias such as telangiectasias and venous lakes. Some of the diseases described here are important skin signs of systemic disease, while others are incidental findings. Additional disorders of blood vessels are covered elsewhere, e.g. infantile hemangiomas ( Ch. 103 ), vascular malformations ( Ch. 104 ), and vascular neoplasms and proliferations ( Ch. 114 ).
Livedo Reticularis
- ▪
A common physiologic finding consisting of a mottled, reticulated vascular pattern
- ▪
May occur secondarily due to an underlying disease, e.g. autoimmune connective tissue disease, antiphospholipid antibody syndrome
- ▪
The pattern varies depending on the underlying cause
- ▪
Appropriate investigations depend on the clinical context and associated findings
Introduction
Livedo reticularis (LR) is an extremely common finding and usually results from a physiologic vasospastic response to cold exposure. Among normal healthy individuals, the predisposition to LR will vary. However, it can also be a reflection of a number of underlying systemic diseases. LR resulting from any cause can vary to some degree with changes in external temperature. Physiologic LR will usually disappear with warming and reappear with cooling whereas other variants may persist to varying degrees with warming.
History
The term “livedo reticularis” was first used by Hebra over a century ago to describe a violaceous skin discoloration caused by an abnormality of the cutaneous circulation. Renault (1883) and later Unna (1896) and Spalteholz (1927) suggested that a cone arrangement of the cutaneous microvasculature served as an explanation for the occurrence and pattern of LR .
Pathogenesis
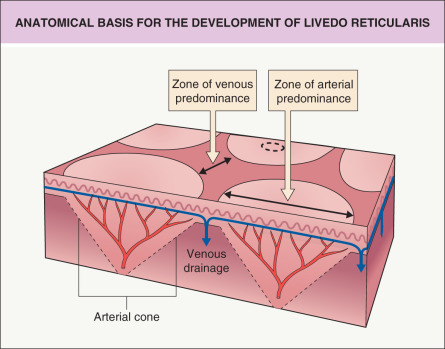
LR results from alterations in blood flow through the cutaneous microvasculature system ( Fig. 106.1 ). The latter consists of arterioles that are oriented perpendicularly to the skin surface. The vessels then divide into capillary beds that in turn drain into a subpapillary plexus. It has been proposed that this arrangement of vessels gives rise to a series of 1–3 cm cones with the ascending arteriole at the apex of each cone. At the edge of the cone, the venous plexus is more prominent and the arterial bed is diminished. Any process that either reduces blood flow to and through the skin or reduces drainage of blood out of the skin will result in the accumulation of deoxygenated blood in the venous plexus, leading to the clinical appearance of LR .
There are a number of causes of LR ( Table 106.1 ), and the clinical pattern of LR can vary with the nature of the underlying cause. A complete fine network is indicative of alterations in blood flow caused by vasospasm or by factors within the blood that alter the viscosity and the flow through the vessels. Vessel wall pathology and intraluminal obstruction are more likely to result in a patchy distribution of LR, depending on the distribution of the underlying pathology.
| CAUSES OF LIVEDO RETICULARIS |
| Congenital livedo reticularis |
|
| Acquired livedo reticularis |
| Vasospasm |
|
| Vessel wall pathology |
|
| Intraluminal pathology |
|
| Other |
|
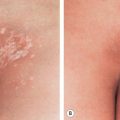
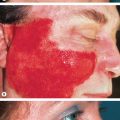
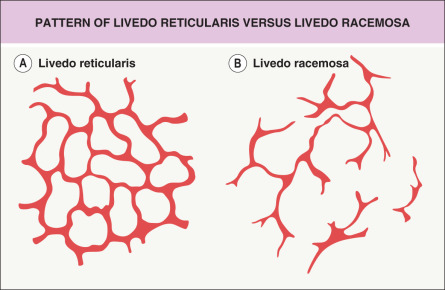
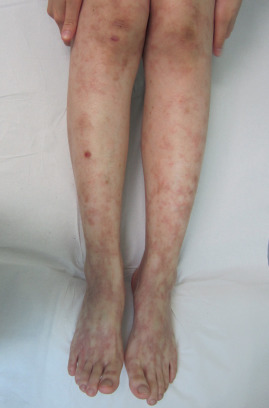
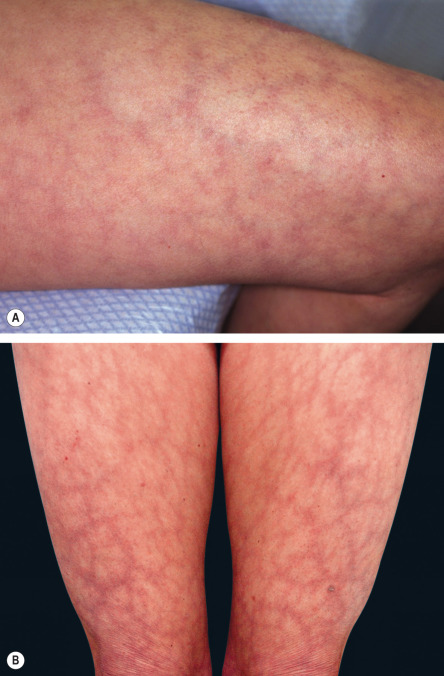
Livedo racemosa refers to a form of LR that has a larger, branching, and more irregular pattern and is often more widespread, affecting both the extremities and the trunk ( Figs 106.2 & 106.3 ). It is generally indicative of several vaso-occlusive disorders including Sneddon syndrome , the antiphospholipid antibody syndrome (APS) , and lymphocytic thrombophilic arteritis .
Clinical Features
Congenital livedo reticularis
Cutis marmorata telangiectatica congenita
Cutis marmorata telangiectatica congenita (CMTC) is characterized by a persistent reticulated vascular pattern that is often limited to one extremity (see Ch. 104 ), but can be more widespread. Lesions are usually noted at birth, and, when the trunk is involved, there may be a sharp cut-off at the midline. Associated anomalies include other vascular malformations, limb asymmetry, and occasionally neurologic or ocular abnormalities . Cutaneous vascular changes can improve during the first few years of life, with 20% of patients showing complete resolution.
Acquired livedo reticularis
Livedo reticularis without systemic associations
Physiologic livedo reticularis/cutis marmorata
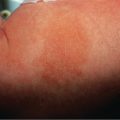
These terms are synonymous and refer to a normal pattern of LR that occurs in response to cold ( Fig. 106.4A ). It is often more marked in neonates, infants, and young children . In adults, it may be associated with a tendency towards acrocyanosis and chilblains.
Primary/idiopathic livedo reticularis
This refers to a persistent fine network of LR that is often widespread, particularly on the lower extremities. While there is some fluctuation with temperature, the LR will usually persist with warming. It is due to persistent vasospasm of arterioles and is not secondary to any underlying cause. However, primary LR is a diagnosis of exclusion and it is important to consider secondary causes (see Table 106.1 ). This is especially true when the LR is extensive .
Livedo reticularis secondary to systemic disease
Livedo reticularis due to vasospasm
Vasospasm is the most common cause of LR, including that seen in association with autoimmune connective tissue diseases (CTD; Fig. 106.4B ). Reflecting a vasospastic tendency, it occurs more commonly in patients with Raynaud phenomenon.
Livedo reticularis due to vessel wall pathology
Vasculitis involving the medium-sized arterioles at the dermal–subcutaneous junction or in the deep dermis is the most common cause of vessel wall pathology associated with LR. Involvement of medium-sized arterioles is characteristic of cutaneous polyarteritis nodosa (PAN) , but can also be seen with systemic PAN and ANCA-associated vasculitides (see Ch. 24 ). Patients with lymphocytic thrombophilic arteritis, in which the peri-arteriolar inflammation is lymphocytic rather than neutrophilic, characteristically develop livedo racemosa (see Fig. 106.3 ) . Whether this disorder represents a distinct entity or a variant of cutaneous PAN is a matter of debate. LR can also be seen in patients with livedoid vasculopathy (see Ch. 23 ) .
Deficiency of adenosine deaminase 2 (DADA2) is an autosomal recessive autoinflammatory disorder (see Ch. 45 ). It has features of both Sneddon syndrome and polyarteritis nodosa, i.e. patients can have both a vasculopathy and vasculitis. Characteristic features include intermittent fevers, early-onset lacunar strokes, hepatosplenomegaly, hypogammaglobulinemia and lymphopenia, as well as cutaneous nodules and livedo racemosa (see Fig. 45.11 ) .
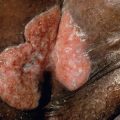
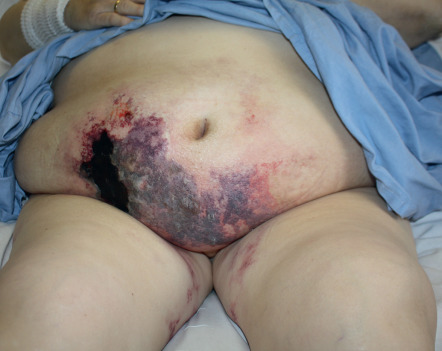
Calciphylaxis consists of calcium deposition in the walls of blood vessels (see Ch. 50 ). It is most commonly seen in the setting of end-stage chronic kidney disease complicated by secondary hyperparathyroidism. Calciphylaxis may initially commence with LR that then becomes purpuric and subsequently necrotic ( Fig. 106.5 ).
Sneddon syndrome is rare and is characterized by widespread livedo racemosa in conjunction with multiple cerebral ischemic episodes leading to progressive neurologic impairment (see Ch. 23 ). It remains uncertain as to whether the vascular pattern results from vasculopathy, vasculitis or coagulopathy (as some patients have antiphospholipid antibodies while others have DADA2), but characteristic changes are seen within affected vessels.
Livedo reticularis due to intraluminal pathology
LR can result either from factors (e.g. within the blood) that slow intravascular blood flow or from complete obstruction of the vessel lumen. Altered blood flow due to an increase in blood viscosity may be secondary to abnormal circulating proteins (e.g. cryoglobulins , cryofibrinogens, cold agglutinins, paraproteins) or an increase in normal blood components (e.g. polycythemia vera , thrombocytosis). A fine, evenly distributed pattern of LR is usually seen. Hypercoagulable states can also be associated with LR, including the APS and protein C , protein S, or antithrombin III deficiencies . LR of the lower extremities is often seen in patients with neurologic conditions that lead to immobility of the lower limbs and stasis.
Complete obstruction of the vessel lumen can result from either emboli (e.g. cholesterol emboli derived from atheromata ) or thromboses within vessels (e.g. APS, heparin or warfarin necrosis). Intracellular crystal deposition is seen in hyperoxaluria and this also gives rise to luminal obstruction and LR . A patchy, discontinuous-pattern LR is seen with intraluminal obstruction and it may subsequently become purpuric with areas of infarction and necrosis.
Other causes of livedo reticularis
There are numerous causes of LR cited in the literature, and, in most, one of the above mechanisms can generally be implicated. The other causes include drugs such as amantadine and norepinephrine (noradrenaline), as well as infections. The latter can lead to: the production of cryoglobulins, cold agglutinins or antiphospholipid antibodies; the induction of immune vasculitis; or septic vasculitis or septic emboli. Neoplasms may also be associated with LR via hypercoagulability or paraproteinemia as well as vasospasm (e.g. pheochromocytoma). Lastly, LR may be seen in several neurologic conditions (e.g. reflex sympathetic dystrophy) as a result of vasospasm or vasodilation (this is in addition to stasis arising from immobility).
Differential Diagnosis
Erythema ab igne is a heat-induced skin disease that begins as a reversible LR, then with continued heat exposure, it evolves into a fixed reticulated hyperpigmentation in the same pattern. When present on the anterior thighs, the possibility of direct contact with a laptop computer should be considered. Various cutaneous eruptions may have a reticulated pattern and might be confused with LR, including reticulated erythematous mucinosis (favors mid central trunk) and some viral exanthems (e.g. erythema infectiosum). Poikilodermatous conditions may also have a reticulate pattern (e.g. dermatomyositis, mycosis fungoides); however, the presence of epidermal changes and telangiectasias will help distinguish these conditions from LR.
Pathology
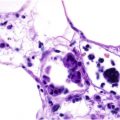
The histology of LR varies depending on the underlying cause. In idiopathic or physiologic forms resulting from vasospasm, no abnormality will be evident. With secondary causes of LR, a number of abnormalities may be seen, including vasculitis, calcium deposition within vessel walls (calciphylaxis; see Fig. 50.6 ), intravascular eosinophilic plugging (monoclonal cryoglobulinemia; see Fig. 23.4 ), intraluminal thromboses (hypercoagulable states), cholesterol clefting (cholesterol emboli; see Fig. 23.5 ), and crystal deposition (oxalosis). In Sneddon syndrome, vessel walls demonstrate endothelial inflammation and subendothelial myointimal hyperplasia with partial or complete occlusion of affected arterioles; however, in order to find these histopathologic changes, it is necessary to sample the affected arterioles. An elliptical biopsy from the paler central “hole” in the net pattern is necessary and serial sectioning may be required (see Fig. 106.1 ).
Treatment
LR is a clinical sign and does not require treatment per se. It is unresponsive to treatments such as vascular laser therapy or vasodilatory medications. Underlying causes require identification and appropriate treatment.
Flushing
- ▪
Flushing is a physiologic response, but in an exaggerated form causes clinical symptoms
- ▪
Common triggers (e.g. heat, emotion, exercise, some foods) will exacerbate flushing of any cause
- ▪
Causes of excessive flushing include exogenous medications, menopause, neurologic disorders, and systemic diseases (e.g. carcinoid syndrome)
Introduction
Flushing is the term used to describe transient and episodic reddening of the skin, most commonly of the face, and less often the neck, ears and upper chest. It is the visible sign of a generalized increase in cutaneous blood flow. The greater visibility and capacitance of the superficial cutaneous vasculature of the face and adjacent areas accounts for the limited distribution . In the evaluation of an affected patient, there are a number of causes to consider, including underlying systemic disorders ( Table 106.2 ).
| CAUSES OF FLUSHING |
|
Pathogenesis
An increase in cutaneous blood flow occurs with relaxation of vascular smooth muscle. This may occur via the autonomic nervous system (usually leading to active vasodilation), endogenous vasoactive agents (such as histamine and serotonin), or exogenous agents ( Table 106.3 ). Alcohol-induced flushing may result from the direct effect of alcohol as well as cutaneous vasodilation from elevated blood levels of acetaldehyde; the latter occurs in those with alcohol dehydrogenase deficiency (prevalent in Asians) and in the “disulfiram reaction” induced by some medications. Flushing from fermented alcoholic drinks may be caused by vasoactive substances such as tyramine. Vasodilation mediated by the autonomic nervous system is often accompanied by eccrine sweating due to a direct effect on both sweat glands and blood vessels (wet flush) . Direct vasodilation by vasoactive agents is usually not associated with increased sweating (dry flush).
| EXOGENOUS AGENTS THAT CAN CAUSE FLUSHING |
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree