Introduction
Advancements in oncoplastic breast surgery have enabled many women to conserve their natural breast tissue and avoid mastectomy. The available techniques can be applied to the majority of women and provide outcomes that range from good to excellent. Techniques related to volume displacement include reduction mammaplasty, adjacent parenchymal rearrangement, and mastopexy, which are usually indicated for women with mammary hypertrophy. Techniques related to volume replacement typically include the use of local or remote flaps and are indicated for women with smaller breast volumes in whom reduction techniques may not be possible. By performing the reconstruction before the radiation, postablative deformities are minimized and adverse events are fewer.
The use of breast implants as a volume replacement procedure during the early years of oncoplasty was fraught with complications and adverse events. One of the obvious caveats with the use of implants was the long-term outcomes due to the presence of an implant in the setting of radiation therapy. Prior studies that reported on outcomes of implant-based oncoplasty demonstrated higher complication rates and less favorable outcomes when compared with oncoplastic reduction mammaplasty. Elton et al demonstrated that the intracavitary placement of an implant was associated with a 27.8% rate of patient dissatisfaction and explantation following radiation therapy.
Recent advancements in radiation oncology have focused on reducing the untoward effects of radiation on the soft tissues. These include hypofractionation, partial breast irradiation, intensity modulation, and three-dimensional (3D) conformal and intraoperative radiation delivery. The benefits of these innovations are that prosthetic devices in the setting of radiation therapy can provide acceptable outcomes in the majority of patients without a dramatic increase in reconstructive failure. Reish et al. demonstrated that radiation therapy in the setting of nipple-sparing mastectomy and prosthetic reconstruction was associated with increased rates of capsular contracture (12% vs 2.3%, p < 0,001) and secondary revision with fat grafting (13.6% vs 3.9%, p < 0.001). Preoperative radiation had an increased likelihood of complications ( p = 0.04), and postoperative radiation had an increased likelihood of explantation (8.9% vs 1%, p = 0.015).
Given the improved outcomes with total mastectomy, prosthetic devices, and radiation therapy, the next advancement was to provide women the option of using prosthetic devices in the setting of oncoplastic breast surgery. The reasons for this are that some women with smaller breasts may choose to avoid the traditional replacement procedures such as a flap due to the risk of complications and prolonged recovery. In addition, they are usually not candidates for displacement procedures such as reduction because of the lack of tissue. From a historical perspective, these patients were given the sole option of a mastectomy to avoid the disfigurement that would occur with breast conservation alone. However, many of these patients did not want to have a mastectomy and therefore posed a unique set of challenges for the aforementioned reasons.
Based on the increased use of prosthetic devices and improved outcomes associated with radiation and implants, the biplanar technique was described that included simultaneous volume displacement and replacement. Instead of using a flap for volume replacement, a small implant would be placed below the pectoralis major muscle. This would occur at the same time as parenchymal rearrangement that would occur above the pectoralis major muscle. Hence, the name biplanar was introduced. Early experience with the biplanar technique was favorable, demonstrating good to excellent results.
A second option, and the focus of this chapter, is one that also incorporates simultaneous volume displacement and replacement; however, rather than using a breast implant, an implantable, resorbable, 3D coil is used. The initial indication for use of this coil was to assist the radiation oncologists for precise localization of the tumor extirpation site following breast conservation therapy (cross). The coil itself comes in a variety of sizes and included metallic studs imbedded to the coil that allows the radiation oncologists to readily identify the coil and target the delivery of radiation therapy more accurately. The coil that is currently in use is known as the BioZorb (Focal Therapeutics, Aliso Viejo, CA, USA).
An incidental benefit of the BioZorb is that it serves as a filler material and partially replaces the volume loss from the tumor extirpation. The difference between this approach and the biplanar approach is that this technique is uniplanar with the volume replacement and displacement occurring above the pectoralis major muscle. The device is placed within the partial mastectomy defect, and the soft tissues above it are closed. With this technique, volume and contour abnormalities can be minimized.
Patient Selection
This technique of simultaneous volume displacement and replacement is best suited for women with small to moderate breast volume in which reduction mammaplasty techniques are not possible and autologous flaps are not possible or declined. The technique is not indicated in patients with a prior history of radiation due the increased likelihood of adverse events with placement of prosthetic devices in a previously radiated field. Patient comorbidities should be assessed to ensure that they are in good general health. In patients with diabetes mellitus, HbA1c levels should be less than 7 and glucose levels on the day of surgery should be less than 200. Patients should avoid tobacco products for 1 month before and following the scheduled procedure.
Beginning the Operation
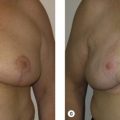
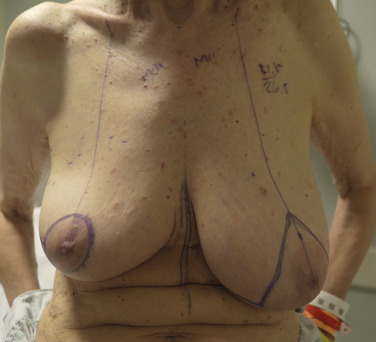
The patient is marked in the preoperative area in the standing position ( Fig. 13.1 ). The preferred incisional approach is a periareolar with a lateral or vertical extension. The lateral periareolar extension is considered for lateral or superior tumors, and the vertical periareolar extension is considered for inferior or medial tumors. These incisional patterns will allow for optimal exposure for all quadrants of the breast. The position of the nipple–areolar complex on the breast mound can be elevated or modified using mastopexy patterns as needed. A scalpel is used to create the incisions through dermis. Electrocautery is used to elevate and separate the subcutaneous layer from the underlying parenchyma in the area of the tumor. It is not recommended to undermine the entire breast parenchyma from the subcutaneous tissues. The partial mastectomy is completed in the standard fashion.
Technique of Coil Placement
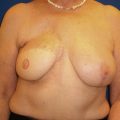
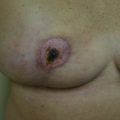
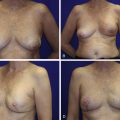
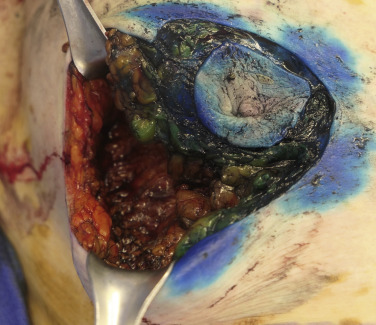
Following the partial mastectomy, the breast defect and the specimen are assessed and measured ( Figs. 13.2 and 13.3 ). Linear and volumetric measurements are important, and the segmental nature of the defect is assessed. When radiolucent markers are placed at the tumor site, radiographic imaging will confirm that the targeted location has been excised ( Fig. 13.4 ). Defects that are square to circular in configuration are best suited for the BioZorb device. If the defects are rectangular and greater than 30% of the estimated volume of the breast, the technique may be less successful because of the difficulties of rearranging a limited amount of tissue due to the risk of devascularization and fat necrosis. The use of fluorescent angiography can be considered to better assess skin and parenchymal perfusion.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree