Diseases of the Hair
Normal human hairs can be classified according to cyclic phases of growth. Anagen hairs are growing hairs; catagen hairs are those undergoing transition from the growing to the resting stage; and telogen hairs are resting hairs, which remain in the follicles for variable periods before they fall out (teloptosis). The lag period between loss of the telogen hair and growth of a new anagen hair has been called kenogen.
Anagen hairs grow for about 3 years (1000 days), with a range between 2 and 6 years. The follicular matrix cells grow, divide, and become keratinized to form growing hairs. Catagen hairs are in a transitional phase, lasting 1 or 2 weeks, in which all growth activity ceases, with the eventual formation of the telogen “club” hair. Many apoptotic cells are present in the outer root sheath of the catagen hair as it involutes. Telogen club hairs are resting hairs, which continue in this state for 3–5 months (≈100 days) before they are released.
Of human hairs plucked from a normal scalp, 85%–90% are anagen hairs, and 10%–15% are telogen hairs. Catagen hairs normally constitute less than 1% of scalp hairs. The scalp normally contains an estimated 100,000 hairs, and the average number of hairs shed daily is 100–150. The hair growth rate of terminal hairs is about 0.37 mm/day. Contrary to popular belief, neither shaving nor menstruation has any effect on hair growth rate. The average uncut scalp hair length is estimated to be 25–100 cm, although exceptional hairs may be as long as 170 cm (70 inches).
Lanugo hair is the fine hair present on the body of the fetus. This is replaced by the vellus and terminal hairs. Vellus hairs are fine and usually light colored and have a narrow hair shaft thinner than the width of the inner root sheath. Terminal hairs are coarse, thick, and dark, except in blond-haired persons. Hair occurs on all skin surfaces except the palms, soles, labia minora, lips, nails, glans, and prepuce. Terminal hairs are typically present on a man’s face, chest, and abdomen, but vellus hairs usually predominate on these sites in women.
Causes of alopecia are generally divided into the broad categories of cicatricial and noncicatricial alopecia. The evaluation should take into account the patient’s age and ethnicity. Examination of hair shafts can establish a diagnosis of trichodystrophy. Hair counts, hair pull, and hair pluck (trichogram) can establish the degree of hair shedding, the type of hair that is shed, and the anagen/telogen ratio. Biopsies can also determine the anagen/telogen ratio and provide information regarding the potential for regrowth, as well as providing a diagnosis. Biopsies are particularly valuable in the evaluation of cicatricial alopecia. Often, a correct diagnosis hinges on a synthesis of clinical, histologic, serologic, and immunofluorescent data.
Noncicatricial Alopecia
Alopecia Areata
Clinical Features.
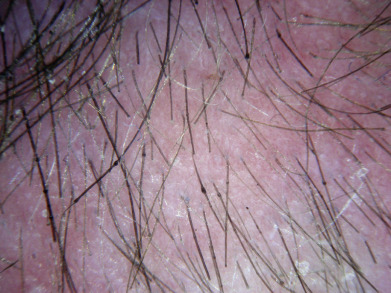
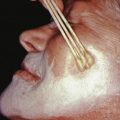
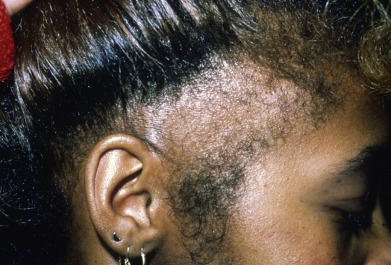
Alopecia areata (in French, pelade) is characterized by rapid and complete loss of hair in one or more round or oval patches, typically 1–5 cm in diameter, usually on the scalp ( Fig. 33.1 ), bearded area, eyebrows, eyelashes, and, less frequently, other hairy areas of the body. A few resting hairs may be found within the patches. Early in the course, there may be sparing of gray hair, and white hairs are rarely affected. Sudden whitening of hair may represent widespread alopecia areata in a patient with salt-and-pepper hair. In about 10% of alopecia areata patients, especially in long-standing cases with extensive involvement, the nails develop uniform pits that may form transverse or longitudinal lines. Trachyonychia, onychomadesis, and red or spotted lunulae occur, but less often. Dermoscopic examination typically demonstrates diffuse, round, or polycyclic perifollicular yellow dots.
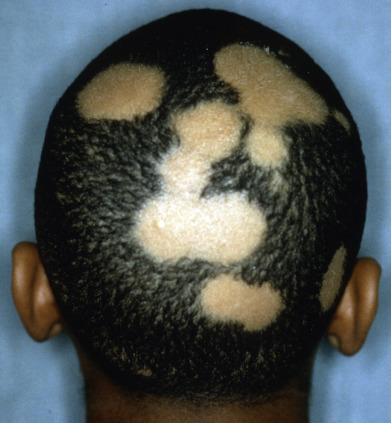
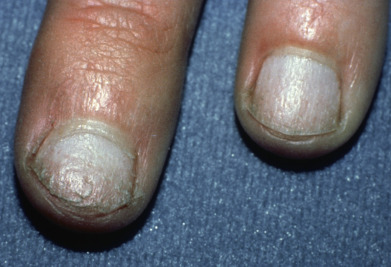
Complete loss of scalp hair is referred to as alopecia totalis, and complete loss of all hair as alopecia universalis. Loss may occur confluently along the temporal and occipital scalp (ophiasis) or on the entire scalp except for this area (sisaipho). Rarely, alopecia areata may present in a diffuse pattern that may mimic pattern alopecia. Clues to the correct diagnosis include a history of periodic regrowth, nail pitting ( Fig. 33.2 ), and the presence of tapered fractures or “exclamation point” hairs. Alopecia areata generally presents as an anagen effluvium, with an inflammatory insult to the hair matrix resulting in tapering of the hair shaft and fracture of anagen hairs. As the hair miniaturizes or converts from anagen to telogen, the remaining lower portion of the hair rises above the level of the scalp, producing the exclamation point hair.
Alopecia areata is associated with a higher incidence than usual of atopic dermatitis, Down syndrome, lichen planus, and autoimmune diseases, such as systemic lupus erythematosus (SLE), thyroiditis, diabetes mellitus, myasthenia gravis, and vitiligo. However, most cases of alopecia areata occur without associated disease, and routine screening for these disorders is of little value unless prompted by signs or symptoms.
Migratory poliosis of the scalp may represent a forme fruste of alopecia areata. Patients with this disorder present with migrating circular patches of white hair, but never lose hair. The histology resembles alopecia areata.
Etiologic Factors.
Oligoclonal and autoreactive T lymphocytes are present in the peribulbar inflammatory infiltrate, and many patients respond to immunomodulating drugs. Affected alopecia areata scalp skin grafted on to nude mice with severe combined immunodeficiency demonstrates loss of infiltrating lymphocytes and hair growth. In this model, injecting T lymphocytes with scalp homogenate can reproduce the alopecia. Follicular melanocytes substitute for scalp homogenates to produce alopecia areata in this model, providing evidence that follicular melanocytes are the targets for activated T cells in this disease. This hypothesis is also supported by the observations that white hair is rarely affected and regrowing hair is often depigmented.
The early phase of hair loss appears to be mediated by type 1 cytokines, including interleukin (IL)–2, interferon (IFN)–γ, and tumor necrosis factor (TNF)–α. The hair bulb normally represents an area of relative immune privilege during anagen, as evidenced by a very low level of expression of major histocompatibility complex (MHC) class Ia antigens. This immune privilege may prevent antigen recognition by autoreactive CD8+ T cells. Alopecia areata may be related to collapse of this immune privilege.
Overall, almost 25% of patients have a positive family history; there are reports of twins with alopecia areata. Patients with “early onset, severe, familial clustering alopecia areata” have a unique and highly significant association with the human leukocyte antigens (HLAs) DR4, DR11, and DQ7. The “later onset, milder severity, better prognostic” subsets of patients have a lower frequency of familial disease and do not share these HLAs. Familial alopecia areata associated with hereditary thrombocytopenia related to mutations in genes on chromosome 17 has been described. R620W (c.1858C>T, a variant of the protein tyrosine phosphatase nonreceptor 22 gene, PTPN22 ) is associated with a variety of autoimmune disorders, including alopecia areata. It is associated with early onset of disease, widespread hair loss, and a positive family history.
Histology.
In early alopecia areata, there is a lymphoid infiltrate in the peribulbar area of anagen or early catagen follicles. Eosinophils may be present in the infiltrate, and lymphocyte-mediated damage to the bulb produces melanin pigment incontinence in the surrounding stroma. The presence of many catagen hairs and pigment casts within the follicular canal can cause histologic confusion with trichotillomania. The follicles eventually miniaturize, appearing as small, dystrophic anagen hairs high in the dermis, often with a persistent lymphocytic peribulbar infiltrate. Fibrous tract remnants beneath the miniaturized bulbs of alopecia areata may contain lymphoid cells, eosinophils, and melanin pigment. With time, the lymphocytes disappear, but focal eosinophils and pigment remain. Finally, only focal melanin pigment remains in the fibrous tract remnants. Every histologic feature of alopecia areata may be seen in syphilis. The presence of plasma cells is suggestive of syphilis, but plasma cells are also lacking in about one third of syphilis biopsies. Plasma cells may be present in biopsies from any form of inflammatory alopecia if the biopsy is taken from the occipital scalp, because this site readily recruits plasma cells.
Differential Diagnosis.
The sharply circumscribed patch of alopecia with exclamation point hairs at the periphery and the absence of scarring are indicative of alopecia areata. Tinea capitis, androgenetic alopecia, early lupus erythematosus (LE), syphilis, congenital triangular alopecia, alopecia neoplastica, and trichotillomania should be kept in mind when alopecia areata is considered. In endemic areas of Southwest Asia, Pheidole ants shear hair shafts during the night, resulting in overnight loss of clumps of hair. The resulting round patches of hair loss closely mimic alopecia areata.
Treatment.
The natural course of the hair loss is highly variable. Some patches will regrow in a few weeks without any treatment. In a series of 63 consecutive responders to a follow-up questionnaire, hair had spontaneously regrown in all but four after 1 year and in all but one after 2 years. The great majority had recovered in 3 months after their only office visit. Therefore anecdotal reports of success must be interpreted carefully in the light of the high rate of spontaneous recovery.
Intralesional injections of corticosteroid suspensions are the treatment of choice for localized, cosmetically conspicuous patches, such as those occurring in the frontal hairline or involving an eyebrow. Injections of triamcinolone, 2–10 mg/mL, are typically given intradermally or in the superficial subcutaneous tissue. Large volumes and higher concentrations of triamcinolone present a greater risk of atrophy. Injection under significant pressure or with a small-bore syringe increases the likelihood of retinal artery embolization. High-strength topical corticosteroids may be used as a safer first-line therapy but are less reliable than injections. Several investigators have reported the use of pulsed oral corticosteroids in rapidly progressing or widespread disease. However, long-term treatment is frequently needed to maintain growth, and the attendant risks should be carefully weighed against the benefits. In a study of 66 patients age 9–60 years, monthly methylprednisolone was administered at a dose of 500 mg/day for 3 days, or 5 mg/kg twice daily over 3 days in children. More than 60% of patients with widespread patchy alopecia responded. Half the patients with alopecia totalis had a good response, whereas a quarter of those with universal alopecia responded. Patients with ophiasic alopecia areata did not respond. Predictors of response include disease duration of 6 months or less, younger than 10 years at disease onset, and multifocal disease.
Oral and topical Janus kinase (JAK) inhibitors, including tofacitanib and ruxolitinib, have demonstrated excellent responses in many patients. The roles of phosphodiesterase 4 inhibitors and platelet-rich plasma are unclear. Induction of contact sensitivity to squaric acid dibutyl ester, dinitrochlorobenzene (DNCB), and diphencyprone can be useful in refractory cases. Topical or oral methoxsalen (psoralen) and ultraviolet A (PUVA) therapy is an option for refractory or widespread lesions. Short-contact topical anthralin 1% cream (applied for 15–20 minutes and then shampooed off) can be of benefit. Topical minoxidil may be combined with other treatments or used as a single agent. Psoriatic doses of methotrexate and sulfasalazine in doses up to 1.5 g three times daily may be beneficial. Cyclosporine has been used alone or combined with other modalities, including PUVA. Biologics have produced mixed, and largely disappointing, results, and alopecia areata has developed during biologic therapy for other conditions. The 308-nm xenon chloride excimer laser (300–2300 mJ/cm 2 /session) has been reported to produce regrowth after 11 and 12 sessions over 9–11 weeks. Periocular pigmentation and iris darkening are associated with use of travoprost, bimatoprost, and latanoprost for eyelash disease. Therapeutic results are mixed. Botanicals, including peony glucosides and glycyrrhizin, demonstrate some promise. In a mouse model, a fusion protein of parathyroid hormone and a bacterial collagen-binding domain produced hair regrowth.
Alopecia areata can cause tremendous psychological stress. Education about the disease process, cosmetically acceptable alternatives (especially information about wigs), and research into innovative therapies should all be made available to the patient. In addition to the information conveyed by the dermatologist, an excellent resource is the National Alopecia Areata Foundation ( www.naaf.org , info@naaf.org ).
Prognosis.
The tendency is for spontaneous recovery in alopecia areata patients who are postpubertal at onset. At first the regrowing hairs are downy and light in color; later they are replaced by stronger and darker hair with full growth. Predictors of a poor prognosis are the presence of atopic dermatitis, childhood onset, widespread involvement, ophiasis, duration of longer than 5 years, and onychodystrophy. Acute diffuse and total alopecia is a newly defined subtype of alopecia areata that occurs in young adults and has a good prognosis.
Açıkgöz G, et al: Pulse methylprednisolone therapy for the treatment of extensive alopecia areata. J Dermatolog Treat 2014; 25: 164.
Apfelbacher CJ: Research questions for the treatment of alopecia areata have been prioritized. Br J Dermatol 2017; 176: 1128.
Ayatollahi A, et al: Platelet rich plasma for treatment of non-scarring hair loss. J Dermatolog Treat 2017; 28: 574.
Barrón-Hernández YL, et al: Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs 2017; 26: 515.
Bayart CB, et al: Topical Janus kinase inhibitors for the treatment of pediatric alopecia areata. J Am Acad Dermatol 2017; 77: 167.
Craiglow BG, et al: Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol 2017; 76: 29.
Gupta AK, et al: What is new in the management of alopecia areata. Skinmed 2016; 14: 375.
Ibrahim O, et al: Treatment of alopecia areata with tofacitinib. JAMA Dermatol 2017; 153: 600.
Jang YH, et al: Systematic review and quality analysis of studies on the efficacy of topical diphenylcyclopropenone treatment for alopecia areata. J Am Acad Dermatol 2017; 77: 170.
Lai VWY, et al: Systemic treatments for alopecia areata: A systematic review. Australas J Dermatol 2018. doi: 10.1111/ajd.12913. [Epub ahead of print] Review. PubMed PMID: 30191561.
Landis ET, Pichardo-Geisinger RO: Methotrexate for the treatment of pediatric alopecia areata. J Dermatolog Treat 2017 Jun 30; ePub ahead of print.
Lim SK, et al: Low-dose systemic methotrexate therapy for recalcitrant alopecia areata. Ann Dermatol 2017; 29: 263.
Liu LY, et al: Tofacitinib for the treatment of severe alopecia areata and variants. J Am Acad Dermatol 2017; 76: 22.
Ngwanya MR, et al: Higher concentrations of dithranol appear to induce hair growth even in severe alopecia areata. Dermatol Ther 2017; 30. E12500.
Triyangkulsri K, et al: Role of janus kinase inhibitors in the treatment of alopecia areata. Drug Des Devel Ther 2018; 12: 2323-2335. doi: 10.2147/DDDT.S172638. eCollection 2018. Review. PubMed PMID: 30100707; PubMed Central PMCID: PMC6067625.
Telogen Effluvium
Telogen effluvium presents with excessive shedding of normal telogen club hairs. This excessive shedding of telogen hairs most often occurs 3–5 months after the premature conversion of many anagen hairs to telogen hairs induced by surgery, parturition, fever, drugs, dieting, or traction. Local patches of early telogen conversion may be induced by papulosquamous diseases affecting the scalp. Alternatively, follicles may remain in prolonged anagen rather than normally cycling into telogen. This occurs during pregnancy. On delivery, many follicles are then released simultaneously into telogen, and shedding occurs 3–5 months later. Prolongation of telogen also occurs during pregnancy and results in an initial wave of hair loss soon after delivery or heralding early termination of a pregnancy. Shortening of the anagen phase occurs in pattern (androgenetic) alopecia and in chronic telogen effluvium. A greater proportion of hairs in telogen at any one time results in a chronic increase in telogen shed. Administration of topical minoxidil may produce a telogen effluvium by premature termination of telogen necessary to initiate anagen in responding follicles. This causes early telogen release and a brief telogen effluvium.
Whatever the cause of the telogen loss, the hair is lost “at the root.” Each hair will have a visible depigmented club-shaped bulb and will lack a sheath ( Fig. 33.3 ).
Telogen shed may be estimated by the pull test: grasping 40 hairs firmly between thumb and forefinger, followed by a slow pull that causes minimal discomfort to the patient. A count of more than 4–6 club hairs is abnormal, but the result is influenced by recent shampooing (2–3 hairs being abnormal in a freshly shampooed scalp), combing, and the phase of telogen effluvium (whether resolving or entering a chronic phase). The clip test may also be useful; 25–30 hairs are cut just above the scalp surface and mounted. Indeterminate and telogen hairs are short and of small diameter. Many hairs of this type may be present in telogen effluvium or pattern alopecia. Trichogram evaluation (50 hairs plucked with Kelly clamp with rubber drains over teeth) can also provide information on the anagen/telogen ratio.
Age, gender, race, and genetic factors influence the normal average daily hair loss in an individual. Again, a full head of hair numbers about 100,000; of these, approximately 100–150 are lost daily. In telogen effluvium, estimates of loss vary from 150 to more than 400. Patients may be instructed to collect and count the hair daily; however, they should make sure they collect all small hairs and those that come out in washing and in the bed, as well as those present on the comb or brush. When the pull test is positive, hair shed counts are not needed. An alternative is to collect all hairs lost during a 1-minute combing session. For this technique, developed by Dr. Jeffrey Miller, the patient combs for 1 minute before shampooing on 3 consecutive days. The patient is instructed to comb from the vertex to the anterior hairline. The normal range of lost hairs with this technique is 10–15. Loss of more than 50 is common in telogen effluvium. Serial 1-minute hair counts can be performed to monitor progress.
Telogen effluvium may be related to protein or other nutrient deprivation ( Fig. 33.4 ). Assessment of dietary habits and determination of iron saturation and ferritin are the simplest ways to determine nutritional status. Iron replacement is advisable if saturation or ferritin is low, but in one study, iron replacement alone did not result in resolution of telogen effluvium. Iron may merely serve as a marker for overall nutritional status. Patients with evidence of deficiency should be given supplements to correct the identified deficiency and encouraged to eat a varied diet. Sources of blood loss, such as menstrual bleeding and gastrointestinal (GI) blood loss, should be investigated. Hypothyroidism, allergic contact dermatitis to hair dyes, and renal dialysis with secondary hypervitaminosis A may also be associated with telogen effluvium. Drug-induced telogen effluvium has been noted with the use of aminosalicylic acid, amphetamines, bromocriptine, captopril, carbamazepine, cimetidine, coumarin, danazol, enalapril, etretinate, levodopa, lithium carbonate, metoprolol, metyrapone, pramipexole, propranolol, pyridostigmine, and trimethadione. Postnatal telogen effluvium of infants may occur between birth and the first 4 months of age. Usually, regrowth occurs by 6 months of age. Telogen counts by Kligman in six infants varied from 64%–87%. He also found a tendency for the alopecia to occur in the male-pattern distribution. Idiopathic chronic telogen effluvium has been described by Whiting in a group of 355 patients (346 women and 9 men) with diffuse generalized thinning of scalp hair. Most were 30–60 years old, and their hair loss started abruptly, with increased shedding and thinning. There was a fluctuating course and diffuse thinning of the hair all over the scalp, accompanied by bitemporal recession. This chronic form is related to shortening of the anagen phase and may respond to 5% minoxidil solution.
Trichodynia is a common symptom in patients with telogen effluvium, as it is in pattern hair loss. Trichodynia may also coexist with signs of depression, obsessive personality disorder, or anxiety.
If a 4-mm punch biopsy is performed, 25–50 hairs are normally present for inspection in transverse (horizontal) sections. If more than 12%–15% of terminal follicles are in telogen, this indicates a significant shift from anagen to telogen. Pattern (androgenetic) alopecia demonstrates miniaturization, variable hair shaft diameter, and an increased proportion of telogen hairs. Traction alopecia and trichotillosis (trichotillomania) result in an increased number of catagen and telogen hairs. Pigment casts, empty anagen follicles, trichomalacia, and catagen hairs help distinguish these entities from simple telogen effluvium.
No specific therapy is required for most patients with telogen effluvium. In the majority of cases, the hair loss will stop spontaneously within a few months, and the hair will regrow. Drug-induced telogen effluvium responds to discontinuation of the offending agent. The prognosis is good if a specific event can be pinpointed as a probable cause. Papulosquamous scalp disorders may precipitate telogen hair loss and should be addressed. Iron and thyroid status should be determined if the course is prolonged or if history or physical examination suggests an abnormality. Patients should be encouraged to eat a balanced diet. In a mouse model, sonic stress can produce catagen. This model may be useful in the study of agents for the treatment of telogen effluvium.
Martínez-Velasco MA, et al: The hair shedding visual scale. Dermatol Ther (Heidelb) 2017; 7: 155.
Mubki T, et al: Evaluation and diagnosis of the hair loss patient. Part I. J Am Acad Dermatol 2014; 71: 415.e1.
Rebora A: Intermittent chronic telogen effluvium. Skin Appendage Disord 2017; 3: 36.
Shin S, et al: Suprabulbar thinning of hair in telogen effluvium. Yonsei Med J 2017; 58: 682.
Anagen Effluvium
Anagen effluvium usually results from hair shaft fracture. It is frequently seen following the administration of cancer chemotherapeutic agents, such as the antimetabolites, alkylating agents, and mitotic inhibitors. These agents result in temporary shutdown of the hair matrix with resultant tapering of the shaft (Pohl-Pinkus constrictions). Trichograms reveal tapered fractures. Only anagen hairs are affected. The 10% of scalp hairs in telogen have no matrix and are unaffected. The loss tends to be diffuse but not complete. Severe loss is frequently seen with doxorubicin, the nitrosureas, and cyclophosphamide. When high doses are given, loss of anagen hairs becomes most apparent clinically in 1–2 months. Hair loss after chemotherapy is usually, but not always, reversible. Permanent alopecia after chemotherapy resembles pattern alopecia histologically. A pressure cuff applied around the scalp during chemotherapy and scalp hypothermia have been reported to prevent such anagen arrest; because the scalp may be a site of metastasis, however, it may be better not to spare the scalp from the effects of chemotherapy. Topical minoxidil has been shown to shorten the period of baldness by an average of 50 days.
In addition to the cytotoxic chemotherapeutic agents, various agents, such as isoniazid (INH), thallium, and boron, may induce anagen effluvium. Anagen effluvium with tapered fractures also occurs in alopecia areata and syphilis. In these diseases, an inflammatory insult to the hair bulb results in tapered fractures.
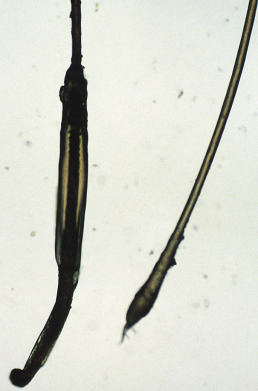
Anagen loss may also occur at the root. Loose anagen syndrome, described by Price in 1989, is a disorder in which anagen hairs may be pulled from the scalp with little effort. It occurs mostly in blond girls and usually improves with age. The syndrome appears to be related to a defect in the hair cuticle. Instead of anchoring the hair firmly, the cuticle simply folds back like a rumpled sock ( Fig. 33.5 ), allowing the hair shaft to be extracted. Woolly hair can be associated with loose anagen hair syndrome. A keratin mutation, E337K in K6HF, was identified in three of nine families studied. Colobomas have also been associated with loose anagen hair.
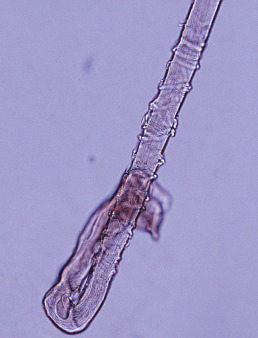
Anagen hairs may be easily extracted from active areas of LE and lichen planopilaris. They usually lack the root sheath that normally surrounds a plucked anagen hair. Anagen effluvium has also been described in lesions of pemphigus.
Pattern Alopecia (Androgenetic Alopecia)
Male-Pattern Baldness.
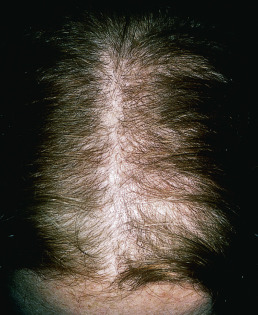
Male-pattern alopecia (common baldness) shows itself during the teens, twenties, or early thirties with gradual loss of hair, chiefly from the vertex and frontotemporal regions. The process may begin at any time after puberty, and the presence of “whisker” or kinky hair may be the first sign of impending male-pattern alopecia. The anterior hairline recedes on each side, in the Geheimratswinkeln (“professor angles”), so that the forehead becomes high. Eventually, the entire top of the scalp may become devoid of hair. Several patterns of this type of hair loss occur, but most common is the biparietal recession with loss of hair on the vertex. The rate of hair loss varies among individuals. Sudden hair loss may occur in the twenties and then proceed relentlessly, though very slowly, for a number of years. The follicles produce finer and lighter hairs with each hair cycle until terminal hairs are eventually replaced by vellus hairs. During evolution of the process, hair shafts vary significantly in diameter. The parietal and occipital areas are usually spared permanently from this process of progressive miniaturization.
Early-onset male-pattern alopecia is related to the androgen receptor gene. There is no doubt that inherited factors and the effect of androgens such as dihydrotestosterone on the hair follicle are important. Arguments for polygenic inheritance include the high prevalence, gaussian curve of distribution in the population, increased risk with number of affected relatives, increased risk in relatives of severely affected women compared with mildly affected women, and greater import of an affected mother than an affected father. The possibility that the early onset (before age 30) and later onset (after 50) forms may be inherited separately by single genes is also hypothesized.
Male-pattern alopecia is dependent on adequate androgen stimulation and appears to be related to the androgen receptor gene. Eunuchs do not develop baldness if they are castrated before or during adolescence. If they are given androgen therapy, baldness may develop. The 5α-reduction of testosterone is increased in the scalp of balding individuals, yielding increased dihydrotestosterone. Androgen-inducible transforming growth factor (TGF)–β1 derived from dermal papilla cells appears to mediate hair growth suppression. In congenital 5α-reductase deficiency, the type 2 isoenzyme is lacking, and baldness does not occur. Pattern alopecia does occur in males with X-linked ichthyosis, indicating that steroid sulfatase is not critical for the production of alopecia.
Progressive shortening of the anagen phase of hair growth is noted as the hair shaft diameter decreases, so hairs not only are narrowing, but also are becoming shorter. A higher proportion of telogen hairs in the affected area results in greater telogen shed. There may also be an increase in the duration of the lag phase between telogen and anagen (the kenogen lag phase).
Histologically, a decrease in anagen and increase in telogen follicles is present. Follicular miniaturization and variability in shaft diameter are noted. These features are particularly evident in transverse sections. Below the level of the miniaturized or telogen follicle, a vascular or fibromucinous fibrous tract remnant is present. These tracts appear numerous in cross section. Many mast cells may be noted in the fibrous tract remnant, but inflammatory cells are absent. Sebaceous glands may be enlarged, and hair thinning may be associated with solar elastosis. Sparse lymphoid inflammation with spongiosis may be noted at the level of the follicular infundibulum. This may represent associated seborrheic folliculitis. A sparse lymphoid infiltrate may also be noted at the level of the hair bulge.
Miniaturized human hair follicles grafted on to immunodeficient mice can quickly regenerate and grow as well as or better than terminal follicles from the same individual. This suggests that even advanced pattern alopecia may be reversible. Partial reversal of pattern alopecia has been noted after chemotherapy or treatment of psoriasis with methotrexate. Unfortunately, available pharmacologic interventions produce little effect in advanced pattern alopecia.
Men with spinal and bulbar muscular atrophy (Kennedy disease), an X-linked neurodegenerative disease caused by an expansion of a polymorphic tandem CAG repeat within the androgen receptor gene, have a decreased incidence of pattern alopecia.
Minoxidil, an oral hypotensive drug that causes hypertrichosis when given systemically, is available as topical solutions (Rogaine). Minoxidil promotes the survival of dermal papilla cells, prolongs anagen phase, and results in enlargement of shaft diameter. Clinically, apparent success is best in early cases (<10 years) of limited extent (bald area <10 cm in diameter on vertex) in whom pretreatment hair density is greater than 20 hairs/cm 2 . Minoxidil is available without a prescription as a solution or foam. Those who respond must continue to use minoxidil indefinitely to maintain a response.
Finasteride, a type 2 5α-reductase inhibitor, given as a 1-mg tablet daily, is effective in preventing further hair loss and in increasing the hair counts to the point of cosmetically appreciable results in men age 18–41 with mild to moderate hair loss at the vertex, in the anterior midscalp, and in the frontal region. Finasteride has been shown to stop hair loss in up to 90% of men for at least 5 years. Approximately 65% of men demonstrate hair regrowth. As with minoxidil, continued use of finasteride is required to sustain benefits. Hair patterning on the temples is not improved. Hair growth will be evident only after 6 months or more of therapy. If no effect is seen after 12 months, further treatment is unlikely to be of benefit. In one study, regimens that included finasteride were more effective than minoxidil alone, and therapeutic efficacy was enhanced by combining the two drugs. Short-term side effects related to finasteride are infrequent; however, the need to take this medication indefinitely suggests that study of long-term side-effect profiles is critical. A prostate cancer prevention trial with a different dosage form of the same drug showed a decrease in the incidence of cancer. However, those cancers that did occur in the treatment group had a higher average Gleason score, possibly because only lower-grade cancers were prevented.
Dutasteride blocks both type 1 and type 2 5α-reductase and is effective in the treatment of male-pattern hair loss. Other treatments that show some promise in preliminary studies include fluridil (topical antiandrogen that suppresses human androgen receptor), topical adenosine, and hormone-enriched topical cell culture medium. Hair transplantation using micrografts of hair follicles from the occipital area to the anterior scalp may satisfactorily recreate hairlines and give excellent cosmetic results. The role of platelet-rich plasma and microneedling is being defined.
Female-Pattern Alopecia (Androgenetic Alopecia in Women)
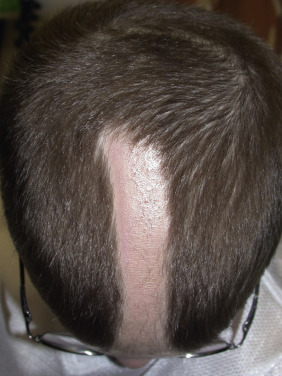
Women generally have diffuse hair loss throughout the apical scalp with the part wider anteriorly. There is typically sparing of the frontal hairline, although a subset of women exhibits a “male” pattern of temporal recession. Although maintenance of the frontal hairline is the rule in women, a progressive decrease in hair density from the vertex to the front of the scalp does occur. The same basic changes—reduced hair density and diameter and diminished anagen and increased telogen hair—occur in women as in men. Sebaceous gland hyperplasia may be present but is less common than in men. Transverse histologic sections demonstrate variability in the size of hair follicles (anisotrichosis).
The cause is now believed to be a genetic predisposition with an excessive response to androgens. Both women and men with pattern alopecia have higher levels of 5α-reductase and androgen receptor in frontal hair follicles than in occipital follicles. Evidence also suggests a hierarchy of androgen sensitivity within follicular units. Follicular miniaturization relates to unrepaired DNA damage and a reduced proliferation rate of matrix keratinocytes. Smoking may be an independent risk factor. Most women with pattern alopecia have normal menses and fertility. If other evidence of androgen excess is present, such as hirsutism, menstrual irregularities, or acne, or if the onset is sudden, evaluation as outlined for hirsutism (see later) should be performed. Topical minoxidil, and oral antiandrogens, such as spironolactone and cyproterone acetate, have been used to treat androgenetic alopecia in women. In one study, cyproterone acetate (CPA) was more effective than minoxidil when there were other signs of hyperandrogenism, hyperseborrhea, and menstrual abnormalities, and when the body mass index was high. When these other factors were absent, minoxidil was the more effective treatment.
Treatment with finasteride is of limited benefit for most women, although the subset with temporal recession may show some benefit. Finasteride treatment is contraindicated in women who may become pregnant. Hair transplantation, wigs, or interwoven hair may give satisfactory cosmetic results. In a pilot study, topical melatonin appeared to prolong anagen phase and may prove to be of some benefit. In some women, telogen effluvium may produce worsening of preexisting pattern alopecia. Reversible causes of telogen effluvium, such as seborrheic dermatitis, nutrient deficiency, and thyroid disease, should be addressed. As in men, the role of platelet-rich plasma and microneedling is being defined.
Adil A, et al: The effectiveness of treatments for androgenetic alopecia. J Am Acad Dermatol 2017; 77: 136.
Alves R, et al: Platelet-rich plasma in combination with 5% minoxidil topical solution and 1 mg oral finasteride for the treatment of androgenetic alopecia. Dermatol Surg 2018; 44: 126.
Czyzyk A, et al: Severe hyperandrogenemia in postmenopausal woman as a presentation of ovarian hyperthecosis. Gynecol Endocrinol 2017; 33: 836.
Dey-Rao R, et al: Genome-wide gene expression dataset used to identify potential therapeutic targets in androgenetic alopecia. Data Brief 2017; 13: 85.
Garg S, et al: Platelet-rich plasma—an “elixir” for treatment of alopecia. Stem Cell Investig 2017; 4: 64.
Girijala RL, et al: Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J 2018; 24(7). pii: 13030/qt8s43026c. PubMed PMID: 30261560.
Ho C, Hughes J: Alopecia, androgenetic. [Updated 2017 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Rodrigues BL, et al: “Treatment of male pattern alopecia with platelet-rich plasma: a double blind controlled study with analysis of platelet number and growth factor levels”. J Am Acad Dermatol 2018. pii: S0190-9622(18)32643-4. doi: 10.1016/j.jaad.2018.09.033. [Epub ahead of print] PubMed PMID: 30287324.
Rudnicka L, et al: Dyslipidemia in patients with androgenetic alopecia. J Eur Acad Dermatol Venereol 2017; 31: 921.
Shah KB, et al: A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology 2017; 9: 14.
Trichotillosis
Trichotillosis (trichotillomania) is the compulsive practice of plucking hair from the scalp, brows, or eyelashes. Typical areas are irregular patches of alopecia that contain hairs of varying length ( Fig. 33.6 ). The scalp has a rough texture, resulting from the short remnants of broken-off hairs. It is seen mostly in girls younger than 10, although boys and all adults may engage in the practice as well. Some patients relate exquisite pain localized to a follicle that can only be relieved by plucking the hair.
When speaking with a patient with characteristic areas of alopecia, rather than asking “if,” one should ask “how” removal of the hair is done. If this fails to uncover a history of hair pulling, shaving a 3-cm 2 area in the involved part of the scalp will result in hairs too short for plucking, and normal regrowth in the “skin window” within 3 weeks. Finally, a biopsy, especially if cut horizontally, may demonstrate empty anagen follicles, catagen hairs, pigment casts within the infundibulum, trichomalacia, and hemorrhage. Alopecia areata shares many of these histologic features, and care must be taken to search for the presence of peribulbar lymphocytes or inflammatory cells within the fibrous tract remnants.
Trichotillosis is usually a manifestation of an obsessive-compulsive disorder but may also be associated with depression or anxiety. It may be associated with compulsive swallowing of the plucked hairs (trichophagia) and may result in formation of a gastric bezoar (Rapunzel syndrome). Behavior modification, psychotherapy, and appropriate psychopharmacologic medication (e.g., clomipramine, olanzapine) may be helpful. N -acetylcysteine was effective in a study in adults, but a separate study showed disappointing results in children. Valproic acid, quetiapine, and naltrexone have been reported as effective in some patients. Bimatoprost has been used when eyelashes are involved.
Barroso LAL, et al: Trichotillomania. An Bras Dermatol 2017; 92: 537.
Bloch MH, et al: N -acetylcysteine in the treatment of pediatric trichotillomania. J Am Acad Child Adolesc Psychiatry 2013; 52: 231.
Falkenstein MJ, et al: Predictors of relapse following treatment of trichotillomania. J Obsessive Compuls Relat Disord 2014; 3: 345.
Grant JE, et al: Placebo response in trichotillomania. Int Clin Psychopharmacol 2017; 32: 350.
Grant JE, et al: The opiate antagonist, naltrexone, in the treatment of trichotillomania. J Clin Psychopharmacol 2014; 34: 134.
Harrison JP, et al: Pediatric trichotillomania. Curr Psychiatry Rep 2012; 14: 188.
Peabody T, et al: Clinical management of trichotillomania with bimatoprost. Optom Vis Sci 2013; 90: e167.
Rothbart R, et al: Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev 2013; 11: CD007662.
Rothbart R, Stein DJ: Pharmacotherapy of trichotillomania (hair pulling disorder). Expert Opin Pharmacother 2014; 15: 2709.
Weidt S, et al: Trichotillomania. Neuropsychiatr Dis Treat 2017; 13: 1153.
Other Forms of Noncicatricial Alopecia
Alopecia syphilitica may have a typical moth-eaten appearance on the occipital scalp ( Fig. 33.7 ), may show a generalized thinning of the hair, or may resemble alopecia areata. Other areas, such as the eyebrows, eyelashes, and body hair, may be involved. The alopecia may be the first sign of syphilis.
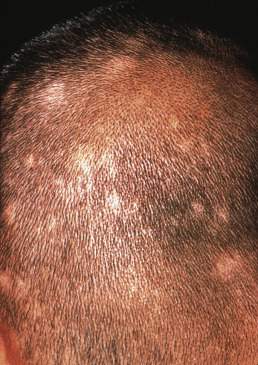
Follicular mucinosis (alopecia mucinosa) most often occurs on the scalp or beard area and manifests as a boggy, red plaque or hypopigmented patch with hair loss. Comedo-like lesions may exude mucin when expressed. Biopsy demonstrates deposition of mucin in the outer root sheath and sebaceous glands. The mucin stains as hyaluronic acid, rather than epithelial sialomucin. Primary cases (unassociated with underlying disease) usually occur as localized lesions of the head or neck. Young people are primarily affected and may demonstrate clonality even in lesions that do not progress clinically. The secondary type is associated with mycosis fungoides–type cutaneous T-cell lymphoma or a chronic inflammatory skin disease. Lesions associated with mycosis fungoides are generally widespread and chronic and occur in older patients. Intralesional steroids and phototherapy have been reported as useful.
Vascular or neurologic alopecia, most often of the lower extremities, may be seen in diabetes mellitus or atherosclerosis. In meralgia paresthetica, there may be alopecia of the anesthetic area of the outer thigh.
Endocrinologic alopecia may occur in various endocrinologic disorders. In hypothyroidism, the hair becomes coarse, dry, brittle, and sparse. The proportion of telogen hairs has been shown to be three to seven times higher than the normal 10%. In hyperthyroidism, the hair becomes extremely fine and sparse. Oral contraceptives (OCs) have been implicated in some cases of androgenetic alopecia. It develops in predisposed women who are usually taking androgenic progestogens. It is advisable to discontinue the androgen-dominant pill and substitute an estrogen-dominant OC. Some women develop telogen effluvium 2–4 months after discontinuing anovulatory agents, which is analogous to postpartum alopecia.
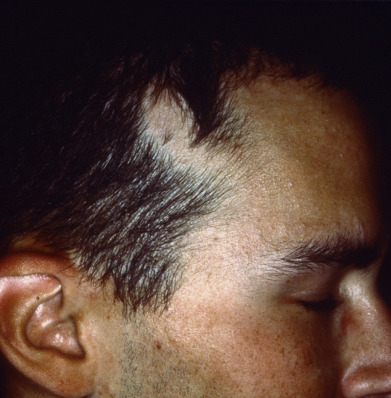
Congenital alopecia occurs as total or partial loss of hair, or a lack of initial growth, accompanied usually by other ectodermal defects of the nails, teeth, and bone. The hair is light and sparse and grows slowly. Congenital triangular alopecia ( Fig. 33.8 ) and aplasia cutis congenita are examples of congenital localized absence of hair. Hidrotic ectodermal dysplasia is a diffuse abnormality of hair associated with dental and nail changes.
Lipedematous alopecia consists of thickening of the scalp that gives the impression of thick cotton batting. The hair may be normal or shortened and sparse. Biopsy shows an increase in thickness of the subcutaneous fat and variable lymphoid inflammation. This disease appears to affect black persons primarily.
Borgia F, et al: Follicular mucinosis with diffuse scalp alopecia treated with narrow-band UVB phototherapy. G Ital Dermatol Venereol 2016; 151: 212.
Gibson LE, et al: Follicular mucinosis in childhood. J Am Acad Dermatol 2013; 69: 1054.
Yasar S, et al: Clinical and pathological features of 31 cases of lipedematous scalp and lipedematous alopecia. Eur J Dermatol 2011; 21: 520.
Cicatricial Alopecia
Cicatricial alopecia appears as areas of hair loss with absence of follicular ostia ( Fig. 33.9 ). Acute lesions may appear as erythematous plaques, perifollicular papules, keratotic follicular spines, or pustules. Deep inflammatory lesions may be boggy or may resemble noncicatricial areata clinically. The inflammatory nature of the lesion may only be evident on biopsy.
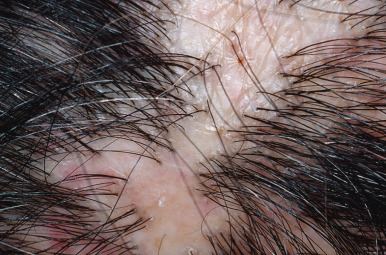
Discoid lupus erythematosus (DLE), lichen planopilaris, sarcoidosis, and folliculitis decalvans are the most common inflammatory causes of cicatricial alopecia. Chronic bacterial and fungal infections may produce inflammatory alopecia that mimics primary scarring alopecia. For example, fungal folliculitis may mimic LE.
Biopsy can confirm the diagnosis and provide prognostic information regarding the potential for new growth. A 4-mm punch biopsy will provide the pathologist with an adequate specimen. Smaller specimens are of limited value. The punch should be placed parallel to the direction of hair growth to avoid transecting follicles, and the punch should be advanced to the deep subcutaneous fat. The biopsy site will typically bleed profusely, but a 4-mm-wide strip of gel foam advanced into the defect will generally provide rapid hemostasis. Sutures are rarely necessary, and because the scar from a sutured biopsy site generally stretches back to the original dimensions of the biopsy, suturing provides little benefit to the patient.
The biopsy should be taken from a well-established lesion that is still active, rather than from the advancing edge. Dermoscopy may be helpful in selecting the biopsy site. The pathologist may prefer vertical or transverse (horizontal) sectioning of the specimen. Each has advantages. Every follicular unit in the specimen will be demonstrated in transverse sections. Vertical sections are superior for demonstrating changes in the surface epidermis, dermoepidermal junction (DEJ), superficial dermis, and subcutaneous fat. In general, the features of androgenetic (pattern) alopecia, telogen effluvium, and trichotillomania are better demonstrated in transverse (horizontal) sections through the specimen. Alopecia areata and syphilitic alopecia are well demonstrated in transverse sections if serial step sections are obtained to demonstrate deeper planes of section, or if the block is cut horizontally in a bread-loaf fashion before embedding. They are equally well demonstrated with serial vertical sections through the block. LE and lichen planopilaris are more easily demonstrated in serial vertical sections.
The diagnostic yield can be enhanced by pairing vertical and transverse sections. If two biopsies are done, one specimen can be bisected vertically for direct immunofluorescence (DIF) and hematoxylin and eosin (H&E) processing. It is most easily split by laying it on its side and bisecting it with a No. 15 blade pushed cleanly through the specimen in a single downward motion. Sawing at the specimen will not produce a satisfactory result. One-half the bisected specimen is placed in formalin and the other half in immunofluorescent media. The second specimen can be bisected for transverse sections in the clinic or left for the laboratory to bisect after processing. If to be bisected in the clinic, it should be placed on its side. The 15 blade should be pushed downward through the specimen in a single motion at the level of the middermis. All pieces for vertical and transverse sections may be placed in a single bottle to be embedded in a single cassette. If a single biopsy specimen is submitted for H&E sections, it can be bisected vertically, then one-half bisected transversely 1 mm above the fat (Tyler technique). This provides the advantages of both vertical and transverse sections with a single specimen.
In LE, the biopsy must be from a lesion of several months’ duration to demonstrate hyperkeratosis, follicular plugging, basement membrane zone thickening, and dermal mucin. Only biopsies from established lesions of lupus will demonstrate reliable immunofluorescence.
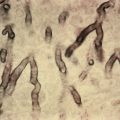
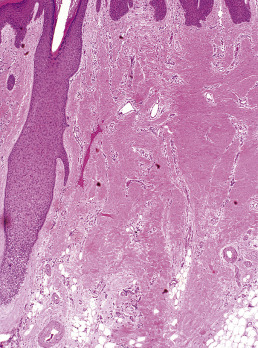
When biopsies of the most active area of alopecia have failed to yield a definite diagnosis, a biopsy from a scarred area may provide additional information. Scars show loss of elastic tissue with the Verhoeff–van Gieson stain. The pattern of elastic tissue loss is the “footprint” of the preceding inflammatory process ( Figs. 33.10 and 33.11 ), and remains the gold standard for evaluation of scarring alopecia, although polarized microscopy and immunofluorescence of H&E-stained sections can also be useful. Lichen planopilaris and folliculitis decalvans both affect the infundibulum. Both result in wedge-shaped superficial dermal scars. DLE results in scarring of both the follicular units and the intervening dermis. Morphea does not produce a scar, but rather hyalinization of collagen bundles with preservation of the elastic fibers. In idiopathic pseudopelade, the fibrous tract remnants are widened, but the elastic tissue sheath at the periphery of the fibrous tract is preserved.
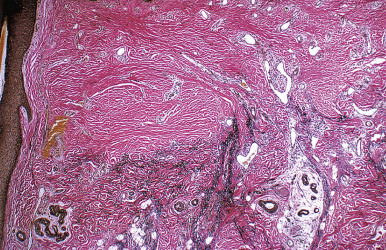
Most patients with cicatricial alopecia experience gradual progression of the alopecia, and the prolonged course of the disease may lead to inappropriate therapeutic complacency. The progressive destruction of hairs will result in ever-expanding areas of permanent alopecia. Therefore cicatricial alopecia must be treated aggressively and early to avoid permanent disfigurement. Surgical revision of the hairless plaque is an option for stable end-stage alopecia, but unless the underlying disease is controlled, surgery may only lead to a flare of the underlying disease with progression of hair loss. Therapy may be forestalled by the inability to establish a definite diagnosis. To help guide therapy for patients who defy diagnosis, work groups of the North American Hair Research Society have proposed a classification scheme based on the type and pattern of inflammation. Some forms of destructive alopecia are lymphocyte mediated; others are suppurative processes. The type of infiltrate and the portion of the pilosebaceous unit affected can be used to guide therapy. This classification system may also allow patients to enroll in clinical trials, even in the absence of a definite diagnosis.
Elston DM: Elastic fibers in scars and alopecia. Am J Dermatopathol 2017; 39: 556.
Fung MA, et al: Elastin staining patterns in primary cicatricial alopecia. J Am Acad Dermatol 2013; 69: 776.
Horenstein MG, et al: Follicular density and ratios in scarring and nonscarring alopecia. Am J Dermatopathol 2013; 35: 818.
Lymphoid-Mediated Disorders
Lupus Erythematosus
Chronic cutaneous (discoid) lupus of the scalp (DLE) is a common cause of cicatricial alopecia. In active disease, anagen hairs may be easily extracted from the involved area. Usually, erythema, atrophy, follicular plugging, and mottled hyperpigmentation and hypopigmentation are present ( Fig. 33.12 ). Patients with chronic cutaneous lupus of the scalp may have accompanying SLE or skin lesions of DLE on other parts of the body. The external ear canal and concha should always be examined because they are common sites for discoid lesions. Occasionally, alopecia occurs in a plaque of tumid lupus. Lupus panniculitis may occasionally result in alopecia in the absence of surface skin changes. SLE is often associated with discoid lesions of the scalp. Patients with SLE may also have short miniaturized “lupus hairs” on the anterior scalp.
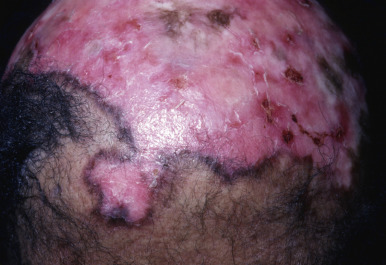
Biopsy of early lesions of DLE is often nondiagnostic. Patchy lymphoid inflammation and perifollicular mucinous fibrosis may be the only histologic findings. Focal vacuolar interface dermatitis may or may not be noted. Active established lesions, present for several months, have a higher diagnostic yield. Active established lesions usually demonstrate hyperkeratosis, follicular plugging, vacuolar interface dermatitis, basement membrane zone thickening, pigment incontinence, and dermal mucin. Perivascular and periadnexal lymphoid infiltrates are patchy and involve the eccrine coil and fibrous tract remnants. Fibrous tract involvement creates dense vertical columns of lymphocytes. The underlying subcutaneous tissue may demonstrate nodular lymphoplasmacytic infiltrates and fibrin or hyaline rings around necrotic fat. Hypertrophic lesions of chronic cutaneous LE often demonstrate lichenoid dermatitis. DIF typically demonstrates continuous granular deposition of immunoglobumin (Ig)G, IgA, IgM, and C3 at the DEJ (“full house” pattern). This pattern is particularly helpful in distinguishing lichenoid hypertrophic LE from lichen planopilaris. Burnt-out lesions of DLE demonstrate loss of elastic fibers throughout the dermis, which differs from the focal peri-infundibular wedge-shaped scars of lichen planopilaris. In SLE, follicular atrophy may be associated with pronounced dermal mucinosis.
Chronic cutaneous lupus may respond to intralesional or potent topical corticosteroids, but systemic therapy is frequently required. Antimalarials, retinoids, dapsone, thalidomide, sulfasalazine, mycophenolate mofetil, and methotrexate have been used successfully. Topical tazarotene and topical calcineurin inhibitors are generally disappointing.
Lichen Planopilaris and Frontal Fibrosing Alopecia
Lichen planopilaris presents with perifollicular erythema and progressive scarring. Small follicular papules may be noted, or the lesion may resemble the ivory-white irregular patches of pseudopelade. In some patients, typical polygonal flat-topped papules are present on the wrists and ankles, and lacy white lesions are noted on the oral and genital mucosa. Widespread follicular papules may be present on the trunk or extremities. In most patients, however, only the scalp is involved. Frontal fibrosing alopecia is a variant of lichen planopilaris. Most patients are older women with bandlike frontotemporal alopecia, hypopigmentation and atrophy ( Fig. 33.13 ), often with “genitalized” kinky hairs. The kinky hairs resemble those in progressive acquired kinking of the hair (a manifestation of early male pattern alopecia) suggesting a hormonal influence. The current epidemic was first described by Dr. Kossard in Australia, but a similar condition was described by the Swedish physician Axel Munthe in 1929, and recounted in his memoirs “The Story of San Michele.”
Graham-Little–Piccardi-Lasseur syndrome includes cicatricial alopecia on the scalp, keratosis pilaris in the skin of the trunk and extremities, and noncicatricial hair loss in the pubis and axillae. It has been described in association with complete androgen insensitivity syndrome, a condition that also presents with noncicatricial alopecia in the axillary and pubic hair.
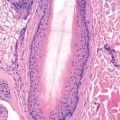
Diagnostic biopsies demonstrate lichenoid interface dermatitis of the follicular unit and sometimes the intervening epidermis. The entire fibrous tract may be filled with cytoid bodies ( Fig. 33.14 ). The changes usually occur focally and may be best visualized with serial vertical sections. Perifollicular mucinous fibrosis is common, and focal perifollicular lymphoid infiltrates tend to involve the infundibulum (infiltrates of LE tend to involve isthmus). DIF may be negative or may reveal cytoid bodies and shaggy linear fibrin at the DEJ.
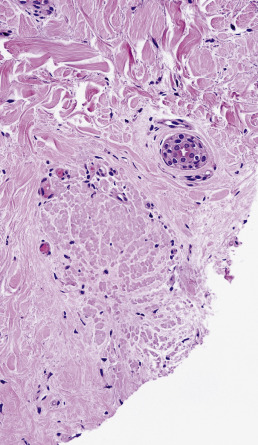
Lichen planopilaris responds to oral and intralesional corticosteroids. Topical corticosteroids or topical calcineurin inhibitors may be adequate in a few patients, but resulting scalp atrophy with prominence of capillary plexus may be misinterpreted as erythema signifying active disease. As in lupus, topical tazarotene and topical macrolactams are generally disappointing. Oral retinoids, antimalarials and excimer laser can be effective, but some reports have noted progression of alopecia with antimalarials despite a reduction in erythema. It is therefore important to follow the size of the alopecic patches, presence of easily extractable anagen hairs, and presence of follicular spines as well as erythema and pruritus. The peroxisome proliferator activated receptor-γ agonist pioglitazone is effective at halting progression in many patients. A 3-month trial of 15 mg followed by 3 months at 30 and 45 mg (if there is no response) should be attempted before the treatment is deemed a failure. Patients should be followed for peripheral edema and other signs of heart failure, but in the authors’ experience, the drug is well tolerated by the majority of patients for the length of time it is employed. Mycophenolate mofetil is generally reliable for patients with refractory disease, and excimer laser can be helpful in refractory disease. Tofacitinib therapy is promising. Biologics have been suggested as therapy, but onset of lichen planopilaris has been noted during etanercept therapy. Dutasteride is often effective as first line therapy in the setting of frontal fibrosing alopecia, and is generally paired with piaglitazone. Oral retinoid therapy has been reported as effective for controlling the facial papules often associated with the disease.
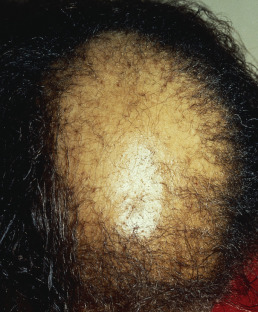
Central Centrifugal Cicatricial Alopecia
Central centrifugal cicatricial alopecia (CCCA) is seen most often in African American women, is slowly progressive, usually begins in the crown, and advances to the surrounding areas ( Fig. 33.15 ). The term is often used as a broad category that includes “hot comb alopecia,” idiopathic pseudopelade, and central elliptical alopecia. Some patients will demonstrate crops of crusts at the periphery of the patches, a feature of folliculitis decalvans. Treatment of CCCA is difficult and often unsatisfactory. Discontinuation of chemical and heat processing and reduction of traction are recommended. Patients with overlapping features of folliculitis decalvans may respond to long-term antibiotic therapy and topical corticosteroids. In such overlapping cases, the histology shows a lymphocytic infiltrate during the chronic stage, but periodic crops of pustules demonstrate a neutrophilic folliculitis.
Neutrophil-Mediated Disorders
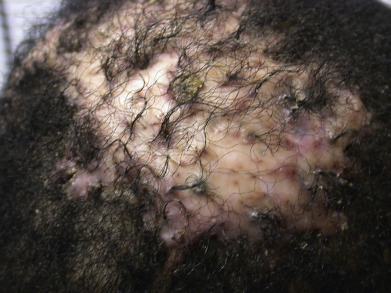
Folliculitis Decalvans
Folliculitis decalvans presents with crops of pustules that result in cicatricial alopecia. Successive crops of pustules, crusts, or erosions lead to expansion of the alopecic patches ( Fig. 33.16 ). Staphylococci are sometimes cultured from the lesions, and some authors have suggested that folliculitis decalvans merely represents a chronic staphylococcal infection. It is more likely that follicular destruction is the result of an abnormal suppurative immune response. Staphylococci and other organisms probably play a role in inciting the response. Erlotinib-induced folliculitis decalvans has been reported. The lesions often respond to long-term treatment with a tetracycline. The improvement may reflect the antineutrophil effects of the drug or its antimicrobial effects. Many patients also respond to other forms of antistaphylococcal therapy, but the lesions generally recur after the antibiotic is discontinued. In contrast, long-term tetracycline treatment generally results in a continued response. Some sustained responses have been noted after combination therapy with rifampin and clindamycin. Rifampin alone may promote the emergence of bacterial resistance. Selenium sulfide shampoo and topical corticosteroids may be useful as adjunctive therapy. Oral retinoids, oral and topical fusidic acid, oral zinc sulfate, photodynamic therapy (PDT) and topical tacrolimus have been reported as successful, and anti-TNF biologics have been used for refractory disease.
A variant of folliculitis decalvans occurs in African American patients who present with pseudofolliculitis of the beard, acne keloidalis nuchae, and scarring alopecia in the vertex and parietal scalp. The scalp demonstrates ingrown hairs, crops of pustules or crusts, and permanent scarring alopecia. Although pseudofolliculitis barbae is generally accepted to be the result of ingrown hairs, the pathogenesis of acne keloidalis nuchae remains in question. Histologically, ingrown hairs are common in advanced lesions. Early lesions may not demonstrate the hair. Some patients merely develop small papules on the nape of the neck, whereas others develop pustules, crusts, and progressive alopecia. This latter group overlaps with folliculitis decalvans, and patients generally respond to treatment with a topical corticosteroid and an oral tetracycline.
Acne Necrotica
Acne necrotica presents with discrete excoriated follicular papules in the scalp. Biopsy demonstrates an inflammatory crust and suppurative folliculitis. Usually, there is no associated scarring alopecia, but occasional cases overlap with folliculitis decalvans.
Erosive Pustular Dermatitis of the Scalp
Pustular dermatitis often presents as expanding eroded patches on the scalp with moist granulation tissue. The lesions often follow trauma or a surgical procedure and tend to be chronic and progressive. They respond best to class I topical corticosteroids. PDT has also been used effectively.
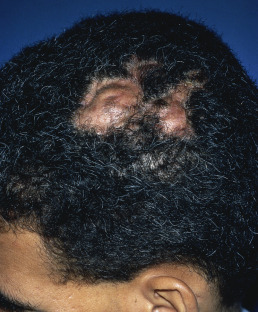
Dissecting Cellulitis (Perifolliculitis Capitis Abscessens et Suffodiens of Hoffman)
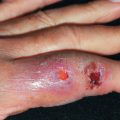
Dissecting cellulitis often coexists with acne conglobata and hidradenitis suppurativa. It may also occur with folliculitis decalvans. The lesions are deep, boggy, and suppurative ( Fig. 33.17 ). They may respond to tetracyclines, retinoids, and intralesional corticosteroids.
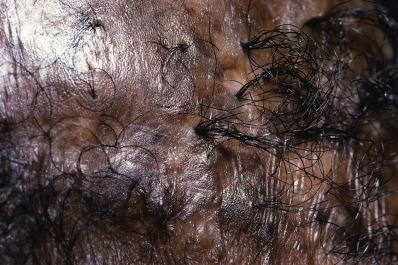
Tufted Folliculitis
Tufted folliculitis presents with doll’s hair–like bundling of follicular units ( Fig. 33.18 ). It is seen in a wide range of scarring conditions, including chronic staphylococcal infection, chronic LE, lichen planopilaris, Graham-Little syndrome, folliculitis decalvans, acne keloidalis nuchae, immunobullous disorders, and dissecting cellulitis. Compound hairs (two or more hairs sharing a common infundibulum) occur physiologically on the occipital scalp and legs and should not be confused with tufted folliculitis.
Other Forms of Permanent Alopecia
Pseudopelade of Brocq
Also known as alopecia cicatrisata, this pseudopelade is a rare form of cicatricial alopecia in which destruction of the hair follicles produces multiple round, oval, or irregularly shaped, hairless, cicatricial patches of varying sizes. They are usually coin sized and are white or slightly pink in color, with a smooth, shiny, marble-like or ivory, atrophic, “onion skin” surface. Interspersed in the patches may be a few spared follicles with hairs growing from them. A clinical inflammatory stage is completely absent. No pustules, crusts, or broken-off hairs are present. The onset, as a rule, is insidious, with one or two lesions appearing on the vertex. The condition affects females three times more often than males and has a prolonged course. In advanced cases, large irregular patches are formed by coalescence of some of the many small macules, a pattern referred to as “footprints in the snow.” The alopecia is permanent and the disease slowly progressive. Most cases of pseudopelade demonstrate scarring in a wedge-shaped pattern in the superficial dermis and represent an end stage of lichen planopilaris. A distinct subset called idiopathic pseudopelade accounts for most patients with CCCA. In these patients, the dermis is contracted into a thin band of dense collagenous tissue. Elastic fibers are intact and quite thick as a result of elastic recoil related to dermal contraction. Fibrous tract remnants are wide and hyalinized with an intact elastic sheath. Lymphoid and neutrophilic inflammation is absent, but loss of the inner and outer root sheaths with subsequent hair fiber granuloma formation is noted. Sebaceous glands are decreased or absent, as they are in most forms of permanent alopecia. DIF is negative.
Traction Alopecia
Traction alopecia occurs from prolonged tension on the hair, either from wearing the hair tightly braided or in a ponytail, pulling the hair to straighten it, rolling curlers too tightly, or from the habit of twisting the hairs with the fingers. Traction alopecia most often involves the periphery of the scalp, especially the temples and above the ears, but a fringe of hair is characteristically present at the frontal and temporal hairline ( Fig. 33.19 ).
Sarcoidosis
Sarcoidosis of the scalp presents with diffuse or patchy hair loss. The involved scalp is often indurated, and a raised peripheral border may be present. The lesions are often red-brown in color and may have an “apple jelly” appearance on diascopy. Biopsy reveals noncaseating granulomas. Treatment is the same as for other forms of sarcoidosis.
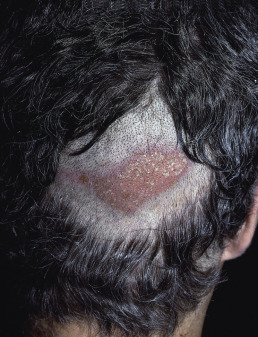
Pressure Alopecia
Pressure alopecia occurs in adults after prolonged pressure on the scalp during general anesthesia, with the head fixed in one position. It may also occur in chronically ill persons after prolonged bed rest in one position ( Fig. 33.20 ), which causes persistent pressure on one part of the scalp. It probably arises because of pressure-induced ischemia.
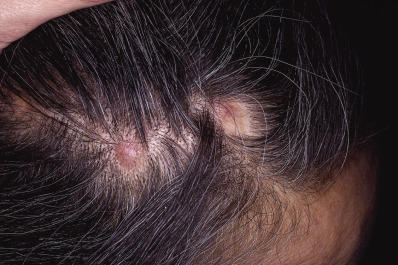
Tumor Alopecia
Tumor alopecia refers to hair loss in the immediate vicinity of either benign or malignant tumors of the scalp. Syringomas, nerve sheath myxomas, and steatocystoma multiplex are benign tumors that may be limited to the scalp and may cause alopecia. Alopecia neoplastica is the designation given to hair loss from metastatic tumors, most often from breast or renal carcinoma ( Fig. 33.21 ).
Keratosis Pilaris Atrophicans
Keratosis pilaris atrophicans includes many forms of keratosis pilaris with cicatricial alopecia. Variants include keratosis pilaris atrophicans faciei, atrophoderma vermiculatum, keratosis follicularis spinulosa decalvans, and ichthyosis follicularis.
Keratosis pilaris atrophicans faciei (ulerythema ophryogenes, keratosis pilaris rubra atrophicans faciei, folliculitis rubra, lichen pilare, xerodermie pilaire symmétrique de la face) begins in infancy as follicular papules with perifollicular erythema. Initially, the lesions are restricted to the lateral eyebrows. With time, they spread to involve the cheeks and forehead. There may be associated keratosis pilaris on the extremities and buttocks. The condition may also be associated with an atopic diathesis, ectodermal dysplasia, or Noonan syndrome.
Atrophoderma vermiculatum (acne vermoulanti, honeycomb atrophy, folliculitis ulerythematosa reticulata, ulerythema acneiforme, folliculitis ulerythematous reticulata, atrophodermia reticulata symmetrica faciei, atrophoderma reticulatum) presents with erythematous follicular papules on the cheeks in childhood. With time, the lesions develop into pitlike depressions (reticulate atrophy). Autosomal dominant inheritance has been described. This condition generally spares the scalp and eyebrows.
Keratosis follicularis spinulosa decalvans is a rare X-linked disorder described by Siemens in 1926. The gene has been mapped to Xp21.2–p22.2. It begins in infancy with keratosis pilaris localized on the face, then evolves to more diffuse involvement. Progressive cicatricial alopecia occurs on the scalp, eyebrows, and sometimes eyelashes. The alopecia starts during childhood, and active disease may remit during the early teenage years. Corneal and conjunctival inflammation, corneal dystrophy, and blepharitis occur, and photophobia is usually a prominent finding.
Ichthyosis follicularis also demonstrates extensive spiny follicular hyperkeratosis, permanent alopecia, and photophobia. Palmar plantar keratosis, nail deformities, atopy, and recurrent cheilitis have been described.
Atrichia With Papular Lesions
Atrichia with papular lesions is a rare autosomal recessive disorder with early onset of atrichia, followed by a papular eruption appearing within the first years of life. The condition has been linked to chromosome 8p21, and mutations have been detected in what is now referred to as the hairless gene. It is discussed in more detail in Chapter 27 .
Bastida J, et al: Treatment of folliculitis decalvans with tacrolimus ointment. Int J Dermatol 2012; 51: 216.
Callender VD, et al: Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia. Arch Dermatol 2012; 148: 1047.
Dlova NC, et al: Central centrifugal cicatricial alopecia. J Investig Dermatol Symp Proc 2017; 18: S54.
Donovan JC, et al: Transversely sectioned biopsies in the diagnosis of end-stage traction alopecia. Dermatol Online J 2013; 19: 11.
Elston CA, et al: Elastic staining versus fluorescent and polarized microscopy in the diagnosis of alopecia. J Am Acad Dermatol 2013; 69: 288.
Elston D: The “Tyler technique” for alopecia biopsies. J Cutan Pathol 2012; 39: 306.
Fertig R, et al: Frontal fibrosing alopecia treatment options. Intractable Rare Dis Res 2016; 5: 314.
Keith DJ, et al: Erlotinib-induced folliculitis decalvans. Clin Exp Dermatol 2013; 38: 924. Lin J, et al: Hypopigmentation in frontal fibrosing alopecia. J Am Acad Dermatol 2017; 76: 1184.
Loganathan E, et al: Complications of hair restoration surgery. Int J Trichology 2014; 6: 168.
Loh SH, et al: Pressure alopecia. J Am Acad Dermatol 2015; 72: 188.
MacDonald A, et al: Frontal fibrosing alopecia. J Am Acad Dermatol 2012; 67: 955.
Mihaljević N, et al: Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges 2012; 10: 589.
Miteva M, et al: Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol 2013; 27: 1299.
Navarini AA, et al: Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol 2011; 147: 1325.
Nic Dhonncha E, et al: The role of hydroxychloroquine in the treatment of lichen planopilaris. Dermatol Ther 2017; 30.
Pirmez R, et al: Successful treatment of facial papules in frontal fibrosing alopecia with oral isotretinoin. Skin Appendage Disord 2017; 3: 111.
Rácz E, et al: Treatment of frontal fibrosing alopecia and lichen planopilaris. J Eur Acad Dermatol Venereol 2013; 27: 1461.
Rodney IJ, et al: Hair and scalp disorders in ethnic populations. J Drugs Dermatol 2013; 12: 420.
Rossi A, et al: Unusual patterns of presentation of frontal fibrosing alopecia. J Am Acad Dermatol 2017; 77: 172.
Shao H, et al: Follicular unit transplantation for the treatment of secondary cicatricial alopecia. Can J Plast Surg 2014; 22: 249.
Spring P, et al: Lichen planopilaris treated by the peroxisome proliferator activated receptor-γ agonist pioglitazone. J Am Acad Dermatol 2013; 69: 830.
Taylor SC, et al: Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis 2017; 100: 31.
Trüeb RM: A comment on frontal fibrosing alopecia (Axel Munthe’s syndrome). Int J Trichology 2016; 8: 203.
Yang CC, et al: Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol Ther 2018; e12656. doi: 10.1111/dth.12656. [Epub ahead of print] PubMed PMID: 30264512.
Ye Y, et al: Non-scarring patchy alopecia in patients with systemic lupus erythematosus differs from that of alopecia areata. Lupus 2013; 22: 1439.
Hair Color
Melanin in the hair follicles is produced in the cytoplasm of the melanocytes. Organelles involved include the endoplasmic reticulum, ribosomes, and Golgi apparatus. Melanocytes producing hair pigment are associated with the hair matrix, and melanogenesis occurs only during anagen. This cyclic melanin synthesis distinguishes follicular melanogenesis from the continuous melanogenesis of the epidermis. With age, cyclic melanocytic activity in the follicular unit declines. By age 40, most individuals show evidence of graying. Graying results primarily from a reduction in tyrosinase activity within hair bulb melanocytes. Defective migration of melanocytes from a diminishing reservoir in the outer root sheath may play a role. Physiologic graying may also be related to reactive oxygen species–mediated damage to nuclear and mitochondrial DNA in bulbar melanocytes. The melanocortin 1 receptor gene (MCR1) is closely related to red hair, freckling, and sun sensitivity.
The pigment in black and dark-brown hair is composed of eumelanin, whereas in blond and red hair, it is pheomelanin. In black hair, the melanocytes contain the densest melanosomes. Brown hair differs only by its smaller melanosomes. Light-brown hair consists of a mixture of the melanosomes of dark hair and the incomplete melanosomes of blond hair. Many of the melanosomes in blond hair develop only on the matrix fibers and not in the spaces between the fibers.
Red hair shows incomplete melanin deposits on the matrix fibers, to produce a blotchy-appearing melanosome. Pheomelanin is distinguished by its relatively high content of sulfur, which results from the addition of cysteine to dopaquinone along the biosynthetic pathway of melanin synthesis.
In gray hair (canities), melanogenic activity is decreased as a result of fewer melanocytes and melanosomes, as well as a gradual loss of tyrosinase activity. Graying of the scalp hair is genetically determined and may start at any age. Usually, it begins at the temples and progresses with time. The beard usually follows, with the body hair graying last. Premature whitening of scalp hair is usually caused by vitiligo, sometimes without recognized, or actually without, lesions of glabrous skin.
Early graying (before age 20 in white or before age 30 in black persons) is usually familial; however, it may occur in progeria and in Rothmund-Thomson, Böök (PHC), and Werner syndromes, as well as after radiation exposure.
In poliosis, gray or white hair occurs in circumscribed patches. This may occur in Waardenburg syndrome and piebaldism, Tietz syndrome, Alezzandrini syndrome, neurofibromatosis, and tuberous sclerosis. Poliosis is also found in association with regressing melanoma, vitiligo, and Vogt-Koyanagi syndrome and may be seen in alopecia areata when the new hairs grow. Migratory poliosis without hair loss may represent a forme fruste of alopecia areata.
Green hair has been traced to copper in the water of a swimming pool. This occurs only in blond or light hair and may be treated with topical ethylenediamine tetraacetic acid (EDTA), penicillamine-containing shampoos, or 1.5% aqueous 1-hydroxyethyl diphosphonic acid. Tars and chrysarobin stain light-colored hair brown.
Changes in hair color occur in various disorders. The hair is blond in phenylketonuria and homocystinuria. Light hair is also seen in oasthouse urine disease (familial methionine malabsorption syndrome), Menkes steely (kinky) hair syndrome, and albinism. In Griscelli and Chédiak-Higashi syndromes, the hair has a silvery sheen. In kwashiorkor, hair assumes a red-blond color and may demonstrate periodic banding (flag sign, segmental heterochromia). Alternating light and dark bands may also occur in iron deficiency anemia and with courses of sunitinib. In vitamin B 12 deficiency and with IFN therapy, whitening may occur. The disorder has been called canities segmentata sideropenica and responds completely to iron supplementation. Triparanol is associated with hypopigmented hair. By changing vellus to terminal hairs, minoxidil causes darkening of hair. Another hypotensive agent, diazoxide, gives the hair a reddish tint. Chloroquine therapy may cause hair whitening, usually in redheads and blonds, but not in brunettes. Pigmentation of the eyelashes and irides has been described with latanoprost. Xanthotrichia (yellow hair) has been noted with selenium sulfide and dihydroxyacetone.
Many black patients with acquired immunodeficiency syndrome (AIDS) have experienced softening, straightening, lightening, and thinning of their hair. Patients with human immunodeficiency virus 1 (HIV-1) infection may also experience elongated eyelashes and telogen effluvium.
Liu F, et al: Colorful DNA polymorphisms in humans. Semin Cell Dev Biol 2013; 24: 562.
Praetorius C, et al: A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell 2013; 155: 1022.
Hair Structure Defects
Examination of hairs for structural defects is greatly facilitated by a method devised by Shelley: putting a piece of double-stick tape on a microscope slide and aligning 5-cm segments of hair in parallel on it. Dermoscopy can be useful in assessing hair morphology. Microscopic mounts of hairs are best examined under a dissecting microscope or polarized light. Gold coating and scanning electron microscopy (SEM) can also be done on hairs so mounted. Hairs from multiple body sites may need to be sampled. This has been documented in Netherton syndrome, where scalp hair can be normal while eyebrow hair demonstrates the characteristic hair shaft defect.
Hair Casts (Pseudonits)
Hair casts represent remnants of the inner root sheath. They often occur in great numbers and may mimic nits in the scalp. Whereas nits are firmly cemented to the hair shaft, however, hair casts slide freely along the shaft. Taeb et al. reviewed 36 published cases and distinguished two groups: girls age 2–8 years with diffuse involvement and no scalp disease, and children and adults with psoriasis, lichen planus, seborrheic dermatitis, traction, or trichotillomania. Keipert made a similar distinction, separating a large group of cases with some keratinizing disorder of the scalp and dark, oddly shaped masses of keratin adherent to or surrounding the hairs, which he called “parakeratotic” hair casts; and lighter-colored tubular casts 2–4 mm long, which he called “peripilar” hair casts. Taeb et al. found 0.025% tretinoin lotion to be effective. False hair casts may occur as a result of hair spray or deodorant concretions. Immunoglobulin casts and cutaneous spicules have been noted in multiple myeloma.
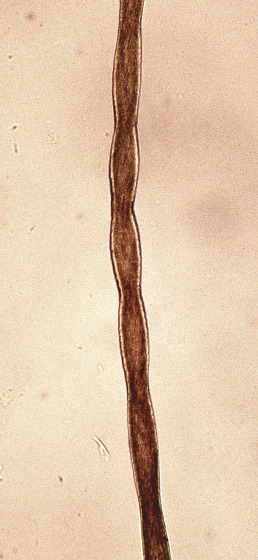
Pili Torti
Also known as “twisted hairs,” pili torti is a malformation of hair characterized by twisting of the hair shaft on its own axis ( Fig. 33.22 ). The hair shaft is segmentally thickened, and light and dark segments are seen. Scalp hair, eyebrows, and eyelashes may be affected. The hairs are brittle and easily broken.
In the classic type, unassociated with other disorders, onset is usually in early childhood; by puberty, it has usually improved. Clinically, pili torti may be associated with patchy alopecia and short, broken hairs. It usually follows a dominant inheritance pattern, although recessive and sporadic cases have been reported. Acquired cases have been described in young women with anorexia nervosa. Pili torti may be seen with associated abnormalities. The Björnstad syndrome consists of congenital deafness of the cochlear type, with pili torti. Both autosomal dominant and autosomal recessive inheritance patterns have been described. BCS1L mutations cause the Björnstad syndrome. The gene encodes an adenosine triphosphatase (ATPase) necessary for the assembly of complex III in mitochondria. BCS1L mutations also cause lethal conditions, including the complex III deficiency and the GRACILE syndrome, with severe multisystem and neurologic manifestations.
Pili torti also may occur in citrullinemia (argininosuccinate synthetase deficiency), Menkes steely (kinky) hair syndrome, Bazex follicular atrophoderma syndrome, ectodermal dysplasias, Crandall syndrome (pili torti, nerve deafness, hypogonadism), Netherton syndrome (along with bamboo hair), with isotretinoin and etretinate therapy, in anorexia nervosa, and in trichothiodystrophy.
Laron syndrome is an autosomal recessive disease with primary insulin-like growth factor 1 deficiency and primary growth hormone insensitivity. Affected children have sparse hair and frontal recession. Pili torti et canaliculi, tapered hair, and trichorrhexis nodosa have been noted.
Pili torti and loose anagen hairs have been described during treatment with erlotinib.
Menkes Steely (Kinky) Hair Syndrome
Pili torti and often monilethrix and trichorrhexis nodosa are all common in the hairs in this sex-linked recessively inherited disorder. It has also been called steely hair disease because the hair resembles steel wool. The characteristic ivory color of the hair appears between 1 and 5 months of age. Drowsiness, lethargy, convulsive seizures, severe neurologic deterioration, and periodic hypothermia ensue, with death at an early age. Hairs become wiry, sparse, fragile, and twisted about their long axis. Osteoporosis and dental and ocular abnormalities are common. The skin is pale and the face pudgy, and the upper lip has an exaggerated “Cupid’s bow” configuration. The occipital horn syndrome, primarily a connective tissue disorder, is a milder variant of Menkes syndrome. Patients have a deficiency of serum copper and copper-dependent enzymes, resulting from mutations in the ATP7A gene. The gene encodes a trans–Golgi membrane–bound copper transporting P-type ATPase. Loss of this protein activity blocks the export of dietary copper from the GI tract and causes the copper deficiency. Low serum copper and ceruloplasmin levels are characteristic, but are not seen in all patients; levels are particularly variable in the first weeks of life. Other tests helpful for screening include the ratio of catechols, such as dihydroxyphenylalanine, to dihydroxyphenylglycol. High levels of the catechols dopa, dihydrophenylacetic acid, and dopamine and low levels of dihydroxyphenylglycol are characteristic. Studies of copper egress in cultured fibroblasts have also been used.
Early detection allows for genetic counseling and institution of copper histidine treatment, which has shown promising results in some infants. Pamidronate treatment is associated with an increase in bone mineral density in children with Menkes disease. In zebra fish, antisense morpholino oligonucleotides directed against the splice-site junctions of two mutant calamity alleles were able to correct the molecular defect. Also, l -threo-dihydroxyphenylserine can correct neurochemical abnormalities in a mouse model. This is a promising area for research.
Trichorrhexis Nodosa
The affected hair shafts fracture easily and may have small white nodes arranged at irregular intervals. These nodes are the sites of fraying of the hair cortex. The splitting into strands produces a microscopic appearance suggestive of a pair of brooms stuck together end to end by their bristles. The hairs soon break at these nodes ( Fig. 33.23 ). The number of these nodes along one hair shaft varies from one to several, depending on its length. These fractured hairs are found mostly on the scalp, often in just a small area or areas, but other sites, such as the pubic area, axillae, and chest, may be involved.

Several categories or types of trichorrhexis nodosa have been described. Proximal trichorrhexis nodosa involves the proximal shafts of the hairs of black patients who traumatize their hair with styling or chemicals. The involved hairs break a few centimeters from the skin surface, resulting in patches of short hair. It appears to occur in genetically predisposed patients. Distal trichorrhexis nodosa affects primarily Asians and white patients; it occurs several inches from the scalp and is associated with trichoptilosis, or longitudinal splitting, known as “split ends.” Acquired localized trichorrhexis nodosa is a common type in which the defect occurs in a localized area a few centimeters across. Diseases that accompany localized trichorrhexis nodosa in which pruritus is a prominent symptom (perhaps caused by scratching and rubbing) include circumscribed neurodermatitis, contact dermatitis, and atopic dermatitis.
The occurrence of trichorrhexis nodosa in some patients with argininosuccinicaciduria has suggested an etiologic connection. Trichorrhexis nodosa has been described in Menkes steely hair syndrome, Netherton syndrome, hypothyroidism, ectodermal dysplasia, the syndrome of intractable infant diarrhea, biotinidase deficiency, and trichothiodystrophy. Trichoschisis, a clean transverse fracture across the hair shaft, is more often present in trichothiodystrophy. The curly hair that may result from isotretinoin therapy has been attributed to extensive trichorrhexis nodosa. Because trauma may induce this hair shaft abnormality, the specificity of this finding in the previous conditions may simply be fortuitous. Treatment is directed toward the avoidance of trauma to the hair.
Trichorrhexis Invaginata
Also known as “bamboo hair,” trichorrhexis invaginata is caused by intussusception of the hair shaft at the zone where keratinization begins. The invagination is caused by softness of the cortex in the keratogenous zone. The softness may be caused by inadequate conversion of –SH to S–S proteins in the cortex. The patient with bamboo hair will have nodose ball-and-socket deformities, with the socket forming the proximal and the ball part forming the distal portion of the node along the hair shaft. This type of hair is associated with Netherton syndrome ( Fig. 33.24 ). Occasionally, only the proximal half of the abnormality is seen; this has been called “golf tee hairs.”