Optimizing Wound Healing and Scar Formation
Reinhard Dolp
Saeid Amini Nik
Marc G. Jeschke
KEY POINTS
Absorbable staples combine the good cosmetic results of sutures with the fast wound closure time of conventional staples.
Adhesive tape and tissue glue are reasonable alternatives for the closure of small wounds.
Laser-assisted wound closure shows great results but is too expensive for routine clinical practice.
Wnt/β-catenin, TGF-β, Hedgehog, and Notch pathways are key mechanisms for wound healing and scar formation.
Intralesional steroid injection and silicone gel sheeting are the most recommended therapies for hypertrophic scars and keloids.
Nutrition plays a key role in wound healing, and special recommendations have to be considered.
Negative pressure dressings and hydrocolloid/hydrogel dressings are excellent in the treatment of complex wounds.
Acellular skin substitutes are a life-saving component in the treatment of severe skin loss.
Cellular skin substitutes are limited by the availability of cells and their high costs.
Cytotherapy is the future in the treatment of wounds and scars.
With claimed annual health care costs for the treatment of chronic wounds of 25 billion dollars (in the United States alone) and a 15 billion dollar market for wound care products,1 the optimization of wound healing and scar formation is essential in times of escalating health care costs.2 Two to three percent of the American population is affected by wound healing disorders that require therapy.1,3 This is not limited to insufficient or absent wound healing, but also includes excessive skin proliferation that can lead to keloids and hypertrophic scars.4 The psychological and physical effects of skin diseases on patients can be devastating, and improving the treatment modalities can drastically improve a patient’s quality of life (see Chapter 24).
Wounds are commonly classified as acute (<3 months) or chronic (>3 months). Recently, it was proposed to change this classification into acute and complex wounds.5 A wound is considered complex if it displays one of the following characteristics: absence of healing within 3 months, infection, compromised viability of superficial tissues, necrosis, circulation impairment, or associated with systemic pathologies. Excessive skin proliferation after dermal injury can be divided into keloids and hypertrophic scars. Keloids are characterized by an abnormally increased deposition of collagen in the dermis that extends beyond the margin of the initial injury, whereas collagen deposition in hypertrophic scars is limited to the initial wound area.
Scar Formation and Wound Healing
In contrast to antenatal skin, postnatal skin usually heals by scar formation; scar tissue is inherently weaker and contains an extracellular matrix (ECM) that is more disorganized.6,7 Posttraumatic inflammation plays a key role in this process (see Chapter 6). Although an inflammatory response is vital to prevent or contain wound site infections, the inflammatory cytokines and growth factors released during this process promote fibrosis and scar formation.7,8,9,10 In addition to this inflammation (that can only take place in a fully developed immune system), the expression profile, concentration of growth factors and cytokines, as well as some ECM components (hyaluronic acid, fibronectin, elastin) differ in adult scar-forming wound healing as compared with embryonic scarless wound healing (see Chapter 27).7,8,11,12,13 Understanding the molecular basis for scar formation and wound healing is essential for developing and applying
novel medical therapies.2 This chapter will explain the most important mechanisms together with the corresponding ways to enhance the process.
novel medical therapies.2 This chapter will explain the most important mechanisms together with the corresponding ways to enhance the process.
Measuring Scars
Despite obvious macroscopic criteria such as wound and scar size and time to wound closure, no consistent methodology exists to precisely determine the effects of therapy upon scar formation and wound healing (see Chapter 28).14 In contrast to intact skin (with its loose, random, basket-weave-like organization of collagen bundles), scar tissue is composed of more parallel and tightly packed collagen bundles (see Chapter 5).15 A total of 85% of the dermis consists of collagen and it dictates dermal elasticity and strength. This makes collagen one of the main targets in the evaluation of wound healing.16,17
Khorasani et al.14 suggested a new method for analyzing scar tissue using fractal dimension (FD) and lacunarity (L) analysis. FD measures the degree to which an object fills a space, with a minimum value of 1 being a straight line and a maximum value of 2 occupying the whole space (=structural density). L determines the degree of structural variance within an object, with a minimal value of 0 representing complete homogeneity and a maximum value of 1 representing absolute heterogeneity (structural heterogeneity).18 This method has already proved itself to be reliable and reproducible in the evaluation of complex biologic structures such as neurons19 and capillary beds.20,21 However, studies of Khorasani et al. were performed on a murine model, and the clinical usefulness of this more precise method of scar tissue determination is yet to be investigated in humans.
