Introduction
In this chapter, we present the use of lateral thoracic skin and subcutaneous tissues as an option for partial breast reconstruction. Lateral thoracic skin and fat are an excellent source of tissue for reconstruction of partial mastectomy defects (after lumpectomy), as well as for augmenting both reconstructed and normal breasts for the purpose of achieving symmetry. The donor site is well concealed and may even serve to improve the lateral chest wall contour. Tissue is harvested on one of three different pedicles, leaving the latissimus dorsi (LD) muscle intact. We find the flaps to be reliable and the technique reproducible, offering an excellent alternative to fat grafting, prostheses, and the LD flap.
Secondary defects in breast reconstruction
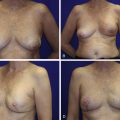
The post-mastectomy defect is frequently treated with autologous breast reconstruction. Despite being unrivaled in restoring symmetry, ptosis, and texture to the reconstructed breast, many patients undergoing reconstruction require secondary or revision procedures in order to achieve symmetry, an acceptable final volume, or to simply correct contour irregularities.
Patients with early-stage breast cancer are increasingly treated with breast conservation therapy. Treatment may comprise lumpectomy and sentinel lymph node dissection with or without axillary node clearance and radiation therapy. However, breast conservation therapy is known to result in a variety of contour irregularities which pose difficult reconstructive challenges. It is widely accepted that between 20% and 30% of patients undergoing breast conservation therapy experience poor or mediocre cosmetic outcomes as a direct result of lumpectomy and radiation fibrosis. An estimated 71% of patients seek treatment for correction of partial mastectomy defects and contour deformities. Often, these defects present the surgeon with a greater reconstructive challenge when compared with reconstruction of a defect resulting from a total mastectomy.
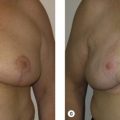
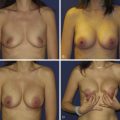
The need for more volume in a reconstructed breast also arises when a reconstructed breast needs to be revised, for example by removal of fat necrosis. This can result in a reconstruction that is smaller than the contralateral breast. Another situation requiring augmentation of volume is when a reconstructed breast is larger than the contralateral normal breast. Lateral thoracic flaps can be used to correct all the aforementioned problems.
Many partial mastectomy defects require only moderate amounts of volume in order to achieve satisfactory correction. While the LD flap has long been the workhorse in transferring autologous tissue to the breast mound in order to reconstruct partial defects, it exacts a high price on the patient. Sacrifice of the LD muscle results in early fatigue with repetitive arm extension as well as weak internal/external rotation of the upper extremity. Since the advent of perforator flaps, it is possible to create muscle-sparing flaps utilizing the lateral thoracic and axillary tissue. Furthermore, the LD flap harvest site is prone to the complication of seroma formation.
An alternative to the myocutaneous LD flap is to simply utilize the lateral thoracic and axillary skin and subcutaneous tissues, based on the perforating blood vessels that supply them. The thoracic skin and fat immediately lateral to the breast provides a logical source for tissue with which to reconstruct the adjacent breast ( Table 7.1 ). Lateral thoracic skin is similar in color and texture to native breast and offers an excellent match. Furthermore, it is possible to harvest large amounts of skin and subcutaneous tissue without violating muscle. Lateral thoracic skin may be applied to breast reconstruction in conjunction with an implant when necessary. The indications and contraindications for use of lateral thoracic flaps are listed in Tables 7.2 and 7.3 .
|
|
|
In many patients the lateral thoracic and axillary skin are present as an unsightly bulge or roll. Harvest of skin from this area provides the additional potential advantage of improving the contour of the lateral chest wall. Harvest of lateral thoracic skin necessarily results in an additional surgical scar; however, the scar is strategically positioned under the arm at the level of the bra line. Thus the donor site is effectively camouflaged in a manner cosmetically acceptable to both the patient and surgeon.
Preoperative history and considerations
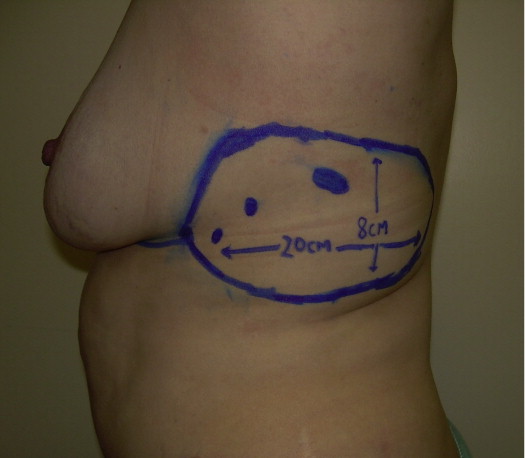
The first consideration is the amount of volume of tissue available. The ‘pinch test’ will help to determine the amount of tissue available. The surgeon must ascertain that a flap of width 7–9 cm is available for harvest with enough subcutaneous fat to provide the volume required. Extensive axillary surgery may be a contraindication to use of the lateral thoracic tissue. If the patient has had an axillary node dissection, it is necessary to review the operative report so that the surgeon knows that the thoracodorsal artery and vein are intact for use as a thoracodorsal artery perforator (TDAP) (Angrigiani) flap. If this cannot be ascertained, a Doppler signal in the appropriate position at the anterior axillary line may represent either an intercostal perforator or a direct cutaneous branch from the axillary artery. Any of these may be pedicle options, and the choice may influence the degree of flap mobility and rotation. Previous implant reconstruction can also sometimes disrupt normal anatomy in the anterior axillary line, rendering intercostal perforators unavailable for use. Liposuction to the lateral thorax is a contraindication to the use of this tissue, and radiation therapy often causes skin changes that limit the skin’s availability.
Flap design
The flap is designed with the patient in the upright position. It is important to mark the patient prior to positioning on the operating table as this provides the surgeon with the opportunity to assess lateral thoracic bulge. Immediately posterior to the lateral breast at the anterior axillary line a handheld 8 MHz Doppler is used to locate signals at the anterior edge of the LD. Signals in this area can be either perforators off of the thoracodorsal, a direct cutaneous branch from the axillary artery, or an intercostal perforator. One, two or three appropriately placed signals are selected and the flap is designed around them. The anterior apex of the flap is adjacent to the lateral breast or breast flap. The flap is oriented horizontally within the bra line as an ellipse with a width of 7–10 cm and length of 15–20 cm ( Fig. 7.1 ).
Relevant anatomy and operative approach
The thoracodorsal artery flap (TDAP)
The TDAP flap represents an adipocutaneous flap supplied by perforating vessels of the thoracodorsal artery. Originally described in 1994, the TDAP was initially applied to forearm and chest reconstruction. The TDAP accomplishes the same reconstructive goals as the LD flap, with the notable advantage that the important LD muscle is completely preserved.
An algorithm for the selection of a pedicle for the transfer of lateral thoracic tissue has previously been detailed by Levine. The thoracodorsal artery takes origin from the subscapular artery, descending along the lateral and deep surface of the LD muscle. Along its course the thoracodorsal artery gives off the serratus branch before terminating in anterior and posterior branches. The anterior and posterior branches yield equal numbers of perforators to supply the overlying skin of the lateral chest wall. A large skin paddle may be designed over any one of the perforators of adequate caliber.
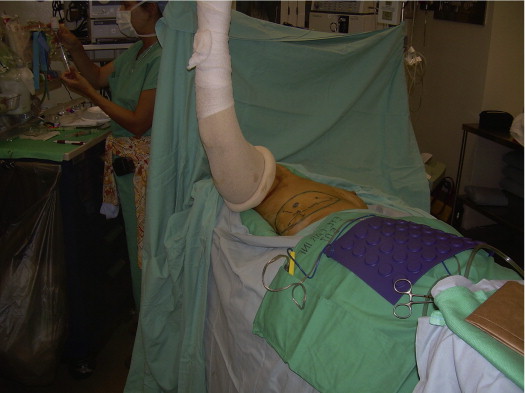
The patient is placed on the operating room table in the supine position, with a bolster under the ipsilateral chest. The ipsilateral arm is prepped into the field with the use of a stockinet cover ( Fig. 7.2 ). Beveling is used to maximize the amount of subcutaneous fat harvested. The flap is elevated from posterior to anterior at the level of the latissimus fascia. When a vessel is encountered it is evaluated for suitability in terms of its size. When an appropriate vessel is selected, the dissection is continued down through the LD. The muscle fibers are gently separated, while preserving the anterior edge of the muscle. When the dissection reaches the thoracodorsal artery and vein, great care must be taken to preserve the thoracodorsal nerve. The dissection continues superiorly toward the axillary vessels; the longer the pedicle dissection, the greater the arc of rotation. The anterior edge of LD must be separated for a sufficient length to enable the flap to be passed through ( Figs 7.3 and 7.4 ). The flap is then placed either behind the breast or reconstructed breast, or used to fill in a contour defect.