Abstract
Eosinophil-associated diseases are a spectrum of disorders in which eosinophils play a central role in the pathophysiology. Clinical manifestations result from activated eosinophils in tissues and/or from eosinophil-derived factors in blood. Cutaneous involvement is common and may be a presenting sign with pruritus, erythema, edema/angioedema, blistering, ulceration, and/or eczema. Fibrosis involving heart, lung, digestive tract, skin, genitourinary tract, other tissues, and organs may develop. Thrombosis with or without thromboembolism and peripheral and/or central neuropathy with chronic or recurrent neurological deficits also may develop. Liver, pancreas, and kidneys are among less common organs showing manifestations. Eosinophils lose their morphological identity in tissue and deposit toxic, proinflammatory products damaging cells, tissues, and organs; their involvement in pathophysiology may not be recognized. Targeted therapies are emerging that will offer significant control of conditions in which eosinophils play a role.
Keywords
Eosinophil, Eosinophil-associated dermatoses, Eosinophil-associated diseases, Eosinophilia, Hypereosinophilia, Hypereosinophilic syndromes (HES), Interleukin (IL)-5 and anti-IL-5
- •
Peripheral blood eosinophilia provides clues to diagnosis, but it is not a diagnostic marker except when levels of peripheral blood eosinophils are in the “hypereosinophilic” range as found in the hypereosinophilic syndromes.
- •
Many diseases with increased peripheral blood eosinophils have accompanying tissue eosinophil infiltration, including skin, often with degranulation and loss of morphological identity of infiltrating eosinophils.
- •
Eosinophils are observed in biopsy specimens of various skin lesions, with and without accompanying peripheral blood eosinophilia, and clinicopathological correlation is needed to arrive at the correct diagnosis; eosinophil-associated dermatoses include drug eruptions, arthropod bite reactions, parasite infestations (“drugs and bugs”), certain autoimmune blistering diseases, Wells syndrome, eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome), and IgG4-related diseases.
- •
Eosinophils commonly disrupt and lose their morphological integrity as they deposit toxic granule proteins and other inflammatory mediators in tissues, prominently in urticarial, eczematous, and pruritic skin lesions; therefore, the presence or absence of intact eosinophils in tissue specimens may not accurately reflect their pathogenic role in disease.
- •
In patients with persistent peripheral blood eosinophilia from any cause, including the hypereosinophilic syndromes, tissue infiltration, and eosinophil-derived effector molecules may cause clinically relevant pathology, including irreversible organ damage.
Eosinophils are leukocytes with cytoplasmic granules that are named for their characteristic staining with the acidic dye, eosin. They circulate in blood as mature cells. Normally, they are not found in human tissues other than blood except in the bone marrow where they develop, in the gastrointestinal tract distal to the esophagus where they likely are eliminated, and in lymphoid tissues where they function in both innate and acquired immunity. Peripheral blood eosinophils are increased in various inflammatory diseases, classically in parasitic infections, allergic diseases and drug reactions, in hematological malignancies, and in some solid tumors. Eosinophils infiltrate tissues in response to certain inflammatory signals, which may or may not be accompanied by peripheral blood eosinophilia. Eosinophils commonly disrupt and lose their morphological identity as they deposit toxic granule proteins, and the presence or absence of intact eosinophils may not accurately reflect a pathogenic role for eosinophils in affected tissues. New therapies that target eosinophils show promise in treating eosinophil-associated diseases.
Classification
Peripheral blood eosinophilia can be transient, episodic, or persistent and can fluctuate between eosinophilia (0.5-1.5 × 10 9 /L) and hypereosinophilia (greater than 1.5 × 10 9 /L). Tissue hypereosinophilia can occur in the absence of increased blood eosinophils, although, often, at least episodic eosinophilia is present. Blood hypereosinophilia can be classified as follows:
- •
Primary hypereosinophilia (clonal/neoplastic) in which eosinophils are neoplastic cells with underlying stem cell, myeloid, or eosinophil neoplasm, as classified by World Health Organization criteria
- •
Secondary hypereosinophilia (reactive) in which eosinophilia is cytokine-driven in most cases by an underlying condition/disease and eosinophils are nonclonal
- •
Hereditary hypereosinophilia (familial) in which a familial clustering of individuals with hypereosinophilia is found without hereditary immunodeficiency and no evidence of neoplastic or reactive conditions underlying the eosinophilia
- •
Hypereosinophilia of undetermined significance in which hypereosinophilia develops with no evidence of underlying cause including no neoplastic or reactive conditions and no family history and with no organ damage; this category may be prodromal to primary or secondary forms.
Various patterns of tissue eosinophil involvement are recognized:
- •
Eosinophil infiltration of few to many intact cells
- •
Intact eosinophils with extracellular eosinophil granule protein deposition proportionate to the numbers of infiltrating eosinophils
- •
Intact eosinophils with extensive extracellular eosinophil granule protein deposition disproportionately greater than the numbers of infiltrating eosinophils
- •
Extensive extracellular eosinophil granule protein deposition with few or no infiltrating eosinophils (in this pattern, eosinophil involvement may not be recognized on histopathology examination).
Eosinophils likely are activated as they infiltrate tissues resulting in the deposition of their toxic granule proteins in many reaction patterns. Virtually every organ, including skin, is affected by inflammatory conditions accompanied by tissue hypereosinophilia.
Pathogenesis
Eosinophils circulate transiently in blood (8 to 18 hours) and are constantly replenished from bone marrow progenitor cells. Major growth factors for eosinophils are interleukin (IL)-5, granulocyte–macrophage colony-stimulating factor (GM-CSF), and IL-3, which are produced and secreted by activated T cells, mast cells, stromal cells, and eosinophils themselves. Eosinophils express cell surface receptors for these cytokines throughout development. These cytokines induce not only proliferation of eosinophil progenitor cells, but migration, adherence, cytokine production, activation, and survival of mature eosinophils. The mobilization of eosinophils from bone marrow into blood is regulated predominantly by IL-5 and eotaxin. Eosinophils derived from inflamed tissues express higher levels of certain cell surface adhesion receptors.
Several lines of investigation indicate that eosinophils are recruited to and activated in tissues by cytokine activity from the Th2 subset of T cells, which produces IL-4, IL-5, IL-10, and IL-13, in addition to cytokines common to Th1 cells, such as GM-CSF and IL-3. Eosinophils themselves elaborate important inflammatory and regulatory cytokines. As a result, eosinophil activation occurs in an autocrine manner. In cytotoxicity assays, eosinophils are maximally activated by GM-CSF, followed by IL-3, IL-5, tumor necrosis factor (TNF)-α, and IL-4, in order of potency.
Upon activation, eosinophils release granule contents into their extracellular spaces via three mechanisms: cytolytic degranulation, piecemeal degranulation, and regulated secretion. Cytolytic degranulation is characterized by cytoplasmic membrane rupture, chromatolysis of nuclei with loss of morphological integrity and identity of eosinophils, and extensive deposition of eosinophil granules and granule products within tissue; this process occurs in many inflammatory disorders, including skin diseases as well as in affected organs in other diseases. The distinctive eosinophil granule proteins, including eosinophil major basic protein (eMBP)1, eMBP2, eosinophil-derived neurotoxin, eosinophil cationic protein, and eosinophil peroxidase, have many biological activities (reviewed in detail in other publications) implicating their roles in pathophysiology with effects on tissues and cells, including basophils, neutrophils, and platelets, as well as infectious organisms including helminths, RNA viruses, and bacteria. Eosinophil granule proteins persist in tissues for long periods of time; eMBP1 up to 6 weeks.
Eosinophils possess receptors for glucocorticoids, which inhibit eosinophil growth and function, and receptor numbers correlate with responses of eosinophils to glucocorticoids. Additionally, glucocorticoids and other anti-inflammatory agents inhibit cytokine-induced expression of adhesion molecules on eosinophils and endothelial cells and, therefore, eosinophil adhesion and transendothelial migration.
Certain specific diseases and syndromes are strongly associated with and/or are defined by peripheral blood and tissue eosinophilia in which cutaneous manifestations are common ( Tables 9-1 and 9-2 ).
| Common/Strong Etiologies | Less Common/Rare Etiologies |
|---|---|
| Adrenal insufficiency (Addison’s disease) | B- and T-cell lymphomas/leukemias |
| Allergic reactions | Chronic graft-versus-host disease |
| Atopic diseases | Chronic inflammatory disorders, including inflammatory bowel disease |
| Drug reactions | Fibrotic reactions |
| Hypereosinophilic syndromes | Fungal infections, allergic bronchopulmonary aspergillosis, and others |
| Immunobullous diseases, particularly pemphigoid | Hodgkin’s disease |
| Parasitosis including ectoparasitic infestations and helminthic infections | Human immunodeficiency virus (HIV) and human T-cell lymphotropic virus (HTLV) I and II |
| IgG4-related diseases | |
| Indolent systemic mastocytosis | |
| Langerhans cell histiocytosis | |
| Sarcoidosis | |
| Solid tumors/malignancy |
| Eosinophilia myalgia syndrome (EMS) and toxic oil syndrome (TOS) | Severe myalgia with hypereosinophilia, often accompanied by neurological symptoms and skin changes; epidemic cases of EMS have been attributed to contaminated l -tryptophan exposure and of TOS to rapeseed oil denatured with aniline |
| Eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) | Necrotizing vasculitis with hypereosinophilia (ANCA1 and ANCA2 subvariants) |
| Gleich’s syndrome | Cyclic recurrent angioedema, hypereosinophilia, and increased IgM levels, often with clonal T cells, one of several possible clinical presentations of secondary/reactive HES |
| Hypereosinophilic syndromes (HES) | Peripheral blood hypereosinophilia, hypereosinophilia-related organ damage; variants classified |
| Hyper-IgE syndromes | Hereditary immunodeficiency syndrome with hypereosinophilia and increased IgE levels, often with eczema and facial anomalies; autosomal dominant variant has STAT3 mutations and autosomal recessive variant has DOCK8 mutations |
| IgG4-related diseases | Spectrum of disorders with fibrosis as a major finding, tissue eosinophilia and increased IgG4 |
| Omenn syndrome | Severe combined immunodeficiency with hypereosinophilia, often with erythroderma, hepatosplenomegaly, and lymphadenopathy and autosomal recessive genetic disease (mutations in RAG1 or RAG2 ) |
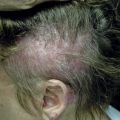
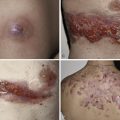
Peripheral blood hypereosinophilia and end-organ damage attributable to tissue hypereosinophilia are diagnostic criteria for the hypereosinophilic syndromes (HES). Dermatologic involvement is the most frequent presenting clinical manifestation in HES ( Table 9-3 ).
| Clinical Manifestations on Initial Presentation | Number Affected ∗ | Description |
|---|---|---|
| Cardiac | 9 | Congestive heart failure (4), valvular abnormality (1), cardiomyopathy (1), pericardial effusion (1), myocarditis (2) |
| Constitutional | 10 | Fever (3), weight loss (6), malaise (7), fatigue (4), night sweats (3), flu-like illness (2) |
| Dermatologic | 70 | Urticaria (6), angioedema (15), pruritus (26), dermatitis (26), erythroderma (1), bullous lesions (1), eosinophilic cellulitis (Wells syndrome) (3), unspecified edema (8), mucosal erosions (3) |
| Gastrointestinal | 26 | Abdominal pain (9), vomiting (6), diarrhea (5) |
| Hematologic | 6 | Deep venous thrombosis (4), anemia (1), superficial thrombophlebitis (1) |
| Neurologic | 9 | Vertigo (2), paresthesia (4), change in mentation (1), aphasia (1), visual disturbances (3) |
| Pulmonary | 47 | Asthma (21), sinusitis (9), rhinitis (2), cough (19), dyspnea (11), recurrent upper respiratory infection (2), pulmonary infiltrates (4), pleural effusion (1) |
| Rheumatologic | 14 | Arthralgia (3), myalgia (9), arthritis (1), myositis (1) |
| Routine laboratory test | 11 | Incidental abnormality found on routine laboratory testing (11) |
∗ Some patients had multiple manifestations, 188 total patients.
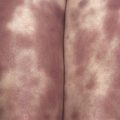
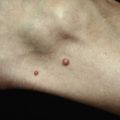
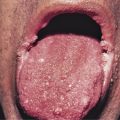
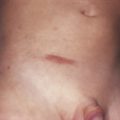
Patients with primary (neoplastic) HES present with signs and symptoms related to the organ systems affected by eosinophilic infiltrates with mucocutaneous lesions in almost 40% and eventually involving almost 70%. Along with skin lesions, the presenting complex may include fever, weight loss, fatigue, and malaise, in addition to increased serum vitamin B12 levels and increased serum tryptase levels. Skin lesions include pruritic erythematous macules, papules, plaques, or nodules on the trunk and extremities or urticaria and angioedema. Mucosal ulcers of the oropharynx or anogenital region associated with primary HES previously had a grim prognosis. Most afflicted patients died within 2 years of presentation; however, these patients are very responsive to imatinib. Embolic events also occur, particularly during the thrombotic stage, and constitute a medical emergency because of their likely serious sequelae; cutaneous involvement with splinter hemorrhages and/or nail fold infarcts may be present and can provide the initial clues to thromboembolic disease. Other cutaneous manifestations of HES include erythema annulare centrifugum-like lesions, retiform purpura, livedo reticularis, and superficial thrombophlebitis ( Table 9-4 ).