Procedural Optimization
Surgical Optimization
Surgical optimization of scar and wound healing starts with choosing a way of opening the skin with the least trauma possible, followed by closing the wound or by preparing it for healing by secondary intention with a technique that achieves the best cosmetic and functional results. It also includes the surgical treatment of aberrant/excessive scar formation after wound closure (see Chapter 12).
Incision Method: Electrocautery/Diathermy
Since the first use of an electrosurgical device in 1926, diathermy has become ubiquitous because of its efficacy, convenience, advantages in hemostasis, and safety as compared with scalpels.22,23 Recent meta-analyses and reviews show no significant difference in the wound infection rate,24,25,26 but suggest a decrease in patient-perceived postoperative pain with the use of diathermy.24 The use of diathermy to cut the epidermis and dermis remains controversial because of the believed inferior scar cosmesis owing to thermal damage. A recent Canadian double-blind randomized trial assessing 66 patients having received abdominal surgery (laparoscopic and open) could not detect any significant difference (on either subjective or objective measures) between epidermal incision with diathermy or with a scalpel, 6 months postoperatively.22 This supports existing data on smaller patient cohorts that also failed to detect an inferior cosmetic result when using diathermy for skin incisions.27,28 Further investigation is needed to elucidate if using diathermy for skin incisions is also legitimate in cosmetically sensitive areas like the head, neck, and breast.22
Wound Closure
The principal aim of skin/wound closure is to minimize tissue damage and inflammation, to promote a rapid acquisition of tissue strength, and good cosmesis. Accurate coaptation of the dermal margins is a key factor in this, and inappropriate eversion or inversion can lead to suboptimal healing.29,30 There are different techniques available for skin/wound closure including sutures, staples, adhesive tape, and liquid adhesives. Sutures and staples are the most commonly used wound closure materials because they provide the often needed mechanical support.31
Absorbable versus Nonabsorbable Sutures
Luck et al.32 could not detect significant differences in cosmesis or infection rate for absorbable vs. nonabsorbable suture materials after 3 months in facial lacerations. Also, no differences regarding the cosmetic outcome could be found in pediatric lacerations after 4 to 5 months.33 However, nonabsorbable monofilament nylon sutures seemed to diminish the risk of hypertrophic scarring in comparison to absorbable sutures in a randomized clinical trial that looked at 60 patients who underwent midline sternotomy for cardiac surgery.34 Skin was closed in a subcuticular continuous fashion either with a 4-0 braided polyglycolic acid suture or with a 4-0 nonabsorbable monofilamentous polypropylene suture that was removed 8 to 10 days after the surgery. Patients were evaluated 6 months after sternotomy. Interestingly, the benefit of the nonabsorbable monofilament suture was only apparent in the upper half of the sternum. The lower half did not show any difference. The authors concluded that increased tension and mobility of the skin in the lower half of the sternotomy led to the inferior scar appearance independently of the suture material. In terms of cost-effectiveness, it has to be considered that nonabsorbable sutures have a higher cost and require an additional physician visit for removal.35
Sutures versus Staples
Compared with sutures, stapling with metal staples is five to seven times faster and produces equivalent cosmetic results when used in an emergency setting.36,37 A 2014 study evaluating 130 women that underwent an emergency cesarean section found skin closure with staples to have a better cosmesis and shorter duration of surgery, with comparable postoperative pain and wound complications.38 Even in cosmetic surgery on more sensitive areas like the neck, no difference between stapling and suturing could
be detected.30 Two out of five randomized controlled trials evaluating chest and leg wounds after cardiovascular surgery found sutures to be superior cosmetically and were associated with fewer complications.39 However, there are no large trials comparing sutures with staples in elective surgeries in cosmetically critical body regions. Staples puncture the epidermis and therefore carry a potential risk of scarring and wound contamination in comparison to absorbable sutures.40 The use of staples has also been associated with increased tension along the incision line, precluding their use for reconstructive flap surgery.41 Stapling has been found to be safer and easier to use.37 Whether the removal of staples or nonabsorbable sutures is more or equally painful for the patient is not clear.36,42 There are also controversial reports about which method is cheaper.37,43
be detected.30 Two out of five randomized controlled trials evaluating chest and leg wounds after cardiovascular surgery found sutures to be superior cosmetically and were associated with fewer complications.39 However, there are no large trials comparing sutures with staples in elective surgeries in cosmetically critical body regions. Staples puncture the epidermis and therefore carry a potential risk of scarring and wound contamination in comparison to absorbable sutures.40 The use of staples has also been associated with increased tension along the incision line, precluding their use for reconstructive flap surgery.41 Stapling has been found to be safer and easier to use.37 Whether the removal of staples or nonabsorbable sutures is more or equally painful for the patient is not clear.36,42 There are also controversial reports about which method is cheaper.37,43
Absorbable staples (tissue half-life of 10 weeks; Insorb) that are placed in the subcuticular tissue without puncturing the epidermis40,44 are designed to combine the good cosmesis of absorbable sutures with the increased wound closure times of a stapler.40 A randomized controlled trial comparing absorbable staples versus absorbable sutures conducted in patients receiving bilateral breast reconstruction found that the wound could be closed faster with staples, while showing a comparable cosmesis.45 Absorbable staples also seem to be an adequate wound closure material in sensitive areas like the face and neck,40 as well as in immunocompromised patients.46 Nevertheless, there are no long-term data regarding absorbable staples and they are a relatively expensive alternative to other wound closure techniques.47
Adhesive Tape
Adhesive tapes contain an adhesive material consisting of iso-octo-acrylate and n-vinyl-pyrrolidone.48 Microporous strips enable the passage of gas and water from the skin surface, reducing the suitable environment for bacterial growth and wound site infections.49,50 In clean contaminated wounds (wounds that are clean, but carry a high risk of contamination in locations such the gastrointestinal, respiratory, or genitourinary tract), a lower rate of infection has been reported in wounds closed with adhesive tape in comparison to sutures.51 The difficulty in approximating the wound edges accurately49 and achieving sufficient adhesiveness, which in turn is dependent on correct usage and the presence of a dry wound site, limits the use of adhesive tape.49,52
Tissue Glue
A liquid adhesive (n-alkyl-alpha-cyanoacrylate tissue adhesives, e.g., Dermabond) polymerizes once applied on the wound, bridging the edges of the wound together.53 The film is water resistant and sloughs off about 9 days after application.53,54 It is supposed to be used in small lacerations replacing sutures that are 5-0 or smaller.54 For the closure of surgical or traumatic wounds, Dermabond was considered to be faster,55,56 less painful,55 and with a similar,57,58 equal,56 or improved59,60 cosmesis compared with standard wound closure methods. No higher rates of dehiscence have been detected.56,59 Whereas the experience of the surgeon is a key factor for a good cosmesis using suturing,61 it seems to be negligible for the outcome with tissue glue.62 Another positive side effect is that liquid tissue adhesive seems to inhibit bacterial growth and wound site infection to a certain degree.60,63,64 Tissue adhesives are now being commonly and successfully used for facial wounds, groin wounds, hand surgery, blepharoplasty, laparoscopic wounds, hair transplantation, and lacrimal punctum closure.63,65 In addition, it can be a good method for wound closure in patients who are at risk for keloid or hypertrophic scar formation.66 Tissue glue is not suitable for patients at risk for delayed wound healing (diabetics or patients with collagen vascular diseases),59 in wounds that are in difficult areas (moist, hairy), or wounds that are complicated by edema, infection, high tension, or bleeding.67,68 Depending on the situation (size of wound, experience of the doctor with different wound closure techniques), liquid tissue adhesives can be more costly than conventional wound closure techniques.52
Laser Therapy
Laser-Assisted Tissue Bonding
Laser techniques to optimize wound healing and scar formation are a promising and highly investigated alternative to currently established procedures.69 In laser tissue welding (LTW), the laser energy is directed to opposed wound margins, causing their partial liquefaction (conversion of photonic energy into heat energy), which is followed by their fusion.52 In this process, collagen fibers from both sides of the wound are intertwined,70 leading to an immediate wound seal, thereby promoting a faster reepithelialization without the need for a large formation of granulation tissue.52,69
In laser tissue soldering (LTS), a solder (a protective proteinaceous barrier such as a semisolid/solid serum albumin) is added to enhance the fusion of opposed wound margins.71 Data suggest that the use of wavelength-specific dye absorbers such as indocyanine green and adhesive proteins such as albumin to LTW may lead to an increased strength and speed of wound closure than that achieved with traditional suture techniques.71,72,73
Laser-assisted tissue bonding is expected to be a fast and efficient technique for wound closure, potentially decreasing scar formation and complications associated with standard surgical alternatives.52 LTS has shown very promising results in various tissues (e.g., skin, vessels) in animal models regarding strength and tissue quality.73,74,75 Temperature-controlled LTS in rats showed comparable histologic and cosmetic results to those of standard sutures.70 In a porcine model, the scars after LTS were almost undetectable 7 days following surgical skin incision.75 A 2001 study compared the results of LTS with conventional suturing for hypospadias repair in 183 boys. The authors reported fewer complications (fistulas and stenosis) in the LTS group and concluded that LTS is an acceptable and faster method of tissue closure in hypospadias repair.76 Furthermore, the reduced need for suture materials appeared to decrease local inflammation.76 However, only a few studies regarding LTW and LTS have been conducted in humans.52 The concerns of low tensile strength, the
potential for excessive thermal damage to the skin, and the high costs of the equipment will likely exclude this technology from use in routine clinical practice.52
potential for excessive thermal damage to the skin, and the high costs of the equipment will likely exclude this technology from use in routine clinical practice.52
Laser-Assisted Scar Treatment
In addition to their applications in connecting wounded tissues, lasers are a promising treatment option for existing keloids and hypertrophic scars (see Chapter 13), though the mechanism of action of laser treatment is still not fully understood. Suggested mechanisms include the thermo-induced breakdown of collagen fibers as well as the mediation of growth factor expression.77,78,79,80,81 In comparison with other treatment options such as intralesional 5-fluorouracil (5-FU) and corticosteroids for keloidal and hypertrophic scars, pulsed dye laser therapy has shown comparable results without side effects (see Chapter 10).77,78 Argon lasers, the first lasers used in the treatment of keloids, were dismissed in favor of carbon dioxide, neodymium-doped yttrium aluminum garnet (Nd:YAG), and pulsed dye lasers because of unsatisfying results.82 As a monotherapy for keloids and hypertrophic scars, nonfractionated carbon dioxide laser ablation was associated with recurrence rates of 90% and higher,83,84 whereas the nonablative Nd:YAG laser showed recurrence rates of 17%.85 Laser-assisted scar treatment also seems to be effective in treating associated symptoms like itching, redness, and pain.77,86 A review article on the treatment of keloids and hypertrophic scars published in the Journal of the American Medical Association stated that pulsed dye lasers are an emerging therapeutic option owing to their high efficacy, yielding fewer adverse effects.82
Recently, using a picosecond pulse width infrared (IR) laser that targets tissue water, Amini-Nik et al.87 demonstrated a significant reduction of thermal damage and scar formation compared with conventional lasers and scalpels.87 In principle, the shorter pulse duration mitigates the destructive photochemical and photothermal effects associated with conventional devices with longer pulse widths.87 This highlights the role that novel surgical modalities might have in minimizing scar formation. However, this study was performed in a rodent model, whose skin and wound healing properties differ from those of humans.88
Pharmacologic Optimization
To understand the pharmacologic targets involved in wound healing and hypertrophic/keloid scar treatment, it is important to have a basic knowledge of important molecular pathways and regulators involved. Those pathways are not isolated entities, instead more and more data reveal an extensive cross-link between them.89
Molecular Pathways Involved in Wound Healing and Scar Formation
Wnt/β-Catenin
Wnts are glycoproteins that are important in embryonic and adult tissue maintenance.90,91,92,93 Depending on the context, Wnt ligands signal through either the canonical (mediated through β-catenin) or the noncanonical (independently of β-catenin) Wnt signaling pathway.92,94 The focus here will be on the canonical Wnt pathway because it plays an important role in skin development and in various aspects of cutaneous wound repair.2,89,95,96,97 Various Wnt glycoproteins (Wnt 1, 3, 4, 5a, and 10b) can be found in cutaneous wounds in the early phase of healing and are present up to 7 days after injury.98,99
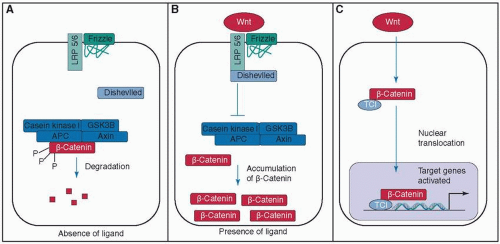
In this signaling pathway, Wnt binds to the membrane receptor Frizzled and the coreceptor low-density lipoprotein receptor-related protein 5/6 (LRP5/6). Once bound, the protein Dishevelled is activated. Dishevelled inhibits a protein complex—consisting of glycogen synthase kinase 3β (GSK-3β), adenomatous polyposis coli protein (APC), casein kinase I, and axin 1—that is responsible for the ubiquitin-mediated degradation of β-catenin. Ultimately Wnt leads to an intracellular accumulation of β-catenin.91,92,93 β-catenin itself is a transcriptional coactivator (bound to T-cell factors; Tcfs) for genes that encode important proteins for wound healing such as fibronectin,90 but it also functions as a structural protein in the adherens junction that mediates cell-cell contacts91,100 (see Fig. 9-1).
The Wnt pathway participates in the dermal accumulation of β-catenin, but it does not seem to be a key mechanism of maintaining it during wound healing.101,102 β-catenin levels can be elevated independently of the Wnt pathway by TGF-β1, a growth factor excreted during early wound healing,103,104,105 as well as by fibronectin, an ECM component, through a β1-integrin-mediated GSK-3β-dependent pathway.101 β-catenin and its target genes are elevated in dermal fibroblasts during the proliferation phase of wound healing, and return to baseline levels during the remodeling phase.106,107 In humans the duration and level of β-catenin activity correlate with wound size108,109 and the number of macrophage cells present in the wound.110 In an uncharacterized process, the Wnt pathway can reprogram or endow other epidermal cells. Ito et al.97 showed that in large wounds the Wnt pathway pushes epithelial cells toward de novo folliculogenesis.
β-catenin is essential for adequate wound repair, though excessive expression is found in human hypertrophic scars and keloids.107,108 A high amount and activity of β-catenin leads to an enlarged and hypercellular dermal compartment with an increased dermal collagen deposition, scarring, and myofibroblast formation.103,111,112 Genetic mutations that cause an unphysiologic stabilization of β-catenin lead to aggressive fibromatosis (desmoid tumor).113 Interestingly the Wnt/β-catenin pathway also seems to have an inhibitory effect: it decreases the reepithelialization after wounding. A nuclear accumulation of β-catenin has been found in the edge of chronic ulcers, and in vitro pharmacologic stabilization of β-catenin inhibited the migration of keratinocytes.114
β-catenin plays a significant role in various cells during the process of wound healing, but its role in macrophages seems to be essential.110 In mice, it has been shown that β-catenin promotes motility and adhesion of macrophages110 by upregulating the expression of cadherins, catenins, AD-AMs (a disintegrin and metalloproteinase), and integrins.110 Macrophages are believed to be an essential mediator of granulation tissue formation after skin trauma.115,116,117,118,119,120,121 These cells express multiple receptors for Wnts,110 and deficient macrophage migration and adhesion has been
associated with poor wound healing.122 If macrophages lacked β-catenin, they also showed less TGF-β1 (a cytokine essential for wound healing; see below).110 Furthermore, in the absence of β-catenin, macrophages were not able to achieve a fibroblast-like phenotype.110 Scarless embryonic wound healing takes place in the absence of a fully developed monocyte lineage118 and a less active Wnt signaling pathway.123
associated with poor wound healing.122 If macrophages lacked β-catenin, they also showed less TGF-β1 (a cytokine essential for wound healing; see below).110 Furthermore, in the absence of β-catenin, macrophages were not able to achieve a fibroblast-like phenotype.110 Scarless embryonic wound healing takes place in the absence of a fully developed monocyte lineage118 and a less active Wnt signaling pathway.123
Manipulation of the Wnt/β-catenin signaling pathway and β-catenin activity could be a promising therapeutic approach in the treatment of deficient wound healing. Nefopam (5-methyl-1-phenyl-1,3,4,6-tetrahydro-2,5-benzoxazocine), a centrally acting nonopioid analgesic, reduced β-catenin levels to baseline even if the cells were highly stimulated with a Wnt ligand.124 These cells showed a decrease in cell proliferation and viability in cell cultures derived from aggressive fibromatosis or hypertrophic wounds without affecting normal fibroblasts.124 When this was used daily for 14 days in a murine model, there was a 50% reduction in scar diameter as compared with the control group. However, the exact mechanism of decreasing β-catenin levels and inhibiting fibroblast cell proliferation is unclear.124 In a murine model a new laser—the picosecond IR laser—showed the ability to ablate tissue at a monocellular level, which causes less tissue trauma and less activation of the Wnt/β-catenin and TGF-β pathways.87 It will be a future challenge to fully understand under which circumstances and in what amount Wnt/β-catenin regulation is beneficial in wound healing.
Growth Factors
In addition to the above-described Wnt/β-catenin pathway, various growth factors and cytokines such as fibroblast growth factors (FGFs), vascular endothelium growth factors (VEGFs), and interleukins (IL) have been linked to the process of wound healing; TGF-β is one of the crucial mediators.2,125,126
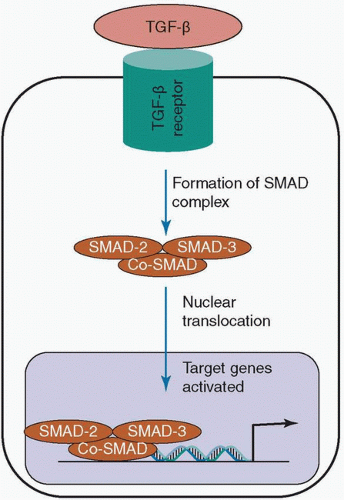
The TGF-β proteins consist of three isoforms—TGF-β1, TGF-β2, and TGF-β3—that are secreted by various cell types such as macrophages and fibroblasts.126,127 Released as inactive precursors and adjacent to TGF-β binding protein, they require activation either through proteases or through conformational changes which are in part caused by cell traction forces of integrins.9,127 Once activated, they are bound to TGF-β receptors. This initiates the formation of a SMAD complex (intracellular proteins that form a trimer of two receptor-regulated SMADs and one co-SMAD when they are activated by an extracellular stimulus) that acts as a transcription factor for various genes that encode important proteins for wound healing such as collagen (see Fig. 9-2).9,10,127,128,129 TGF-β can also act through non-SMAD-dependent mechanisms including TGF-β-associated kinase I, mitogen-activated protein kinase, and GTPases, but the exact function of this pathway and its effect on wound healing are yet to be elucidated.130,131
The different isomers of the TGF-β superfamily have different effects on wound healing and scar formation (see Table 9-1). TGF-β1 promotes the proliferation of fibroblasts, their synthesis of collagen I and fibronectin as well as wound contraction, but inhibits keratinocyte migration and therefore wound reepithelialization132,133,134,135,136,137,138. TGF-β3 seems to have antiscarring effects, whereas TGF-β2 is required for hair follicle development.11,139,140 Deficiencies in the TGF-β pathway lead to poor wound healing including a thin disorganized dermis, fewer fibroblasts, fibroblasts with cytoskeletal abnormalities, and a reduction of granulation tissue through reduced ECM deposition.136,141,142,143 Interestingly, a deficiency in the TGF-β pathway also leads to smaller wound areas and less inflammation.141,142
The expression of TGF-β proteins by fibroblasts does not only affect keratinocytes in a unidirectional manner; rather, keratinocytes also have the ability to downregulate TGF-β1 secretion.138,144 Excessive amounts of TGF-β1 lead to hypertrophic scars mediated either through the above-described β-catenin pathway or through connective tissue growth factor (CTGF; CCN2).103,104,145,146,147,148,149,150 Topical
TGF-β1 application on murine wounds causes accelerated healing and reepithelialization.132 Interestingly, antagonizing TGF-β can also result in accelerated wound closure and increased keratinocyte proliferation and migration.140,151,152 It seems to be of great importance at which exact point the TGF-β pathway is altered, suggesting a complex system of networks in this pathway that are yet to be elucidated.2 In scarless embryonic wound healing, the ratio of TGF-β1 to TGF-β3 is lower as compared with what has been found in adults.8,11,153 Applying TGF-β1 to embryonic wounds (in animal models) promotes scar formation, whereas TGF-β3 reduced it.139,153 The promising results of wound treatment with tissue growth factor in animal models has led to various clinical trials. Despite positive preliminary data,154 all of the clinical studies failed to establish a clinical benefit for TGF therapy in wound healing.2,155,156,157,158,159 Additionally, TGF also contributes to the formation of hypertrophic scars.105,160,161
TGF-β1 application on murine wounds causes accelerated healing and reepithelialization.132 Interestingly, antagonizing TGF-β can also result in accelerated wound closure and increased keratinocyte proliferation and migration.140,151,152 It seems to be of great importance at which exact point the TGF-β pathway is altered, suggesting a complex system of networks in this pathway that are yet to be elucidated.2 In scarless embryonic wound healing, the ratio of TGF-β1 to TGF-β3 is lower as compared with what has been found in adults.8,11,153 Applying TGF-β1 to embryonic wounds (in animal models) promotes scar formation, whereas TGF-β3 reduced it.139,153 The promising results of wound treatment with tissue growth factor in animal models has led to various clinical trials. Despite positive preliminary data,154 all of the clinical studies failed to establish a clinical benefit for TGF therapy in wound healing.2,155,156,157,158,159 Additionally, TGF also contributes to the formation of hypertrophic scars.105,160,161
Table 9-1 Effects of TGF in Wound Healing and Scar Formation | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Today, only a few growth factors are approved for clinical treatment and their indications are very limited. Human recombinant platelet-derived growth factor-BB (PDGF-BB; becaplermin) is used for the treatment of chronic diabetic ulcers on the limbs. It led to a 15% increase in healing compared with standard wound care alone in those patients.162,163,164 Health Quality Ontario stated that the efficacy of growth factors and granulocyte colony-stimulating factor (G-CSF) in enhancing the healing of chronic pressure ulcers has not been established yet.165 PDGF (Regranex; approved by Health Canada for the treatment of diabetic ulcers on the lower extremities) was associated with an increased rate of deaths from cancer.165 In Japan, FGF-2 is used postoperatively to reduce hypertrophy and widening of scars.2 Next to its antiscarring effects,166 FGF-2 showed promising results in the treatment of life-threatening, large disruptions of the skin integrity as in severe burns.167. More sophisticated, well-designed clinical trials will be necessary to evaluate the efficiency of growth factors in the treatment of wounds and scars.125,168
Hedgehog
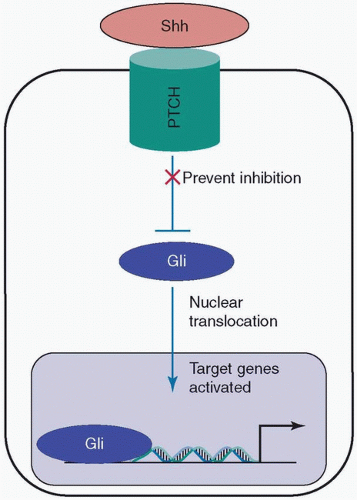
The Hedgehog pathway plays important roles in embryonic skin development, angiogenesis, the regulation of epidermal stem cells, and the development of skin appendices in adults.169,170,171,172,173,174,175 Among the three existing Hedgehog proteins—Indian Hedgehog (Ihh), Sonic Hedgehog (Shh), and Desert Hedgehog (Dhh)—Shh seems to be the main contributor to wound healing and scar formation.169,176 Shh binds to Protein patched homolog (PTCH)—a transmembrane protein. PTCH is a tumor suppressor that prevents the activation of Gli transcription factors (see Fig. 9-3). Shh removes the inhibition of PTCH and activates Gli transcription factors that promote the encoding of a multitude of different proteins such as cyclin D2, a key regulator of the cell cycle.169,176 The exact role of the Hedgehog pathway in wound healing and scar formation is relatively unknown.
Shh can be found in regenerated hair follicles after wounding, but not in the epidermis or keratinocytes.177 Nevertheless, if diabetic mice are treated topically with Shh they show better reepithelialization and wound healing. The dermis is thicker, more collagen-rich, and better vascularized.178 Shh stimulates the proliferation of fibroblasts and promotes the recruitment of bone marrow-derived endothelial progenitor cells and the excretion of VEGF.178 If the Shh pathway is blocked, mice showed delayed wound healing, reduced granulation tissue formation, and decreased vascularization.179
Notch
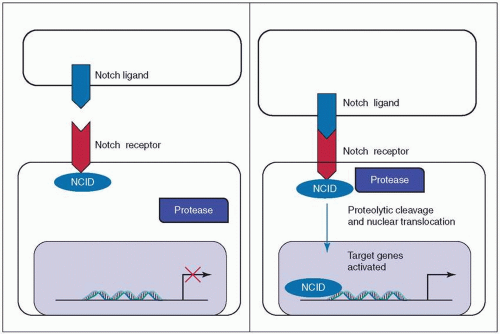
The Notch pathway in mammals is activated by the binding of a transmembrane ligand (members of the Delta-like and Jagged families) to one of the four Notch receptors of an adjacent cell.180,181,182 This binding causes the liberation of the Notch intracellular domain (NCID) by sequential
proteolytic cleavage. The NCID then translocates to the nucleus where it regulates gene expression (see Fig. 9-4).180,181,182
proteolytic cleavage. The NCID then translocates to the nucleus where it regulates gene expression (see Fig. 9-4).180,181,182
Notch plays a role in the development and maintenance of the epidermis and blood vessels.180,181,182,183 It ensures the correct skin stratification during skin development.183,184,185
Downregulation of the Notch pathway leads to delayed wound healing, and stimulates increased wound closure in mice.186 This might be because of an activating effect on the endothelial cells and the fibroblasts, increasing vascularization and collagen deposition.186,187,188 In addition, recent data suggest that the Notch pathway promotes inflammatory activity by enhancing the recruitment of macrophages and the secretion of inflammatory cytokines.187 However, the role of the Notch pathway in wound healing and scar formation is relatively unknown, as well as its impact on the different cell types contributing to this process (like macrophages, keratinocytes, and fibroblasts).2
Nonsteroidal Anti-inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely used oral drugs to suppress inflammation.189 They inhibit the activity of cyclooxygenase (COX), which is needed for the synthesis of prostaglandins and proinflammatory cytokines like IL-1β.190,191 NSAIDs minimize swelling and pain by reducing the inflammatory response after skin injury.192 Diclofenac also has a moderate antibacterial activity (in vitro and in vivo) because of its ability to inhibit DNA synthesis in bacteria.193 Despite those positive aspects of NSAIDs, aspirin administration is associated with impaired wound healing.194 Postoperative diclofenac treatment significantly inhibited collagen deposition in murine subcutaneous granulation tissue.190 It has been postulated that NSAIDs decrease the degradation
of collagen in the early phase of wound healing because of their anti-inflammatory properties and inhibition of matrix metalloproteinases (MMPs),195,196,197 whereas they inhibit collagen synthesis in later stages.196
of collagen in the early phase of wound healing because of their anti-inflammatory properties and inhibition of matrix metalloproteinases (MMPs),195,196,197 whereas they inhibit collagen synthesis in later stages.196
Antihistamines
Histamine is an important mediator of inflammatory skin reactions.198,199,200 It is mainly released by basophils and mast cells.200,201,202 The complex influence of histamine on the cellular and humoral immune system is not fully understood,203 but studies suggest that it plays an important role in restoring the mechanical integrity of the skin barrier and in pathogen clearance at multiple levels.204 Histamine-induced wound closure is mediated by all classic histamine receptors (HRs), with HR1 being the main one.204
If exposed to histamine, keratinocytes have shown an altered expression profile of multiple genes204 including an upregulation of genes involved in the migration of epithelial cells like β5-integrin,205 and a downregulation of the tumor suppressor gene p53.204 This leads to a dose-dependent acceleration of reepithelialization in histamine-exposed keratinocytes.204 Antagonizing HR1 results in delayed skin healing and reduces the breaking strength of collagen.206 The antiproliferative and anti-inflammatory properties of HR1 antagonists207,208 are promising for the potential treatment of keloidal and hypertrophic scars. Pheniramine, an antihistamine, reduced the proliferation and DNA and collagen synthesis rate in fibroblasts from abnormal scars, but also affected, to a lesser extent, fibroblasts from regular skin.209 Tranilast, a histamine antagonist, reduced the synthesis of collagen in keloidal fibroblasts through suppression of the release of TGF-β1, but does not affect normal fibroblasts.210 This drug is approved and has been used in Korea and Japan for the treatment of hypertrophic scars since 1993.211
Immune Suppressants and Modulators
Intralesional Steroid Injection
Intralesional steroid injection (triamcinolone acetonide suspension) has been considered a mainstay in the prophylaxis and treatment of hypertrophic and keloid scars for decades (see Chapter 10).82 It leads to the degeneration of collagen bundles as well as to the inhibition of fibroblast growth.212 In a 10-year follow-up study of keloid and hypertrophic scar patients, intralesional triamcinolone acetonide caused a 64% reduction in scar size and a 72% reduction in symptoms. Keloidectomy in combination with β-radiation showed a similar effect on the reduction of scar size (75%), whereas β-radiation alone was inferior to steroid injection and surgery (11% scar size reduction, 55% symptom control).213
Interferons
Interferons (INFs) are potent inhibitors of human fibroblast collagen production, with INF-γ being more potent than INF-α.214 Intralesional injection of INF-γ used as a monotherapy led to a reduction in keloid size in 75% of patients.215 As an adjunct to scar excision, INF-α reduced the recurrence rate of keloids from 58% to 19%.216 Disadvantages of INF include adverse effects such as flu-like symptoms and high cost.82
Imidazoquinolines
Imidazoquinolines (imiquimod and resiquimod) are toll-like receptor (TLR) 7 and 8 agonists that induce key cytokines for wound healing like INF-α.214,217 Data on the utility of topical application of imiquimod for the prevention of keloid recurrence after excision have been conflicting. The application of 5% imiquimod cream after218 or before219 surgical removal of keloids resulted in a good cosmesis and no recurrences at 4 and 12 months. However, less successful imiquimod treatment has also been reported. A study evaluating postsurgical administration of 5% imiquimod after surgical therapy of keloids of the trunk had a recurrence rate of 80% (8 out of 10).220 In addition, pigmentary alteration is a common side effect.218
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree