Bacterial infections in the skin often have distinct morphologic characteristics that should alert the clinician that a potentially treatable and reversible condition exists. These cutaneous signs may be an indication of a generalized systemic process or simply an isolated superficial infection. Patients who have immunodeficiencies or are immunosuppressed may acquire severe or refractory pyogenic infections. Patients with atopic dermatitis are also predisposed to bacterial infections due to down regulation of antimicrobial peptides. The categorization of bacterial infections in this chapter first addresses diseases caused by gram-positive bacteria, followed by those caused by gram-negative bacteria, and then several miscellaneous diseases caused by the Rickettsiae , mycoplasmas, Chlamydiae , and spirochetes.
Chon SY, et al: Antibiotic overuse and resistance in dermatology. Dermatol Ther 2012; 25: 55.
Dawson AL, et al: Infectious skin diseases. Dermatol Clin 2012; 30: 141.
Dumville JC, et al: Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev 2013; 3: CD003949.
Grice EA, et al: Topographical and temporal diversity of the human skin microbiome. Science 2009; 324: 1190.
Holmes AH, et al: Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet 2016; 387: 176.
Mistry RD: Skin and soft tissue infections. Pediatr Clin North Am 2013; 60: 1063.
Nakatsuji T, et al: Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Science Translational Medicine 2017; 9.
Infections Caused by Gram-Positive Organisms
Staphylococcal Infections
The skin lesions induced by the gram-positive staphylococci usually appear as pustules, furuncles, or erosions with honey-colored crusts. However, bullae, widespread erythema and desquamation, or vegetating pyodermas may also be due to Staphylococcus aureus infection. Purulent purpura may indicate bacteremia or endocarditis that can be caused by S. aureus or, in immunocompromised patients, S. epidermidis. Two distinctive cutaneous lesions that occur with endocarditis are the Osler node and Janeway lesion or spot. The Osler node is a painful, erythematous nodule with a pale center located on the fingertips. The Janeway spot is a nontender, angular hemorrhagic lesion of the soles and palms ( Fig. 14.1 ). These lesions are likely caused by septic emboli leading to clogging of the distal vessels.

From 20%–40% of adults are colonized with S. aureus in the nares but the bacteria can also colonize hands and perineum, so this estimate is likely underestimating staphylococcal colonization. One study found that if only nares were cultured, 40% of carriage was missed and perineal colonization correlated better with the strain isolated from the abscess. Carriers are particularly prone to infections with S. aureus because of its continuous presence on the skin and nasal mucosa. Spread of infection in the hospital setting is frequently traced to the hands of a health care worker. Proper handwashing or sanitizing technique is essential in preventing spread of infection. Human immunodeficiency virus (HIV)–infected patients are at least twice as often nasal carriers, and they tend to harbor S. aureus in higher frequency and density at other sites of the body, thus predisposing them to skin and systemic infection.
Antibiotic resistance has become more common in S. aureus. Methicillin-resistant S. aureus (MRSA) is an important pathogen in nosocomial and community-acquired skin infections. MRSA infection may be suspected from knowledge of local patterns of resistance, lack of response to initial methicillin-sensitive S. aureus (MSSA)–directed therapy (e.g., cephalexin), and factors predisposing to colonization and infection with this organism. Predisposing factors for MRSA include age (>65), exposure to others with MRSA infection, prior antibiotic therapy, trauma to the skin, rectal or nasal colonization, crowded households, child care attendance, contact sports, chronic skin disease, pets, and recent hospitalization or chronic illness. The bacteria can be found on bed linens, remote controls, hand towels, and other inanimate objects, making decolonization very challenging. The primary treatment for an abscess remains drainage although newer literature suppports the addition of oral antibioitcs that may help lessen recurrence. Ideally, antibiotic choice is guided by culture and sensitivities. Often clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX, alone or combined with rifampin), and doxycycline are effective. TMP-SMX and doxycycline do not cover group A streptococci; therefore if a mixed infection is suspected, adding cephalexin or penicillin is necessary, or clindamycin alone may treat both pathogens. Complicated infections with the most resistant strains may require antibiotics that nearly universally cover S. Aurues such as vancomycin, linezolid or tedizolid. Bacterial culture is the gold standard for establishing antibiotic susceptibility profiles and is increasingly necessary as resistance becomes more common. Superficial and localized infections may only require topical antibiotic therapy. Systemic antibiotics combined with topical therapy are recommended for patients with more widespread and deeper infections. A semisynthetic penicillin or a first-generation cephalosporin is recommended, unless MRSA is suspected, as detailed earlier. Treatment should generally be given for 7–10 days, although longer courses may sometimes be necessary
Primary Cutaneous Staphylococcal Infections
Staphylococcus can affect the skin locally and the therapy and prevention are similar so will be discussed together at the end of the section.
Impetigo
The bullous variety of impetigo caused by S. aureus occurs characteristically in newborns and young children, although it may occur at any age. The majority is caused by phage types 71 or 55 coagulase-positive S. aureus or a related group 2 phage type. Bullous impetigo may be an early manifestation of HIV infection. The neonatal type is highly contagious and can spread rapidly nurseries and day cares. In most cases, the disease begins between the fourth and tenth days of life with the appearance of bullae, which may appear on any part of the body but have a predilection for the face and perineum. Sources of the primary infection may be the umbilical stump, circumcision, or other area of skin breakdown. Constitutional symptoms are absent at first, but staphylococcal scalded skin or more rarely disseminated infection can occur in neonates. Bacteremia, pneumonia, or meningitis rarely may develop. Any infection in a neonate under 6 weeks should be treated more aggressively, with strong consideration given for oral antibiotics. Herpes simplex infections in neonates can be lethal, so if there is any suspicion for herpes simplex virus (HSV), polymerase chain reaction (PCR) should be done to rule this out.
In warm climates particularly, adults may have bullous impetigo, most often in the axillae or groins, but also on the hands. The lesions are strikingly large, fragile bullae, suggestive of pemphigus and intact bullae may not be present ( Fig. 14.2 ). When these rupture, they leave circinate, weepy, or crusted lesions, and in this stage it may be called impetigo circinata. Children with bullous impetigo ( Fig. 14.3 ) may give a history of an insect bite at the site of onset of lesions. Smaller vesicles can be seen with streptococcal infections or herpes simplex. Impetigo contagiosa is a term used to encompass vesicles that might be from a staphylococcal, streptococcal, or combined infection ( Fig. 14.4 ). It is characterized by discrete, thin-walled vesicles that rapidly become pustular and then rupture especially in the setting of atopic dermatitis. Impetigo on the scalp is a frequent complication of pediculosis capitis. In streptococcal-induced impetigo, regional lymphadenopathy is common, but not serious.



Most studies find that 50%–70% of cases are caused by S. aureus, with the remainder from either S. pyogenes or a combination of these two organisms. Streptococci may represent an early pathogen in the development of impetigo, with staphylococci replacing streptococci as the lesion matures. Group B streptococci are associated with newborn impetigo, and groups C and G are rarely isolated from impetigo, unlike the usual group A.
Impetigo occurs most frequently in early childhood, although all ages may be affected. It occurs in the temperate zone, mostly during the summer in hot, humid weather. Common sources of infection for children are pets, dirty fingernails, and other children in schools, day care centers, or crowded housing areas; sources for adults include infected children and self-inoculation from nasal or perineal carriage. Impetigo often complicates pediculosis capitis, scabies, HSV, insect bites, poison ivy, eczema, and other exudative, pustular, or itching skin diseases.
Group A β-hemolytic streptococcal skin infections (but not those from S. aureus ) are sometimes followed by acute glomerulonephritis (AGN). Nephritogenic streptococci are generally associated with impetigo rather than with upper respiratory tract infections. The important factor predisposing to AGN is the serotype of the streptococcus producing the impetigo. Type 49, 55, 57, and 60 strains and strain M-type 2 are related to nephritis.
The incidence of AGN with impetigo varies from about 2%–5% (10%–15% with nephritogenic strains of streptococcus) and occurs most frequently in childhood, generally before age 6. The prognosis in children is mostly excellent, but in adults it is not as good. Treatment, however early and appropriate, is not believed to reduce the risk of AGN.
Impetigo may simulate several diseases. The circinate patches can be mistaken for tinea, but clinically are more crusted and eroded whereas tinea is scaling. Impetigo may be mistaken for Toxicodendron contact dermatitis due to the vesicles and bullae seen in both. Contact dermatitis is often in linear patterns and on exposed areas whereas impetigo tends to be discrete individual round bullae and erosions. Impetigo is also not associated with the eyelid puffiness, the linear lesions, or the itchiness typically present in dermatitis. In ecthyma, the lesions are crusted ulcers.
When treating impetigo, it is necessary to soak off the crusts frequently, after which an antibacterial ointment should be applied. Applying antibiotic ointment as a prophylactic to sites of skin trauma will prevent impetigo in high-risk children attending day care centers. In one study, infections were reduced by 47% with antibiotic ointment versus 15% with placebo. Additionally, if recurrent staphylococcal impetigo develops, a culture of the anterior nares may yield this organism. Such carrier states may be treated by application of mupirocin ointment to the anterior nares twice daily or by a 10-day course of rifampin, combined with dicloxacillin (for MSSA) or TMP-SMX (for MRSA).
Folliculitis
Folliculitis is the infection of hair follicles. S. aureus is the most common cause of folliculitis.
Superficial pustular folliculitis (impetigo of Bockhart) is a superficial folliculitis with thin-walled pustules at the follicle orifices. Susceptible locations are the extremities and scalp, although it is also seen on the face, especially periorally. These fragile, yellowish white, domed pustules develop in crops and heal in a few days.
Staphylococcal folliculitis can affect any hair-bearing areas, often on the trunk and extremities, but may affect areas such as the eyelashes, axillae, pubis, and thighs ( Figs. 14.5 and 14.6 ). On the pubis, it may be transmitted among sexual partners, and “mini” epidemics of folliculitis and furunculosis of the genital and gluteal areas may be considered a sexually transmitted disease (STD). In the beard area, it is called sycosis vulgaris (sycosis barbae).


Sycosis vulgaris, also known as “barber’s itch” or sycosis barbae, is a perifollicular, chronic, pustular staphylococcal infection of the bearded region characterized by inflammatory papules and pustules, and a tendency to recurrence ( Fig. 14.7 ). The disease begins with erythema and burning or itching, usually on the upper lip near the nose. In 1 or 2 days, one or more pinhead-sized pustules, pierced by hairs, develop. These rupture after shaving or washing and leave an erythematous spot, which is later the site of a fresh crop of pustules. In this manner, the infection persists and gradually spreads, at times extending deep into the follicles. A hairless, atrophic scar bordered by pustules and crusts may result. Marginal blepharitis with conjunctivitis is usually present in severe cases of sycosis.

Sycosis vulgaris is to be distinguished from tinea, acne vulgaris, pseudofolliculitis barbae, and herpetic sycosis. Tinea barbae rarely affects the upper lip, which is a common location for sycosis. In tinea barbae, involvement is usually in the submaxillary region or on the chin, and spores and hyphae are found in the hairs. Pseudofolliculitis barbae manifests torpid papules at sites of ingrowing beard hairs in black men. In HSV infection, duration is usually only a few days, and even in persistent cases there are vesicles, which help to differentiate HSV from sycosis vulgaris.
Staphylococcal folliculitis has also been reported frequently in patients with acquired immunodeficiency syndrome (AIDS) and may be a cause of pruritus. An atypical, plaquelike form has been reported. Treatment of folliculitis is addressed later in this chapter.
Furunculosis
A furuncle, or boil, is an acute, round, tender, circumscribed, perifollicular staphylococcal abscess that generally ends in central suppuration ( Fig. 14.8 ). A carbuncle is merely two or more confluent furuncles, with separate heads.

The lesions begin in hair follicles and often continue for a prolonged period by autoinoculation. Some lesions disappear before rupture, but most undergo central necrosis and rupture through the skin, discharging purulent, necrotic debris. Sites of predilection are the nape, axillae, and buttocks, but furuncles may occur anywhere. The integrity of the skin surface may be impaired by irritation, pressure, friction, hyperhidrosis, dermatitis, dermatophytosis, shaving, and other factors. Local barrier compromise predisposes to infection by providing a portal of entry for the ubiquitous S. aureus. The proximate cause is either contagion or autoinoculation from a carrier focus, usually in the nose or perineum. Furuncles are caused by invasion deeper into the skin and are commonly associated with MRSA. Hospital-acquired MRSA is often more resistant to antibiotics, although in recent years multidrug-resistant strains have been seen in patients who seemingly have no risk factors.
Certain systemic disorders may predispose to furunculosis: alcoholism; malnutrition; blood dyscrasias; disorders of neutrophil function; iatrogenic or other immunosuppression (e.g., AIDS); and diabetes ( Fig. 14.9 ). Patients with several of these diseases, as well as those receiving renal dialysis or isotretinoin or acitretin therapy, are often nasal carriers of S. aureus. Additionally, atopic dermatitis also predisposes to the S. aureus carrier state, which helps explain the observed increases in the incidence of infections in these diseases. Epidemics of staphylococcal infections occur in hospitals. Marked resistance to antibacterial agents in these cases is common. Attempts to control these outbreaks center on meticulous handwashing. In nurseries, a fall in neonatal colonization and infections with S. aureus and non–group A streptococci may be achieved by using a 4% solution of chlorhexidine for skin and umbilical cord care.

Folliculitis may be treated with topical therapy or antibacterial washes. Topical antibiotics such as bacitracin, mupirocin, retapamulin ointment, and topical clindamycin are topical antistaphylococcal antibiotics. In addition to treating the individual pustules, benzoyl peroxide washes, dilute sodium hypochlorite baths ( ![]() US cup bleach added to a 40-gallon tub of bathwater), and chlorhexadine washes are antimicrobials that can be helpful to decolonize the skin surface. Skin surface staphylococcal carriage in abrasions and eczematous areas may be addressed with these topical antibiotics, topical chlorhexidine washes, or dilute sodium hypochlorite (bleach) baths. Mupirocin ointment applied to the anterior nares daily for 5 days along with bleach baths may help prevent recurrence.
US cup bleach added to a 40-gallon tub of bathwater), and chlorhexadine washes are antimicrobials that can be helpful to decolonize the skin surface. Skin surface staphylococcal carriage in abrasions and eczematous areas may be addressed with these topical antibiotics, topical chlorhexidine washes, or dilute sodium hypochlorite (bleach) baths. Mupirocin ointment applied to the anterior nares daily for 5 days along with bleach baths may help prevent recurrence.
Early furuncles are incipient and acutely inflamed, and incision may not be possible because the abscess may not have collected yet; warm compresses and oral antibiotics are administered. When a furuncle has become localized and shows definite fluctuation, incision with drainage is indicated. When the inflammation is acute, hot wet soaks with aluminum acetate solution diluted 1 : 20 are beneficial. An anhydrous formulation of aluminum chloride (Drysol, Xerac-AC) is effective when used once nightly for chronic folliculitis, especially of the buttocks. Although drainage alone is effective for many lesions, a recent randomized trial showed that even in abscesses smaller than 5 cm antibiotic therapy with either clindamycin or TMP-SMX in addition to incision and drainage both increased initial cure rate and decreased rebound rate. In furuncles of the external auditory canal, upper lip, and nose, spontaneous drainage is common and a trial of antibiotic therapy may be attempted before surgical incision and drainage. In these patients, antibiotic ointment should be applied and antibiotics given internally. Warm saline-solution compresses should be applied liberally.
For folliculitis, a first-generation cephalosporin or penicillinase-resistant penicillin (e.g., dicloxacillin) is indicated, unless MRSA is suspected (see earlier). Methicillin-resistant and in some parts of the world vancomycin-resistant strains occur and, if suspected, are treated with trimethoprim-sulfamethoxazole, clindamycin, doxycycline, minocycline or linezolid based on cultures. In patients with staphylococcal infections unresponsive to empiric therapy, antibiotic-resistant strains should be suspected and sensitivities checked.
Despite treatment, recurrences of some boils may be anticipated. Usually, no underlying predisposing disease is present; rather, autoinoculation and intrafamilial spread among colonized individuals are responsible.
One of the most important factors in prevention is to avoid autoinoculation. It is important to emphasize that the nasal carrier state predisposes to chronic furunculosis. The skin surface in the region of the furuncles may be a source of colonization, especially if there are cuts, excoriation, or eczematous changes. In addition, the hazard of contamination from the perianal and intertriginous areas must be considered. In general, indications for elimination of the carriage state are recurrent infection, evidence of spread to others, and high-risk individuals in the household. In addition the tubs of moisturizer used by atopic dermatitis patients can be a source of re-inoculation.
Routine precautions to take in attempting to break the cycle of recurrent folliculitis and furunculosis include a daily 4% for a week chlorhexidine wash, with special attention to the axillae, groin, and perianal area; laundering of bedding and clothing on a daily basis initially; use of bleach baths; and frequent handwashing. Additionally, the application of mupirocin ointment twice daily to the nares of patients and family members every fourth week has been found to be effective. Rifampin for 10 days, combined with dicloxacillin for MSSA or TMP-SMX for MRSA, or low-dose clindamycin for 3 months may also be effective in eradicating the nasal carriage state. The use of bacitracin ointment inside the nares twice daily throughout the course of isotretinoin therapy eliminates, or greatly reduces, the risk of inducing nasal carriage of S. aureus and thus staphylococcal infections.
Pyogenic Paronychia
Paronychia is an inflammatory reaction involving the folds of the skin surrounding the fingernail ( Fig. 14.10 ). It is characterized by acute or chronic tender, and painful swelling of the tissues around the nail that at first will be red and then pustular, caused by an abscess in the nailfold. When the infection becomes chronic, horizontal ridges appear at the base of the nail. With recurrent bouts, new ridges appear.

The primary predisposing factor that is identifiable is separation of the eponychium (cuticle) from the nail plate. The separation is usually caused by trauma as a result of moisture-induced maceration of the nailfolds from frequent wetting of the hands. The relationship is close enough to justify treating chronic paronychia as a work-related condition in bartenders, food servers, nurses, and others who often wet their hands. The moist grooves of the nail and nailfold become secondarily invaded by pyogenic cocci and yeasts. Alternatively children with atopic dermatitis can inoculate bacteria under the nail by scratching infected atopic dermatitis resulting in a paronychia of the distal finger under the nail. The causative bacteria are usually S. aureus, Streptococcus pyogenes, Pseudomonas species, Proteus species, or anaerobes. The pathogenic yeast is most frequently Candida albicans.
The bacteria usually cause acute abscess formation (Staphylococcus), or erythema and swelling (Streptococcus), and C. albicans most frequently causes a chronic swelling. If an abscess is suspected, applying light pressure with the index finger against the distal volar aspect of the affected digit will better demonstrate the extent of the collected pus by inducing a well-demarcated blanching. Smears of purulent material will help confirm the clinical impression and drain the abscess allowing more rapid improvement. Myrmecial warts may mimic paronychia. Subungual black macules, followed by edema, pain, and swelling, have been reported as a sign of osteomyelitis caused by S. aureus or Streptococcus viridans, in children with atopic dermatitis.
Treatment of pyogenic paronychia consists mostly of protection against trauma and concentrated efforts to keep the affected fingernails meticulously dry. Rubber or plastic gloves over cotton gloves should be used whenever the hand must be placed in water. Acutely inflamed pyogenic abscesses should be incised and drained. The abscess may often be opened by pulling the nailfold away from the nail plate but sometime the area must be cleansed with alcohol and a needle or blade be used to drain the abscess. In acute suppurative paronychia, especially if stains show pyogenic cocci, a semisynthetic penicillin or a cephalosporin with excellent staphylococcal activity should be given orally. If these are ineffective, MRSA or a mixed–anaerobic bacteria infection should be suspected. Treatment dictated by the sensitivity of the cultured organism will improve cure rates. Rarely, long-term antibiotic therapy may be required.
Although Candida is the most frequently recovered organism in chronic paronychia, topical or oral antifungals lead to cure in only about 50% of cases. If topical corticosteroids are used to decrease inflammation and allow for tissue repair, there may be a higher cure rate. Often, an antifungal liquid such as miconazole is combined with a topical corticosteroid cream or ointment in candidal chronic paronychia.
Botryomycosis
Botryomycosis is an uncommon, chronic, indolent disorder characterized by nodular, crusted, purulent lesions ( Fig. 14.11 ). Sinuses that discharge sulfur granules are present. These heal with atrophic scars. The granules most frequently yield S. aureus on culture, although cases caused by Pseudomonas aeruginosa, Escherichia coli, Proteus, Bacteroides, and Streptococcus have been reported. Botryomycosis often occurs in patients with altered immune function, such as those with neutrophilic defects. Other predisposing factors include diabetes, HIV infection, alcoholism, and Job syndrome. Appropriate antibiotics, surgical drainage, and surgical excision are methods used to treat botryomycosis.

Blastomycosis-like Pyoderma
Large, verrucous plaques with elevated borders and multiple pustules occur. Most patients with blastomycosis-like pyoderma have some underlying systemic or local host compromise. Bacteria such as S. aureus, P. aeruginosa, Proteus, E. coli, or streptococci may be isolated. Antibiotics appropriate for the organism isolated are curative; however, response may be delayed and prolonged therapy required. Acitretin may also be useful.
Invasive and Systemic Staphylococcal Infections
Pyomyositis
S. aureus abscess formation within the deep, large, striated muscles usually presents with fever and muscle pain and is called pyomyositis. It is typically hematogenous in origin. Pyomyositis is more common in the tropics, where it may affect adults but most frequently occurs in children. In temperate climates, it occurs in children and patients with AIDS. The most frequent site in tropical disease is the thigh, whereas in HIV-infected patients, the deltoid muscle is most often involved, followed closely by the quadriceps. Swelling and occasionally erythema or yellow or purplish discoloration are visible signs of pyomyositis, but these are late findings. Non– S. aureus infections may also cause this same clinical picture. Magnetic resonance imaging (MRI) with gadolinium injection will help delineate the extent of disease. Drainage of the abscess and appropriate systemic antibiotics are the recommended treatment.
Staphylococcal Scalded Skin Syndrome
Staphylococcal scalded skin syndrome (SSSS) is a generalized, confluent, superficially exfoliative disease, occurring most often in neonates and children under 5 years of age. It occurs rarely in adults, usually with renal compromise or immunosuppression as a predisposing factor. SSSS is a febrile, rapidly evolving, desquamative infectious disease in which the skin exfoliates in sheets. SSSS is differentiated from Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) based on the level of the epidermal separation and the fact that SSSS does not affect mucous membranes. The skin does not separate at the dermoepidermal junction, as in toxic (drug-induced) TEN, but within the granular layer. The lesions are thus much more superficial and less severe than in TEN, and healing is much more rapid. SSSS is caused by exfoliative exotoxins types A and B, elaborated by the staphylococcus in remote sites. Usually, staphylococci are present at a distant focus, such as the pharynx, nose, ear, or conjunctiva. Septicemia or a cutaneous infection may also be the causative focus.
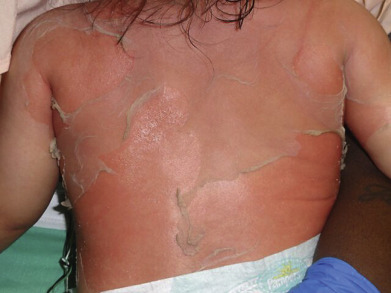
Clinical manifestations of SSSS begin abruptly with skin redness and tenderness concentrated in the perioroficial areas of the face along with the intertriginous areas (neck, groin, axillae and inguinal folds). Fever is variable and the peeling first starts usually around the eyes, nose and mouth. The redness is often described as simulating a sunburn. The areas of red eventually slough with large sheets of skin being lost ( Fig. 14.12 ). There is sparing of the palms, soles, and mucous membranes. Nikolsky sign is positive and blisters will often form where children are picked up or where electrocardiogram leads are attached and removed. Generalized exfoliation follows within the next hours to days, with large sheets of epidermis separating. Rarely true bullae can form if the sloughing skin is not disrupted. Group 2 S. aureus, usually phage types 71 or 55, is the causative agent in most cases. If taken, cultures should be obtained from a primary site of infection if one can be identified or the nares, perianal area or periocular areas. The red skin and desquamation are sterile because the split is caused by the distant effects of the exfoliative toxins, unlike in bullous impetigo, where S. aureus is present in the lesions.
Rapid diagnosis of SSSS can be made by examining frozen sections of a blister roof and observing that the full thickness of the epidermis is not necrotic, as in TEN, but rather is cleaved below the granular layer. The exfoliative toxins A, B, and D specifically cleave desmoglein 1, the antigenic target of autoantibodies in pemphigus foliaceus, thus accounting for the clinical and histologic similarity to pemphigus observed in SSSS and bullous impetigo. In mucous membranes, desmoglein 3 is more prominent than desmoglein 1 and thus because the toxin only targets desmoglien 1 mucous membranes are characteristically spared in SSSS. Treatment of choice is a penicillinase-resistant penicillin such as dicloxacillin combined with fluid therapy and general supportive measures. Addition of clindamycin may help target the bacteria’s ability to form the toxin due to its antiribosomal mechanism but as clindamycin resistance is becoming more common, clindamycin should not be used alone without cultures that prove susceptibility to clindamycin. Recent studies have shown SSSS is more commonly caused by MSSA. If MRSA is cultured and response is sluggish, antibiotics directed according to the susceptibility of the recovered organism are needed. The prognosis is excellent in children, but mortality rates in adults can reach 60%.
Gram-Positive Toxic Shock Syndromes
Toxic shock syndrome (TSS) is an acute, febrile, multisystem illness, with one of its major diagnostic criteria being a widespread macular erythematous eruption. It is usually caused by toxin-producing strains of S. aureus, most of which were initially isolated from the cervical mucosa in menstruating young women. Patients who don’t have TSST-1 toxin antibodies are more susceptible to TSS. Currently, cases are most often caused by infections in wounds, catheters, contraceptive diaphragms, infections of bone, lung, or soft tissue or nasal packing. Mortality in these nonmenstrual cases is higher (up to 20%) compared with menstrual-related cases (<5%), probably as a result of delayed diagnoses. Also, a similar syndrome has been defined in which the cause is group A, or rarely group B, streptococci. This latter multiorgan disease has systemic components similar to classic staphylococcal TSS; however, the infection is usually a rapidly progressive, destructive soft tissue infection such as necrotizing fasciitis. Underlying chronic illness, recent varicella, and use of nonsteroidal antiinflammatory drugs (NSAIDs) may predispose to TSS. It has a case-fatality rate of 30%. The streptococci are usually of M-types 1 and 3, with 80% of the isolates producing pyrogenic exotoxin A.
The Centers for Disease Control and Prevention (CDC) case definition of staphylococcal TSS includes a temperature of 38.9°C (102°F) or higher, an erythematous eruption, desquamation of the palms and soles 1–2 weeks after onset ( Fig. 14.13 ), hypotension, and involvement of three or more other systems: gastrointestinal (GI; vomiting, diarrhea), muscular (myalgias, increased creatinine kinase level), mucous membrane (hyperemia), renal (pyuria without infection or raised creatinine or blood urea nitrogen levels), hepatic (increased bilirubin/serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase), hematologic (platelets <100 000/mm 3 ), or central nervous system (CNS; disorientation). In addition, serologic tests for Rocky Mountain spotted fever, leptospirosis, and rubeola, and cultures of blood, urine, and cerebrospinal fluid (CSF) should be negative. Procalcitonin, an indicator of severe bacterial infection, may be a biologic marker for the toxic shock syndromes. Bulbar conjunctival hyperemia and palmar edema are two additional clinical clues. Streptococcal TSS is defined by isolation of group A β-hemolytic streptococci, hypotension, and two or more of the following: renal impairment, coagulopathy, hepatic involvement, acute respiratory distress syndrome, a generalized erythematous macular eruption that may desquamate, and soft tissue necrosis, myositis, or gangrene.

Histologic findings are spongiosis and neutrophils scattered throughout the epidermis, individual necrotic keratinocytes, perivascular and interstitial infiltrates composed of lymphocytes and neutrophils, and edema of the papillary dermis. TSS must be differentiated from viral exanthems, Kawasaki disease, scarlet fever, recurrent toxin-mediated perianal erythema, drug eruptions, Rocky Mountain spotted fever, systemic lupus erythematosus (SLE), TEN, and SSSS.
Treatment of TSS consists of systemic antibiotics such as vancomycin, which may be combined with nafcillin, in critically ill adult patients; vigorous fluid therapy to treat shock; and drainage of the S. aureus –infected site.
Agarwal V, et al: Pyomyositis. Neuroimaging Clin North Am 2011; 21: 975.
Albrecht VS, et al: Staphylococcus aureus colonization and strain type at various body sites among patients with a closed abscess and uninfected controls at U.S. emergency departments. J Clin Microbiol 2015; 53: 3478.
Al-Najar M, et al: Primary extensive pyomyositis in an immunocompetent patient. Clin Rheumatol 2010; 29: 1469.
Antoniou T, et al: Prevalence of community-associated methicillin-resistant Staphylococcus aureus colonization in men who have sex with men. Int J STD AIDS 2009; 20: 180.
Atanaskova N, et al: Innovative management of recurrent furunculosis. Dermatol Clin 2010; 28: 479.
Berk DR, et al: MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann 2010; 39: 627.
Braunstein I, et al: Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol 2014; 31: 305.
Burdette SD, et al: Staphylococcus aureus pyomyositis compared with non– Staphylococcus aureus pyomyositis. J Infect 2012; 64: 507.
Caum RS, et al: Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357: 380.
Datta R, et al: Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis 2008; 47: 176.
Daum RS, et al: A placebo-controlled trial of antibiotics for smaller skin abscesses. New England Journal of Medicine. 2017 Jun 29; 376(26): 2545-55.
Demos M, et al: Recurrent furunculosis. Br J Dermatol 2012; 167: 725.
Durupt F, et al: Prevalence of Staphylococcus aureus toxins and nasal carriage in furunculosis and impetigo. Br J Dermatol 2007; 157: 43.
Elliott DJ, et al: Empiric antimicrobial therapy for pediatric skin and soft-tissue infections in the era of methicillin-resistant Staphylococcus aureus. Pediatrics 2009; 123: e959.
Elston DM: How to handle a CA-MRSA outbreak. Dermatol Clin 2009; 27: 43.
Forcade NA, et al: Antibacterials as adjuncts to incision and drainage for adults with purulent methicillin-resistant Staphylococcus aureus (MRSA) skin infections. Drugs 2012; 72: 339.
Fritz SA, et al: Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S. aureus. JAMA Pediatr 2014; 168: 1030.
Garcia C, et al: Staphylococcus aureus causing tropical pyomyositis, Amazon Basin, Peru. Emerg Infect Dis 2013; 19: 123.
Gutierrez K, et al: Staphylococcal infections in children, California, USA, 1985–2009. Emerg Infect Dis 2013; 19: 10.
Kato M, et al: Procalcitonin as a biomarker for toxic shock syndrome. Acta Derm Venereol 2010; 90: 441.
Kirkland EB, et al: Methicillin-resistant Staphylococcus aureus and athletes. J Am Acad Dermatol 2008; 59: 494.
Lappin E, et al: Gram-positive toxic shock syndromes. Lancet Infect Dis 2009; 9: 281.
Le Bihan C, et al: Staphylococcus aureus transmission in the intensive care unit. Ann Infect 2017; 1: 3.
Low DE: Toxic shock syndrome. Crit Care Clin 2013; 29: 651.
Mitsionis GI, et al: Pyomyositis in children. J Pediatr Surg 2009; 44: 2173.
Neylon O, et al: Neonatal staphylococcal scalded skin syndrome. Eur J Pediatr 2010; 169: 1503.
Otto M: Staphylococcus aureus toxins. Curr Opin Microbiol 2014; 17: 32.
Ouchi T, et al: A case of blastomycosis-like pyoderma caused by mixed infection of Staphylococcus epidermidis and Trichophyton rubrum. Am J Dermatopathol 2011; 33: 397.
Patel GK, et al: Staphylococcal scalded skin syndrome. Am J Med 2010; 123: 505.
Patrizi A, et al: Recurrent toxin-mediated perineal erythema. Arch Dermatol 2008; 144: 239.
Piechowicz L, et al: Outbreak of bullous impetigo caused by Staphylococcus aureus strains of phage type 3C/71 in a maternity ward linked to nasal carriage of a healthcare worker. Eur J Dermatol 2012; 22: 252.
Rertveit S, et al: Impetigo in epidemic and nonepidemic phases. Br J Dermatol 2007; 157: 100.
Ritting AW, et al: Acute paronychia. J Hand Surg Am 2012; 37: 1068.
Rubenstein E, et al: Botryomycosis-like pyoderma in the genital region of a human immunodeficiency virus (HIV)–positive man successfully treated with dapsone. Int J Dermatol 2010; 49: 842.
Scheinpflug K, et al: Staphylococcal scalded skin syndrome in an adult patient with T-lymphoblastic non-Hodgkin’s lymphoma. Oncologie 2008; 31: 616.
Sica RS, et al: Prevalence of methicillin-resistant Staphylococcus aureus in the setting of dermatologic surgery. Dermatol Surg 2009; 35: 420.
Van Rijen M, et al: Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev 2008; 4: CD006216.
Wilkins AL, et al: Toxic shock syndrome. J Infect 2017; 74: S147.
Streptococcal Skin Infections
Specific diseases caused by direct infection with Streptococcus pyogenes and its toxins, as discussed in this chapter, also have immune-mediated consequences, including acute rheumatic fever, chronic rheumatic heart disease, guttate psoriasis and acute poststreptococcal glomerulonephritis. The first two only occur after pharyngitis or tonsillitis but guttate psoriasis can occur after perianal streptococcal infection especially in younger children. Although most of such complications occur in resource-poor countries, the global burden of these sequelae is significant.
Ecthyma
Ecthyma is an ulcerative streptococcal or less commonly staphylococcal pyoderma. The disease begins with a vesicle or vesicopustule, which enlarges and in a few days becomes thickly crusted. The vesicles of streptococcal ecthyma can mimic HSV especially in the setting of atopic dermatitis. When the crust is removed, there is a superficial, saucer-shaped ulcer with a raw base and elevated edges ( Fig. 14.14 ). In urban areas, these lesions are caused by S. aureus and are seen in intravenous drug users and HIV-infected patients.

The lesions tend to heal after a few weeks, leaving scars, but rarely may proceed to gangrene when resistance is low. Debilitated patients often have a focus of pyogenic infection elsewhere. Local adenopathy may be present. Uncleanliness, malnutrition, and trauma are predisposing causes.
Treatment includes cleansing with soap and water after soaking off the crust with compresses, followed by the application of mupirocin, retapamulin, or bacitracin ointment, twice daily. Oral dicloxacillin or a first-generation cephalosporin is also indicated, with adjustments made according to the cultured organism’s susceptibilities.
Scarlet Fever
Scarlet fever is a diffuse, erythematous exanthem that occurs during the course of streptococcal pharyngitis. It affects primarily children, who develop the eruption 24–48 hours after onset of pharyngeal symptoms. The tonsils are red, edematous, and covered with exudate. The tongue has a white coating through which reddened, hypertrophied papillae project, giving the so-called white strawberry tongue appearance (as opposed to the red strawberry tongue of Kawasaki that lacks an exudate). By the fourth or fifth day the coating disappears, the tongue is bright red, and the red strawberry tongue remains.
The cutaneous eruption begins on the neck, then spreads to the trunk and finally the extremities ( Fig. 14.15 ). Within the widespread erythema are 1–2 mm papules, which give the skin a rough, sandpaper quality. There is accentuation over the skinfolds, and a linear petechial eruption, called Pastia lines, is often present in the antecubital and axillary folds. There is facial flushing and circumoral pallor. A branny desquamation occurs as the eruption fades, with peeling of the palms and soles taking place about 2 weeks after the acute illness. The latter may be the only evidence that the disease has occurred.

The eruption is produced by erythrogenic exotoxin-producing group A streptococci. Cultures of the pharynx will recover these organisms. Rarely, scarlet fever may be related to a surgical wound or burn infection with streptococci. An elevated antistreptolysin O or DNase B titer may provide evidence of recent infection if cultures are not taken early. A condition known as staphylococcal scarlatina has been described that mimics scarlet fever; however, the strawberry tongue is not seen.
Penicillin, erythromycin, or dicloxacillin treatment is curative for scarlet fever, and the prognosis is excellent.
Recurrent Toxin-Mediated Perineal Erythema
This condition manifests as a perineal, erysipelas-like erythema that resolves with desquamation. Strawberry tongue, erythema of the hands with desquamation, and a mild fever 1 or 2 days before the eruption are other signs. In some patients, a staphylococcal or streptococcal pharyngitis, impetigo, or perianal streptococcal dermatitis is present. There may be recurrences in individual patients. Streptococcal pyrogenic exotoxins A and B or TSS toxin 1 may be responsible for the skin findings.
Erysipelas
Also once known as St. Anthony’s fire and ignis sacer, erysipelas is an acute β-hemolytic group A streptococcal infection of the skin involving the superficial dermal lymphatics. Occasional cases caused by streptococci of group C or G are reported in adults. Group B streptococcus is often responsible in the newborn and may be the cause of abdominal or perineal erysipelas in postpartum women. It is characterized by local redness, heat, swelling, and a highly characteristic raised, indurated border ( Fig. 14.16A ). The onset is often preceded by prodromal symptoms of malaise for several hours, which may be accompanied by a severe constitutional reaction with chills, high fever, headache, vomiting, and joint pains. There is usually a polymorphonuclear leukocytosis of 20,000 cells/mm 3 or more. However, many cases present solely as an erythematous lesion without associated systemic complaints.


The skin lesions may vary from transient hyperemia followed by slight desquamation to intense inflammation with vesicles or bullae. The eruption begins at any one point as an erythematous patch and spreads by peripheral extension. In the early stages, affected skin is bright red, hot to the touch, branny, and swollen. A distinctive feature of the inflammation is the advancing edge of the patch. This is raised and sharply demarcated and feels like a wall to the palpating finger. In some cases, vesicles or bullae that contain seropurulent fluid occur and may result in local gangrene.
The legs and face are the most common sites affected. On the face, the inflammation generally begins on the cheek near the nose or in front of the lobe of the ear and spreads upward to the scalp, with the hairline sometimes acting as a barrier against further extension. On the legs, edema and bullous lesions are prominent features in many patients ( Fig. 14.16B ). Septicemia, deep cellulitis, necrotizing fasciitis, and abscess formation may be complications, especially in obese patients and those with chronic alcohol abuse. Predisposing causes are surgical wounds, which may lead to gluteal and thigh involvement; fissures in the nares, in the auditory meatus, under the earlobes, on the anus or penis, and between or under the toes, usually the little toe; abrasions or scratches; venous insufficiency; obesity; lymphedema; and chronic leg ulcers.
Recognition of erysipelas generally is not difficult. It may be confused with contact dermatitis from plants, drugs, or dyes and with angioneurotic edema, but with each of these, fever, pain, and tenderness are absent and itching is severe. A butterfly pattern on the face may mimic lupus erythematosus, and ear involvement may suggest relapsing polychondritis.
Systemic penicillin is rapidly effective although since S. Aureus cellulitis maybe in the differnetial diagnosis, use of an antibiotic that will cover both is often indicated. Improvement in the general condition occurs in 24–48 hours, but resolution of the cutaneous lesion may require several days. Vigorous treatment with antibiotics should be continued for at least 10 days. Locally, ice bags and cold compresses may be used. Leg involvement, especially when bullae are present, will more likely require hospitalization with intravenous antibiotics. Elderly patients, those with underlying immunocompromise, a longer duration of illness before presentation, and patients with leg ulcers will require longer inpatient stays. A small group will have recurrent disease, in whom long-term antibiotic prophylaxis may be beneficial.
Cellulitis
Cellulitis is a suppurative inflammation involving the subcutaneous tissue. Often, cellulitis follows a wound. On the leg, tinea pedis is a common portal of entry. Mild local erythema and tenderness, malaise, fever and chills may be present but are not necessary for diagnosis. The erythema rapidly becomes intense and spreads ( Fig. 14.17 ). The area may infiltrated and pit on pressure. The central part may become nodular and surmounted by a vesicle that ruptures and discharges pus and necrotic material. Streaks of lymphangitis may spread from the area to the neighboring lymph glands ( Fig. 14.18 ). Gangrene, metastatic abscesses, and severe sepsis may follow. These complications are unusual in immunocompetent adults, but children and immunocompromised adults are at higher risk.


The diagnosis of bacterial cellulitis is usually made on clinical grounds. It is uncommon for blood studies, including cultures, and skin biopsies or aspirates to be positive. If, however, an open wound is present, a culture may be positive. Streptococci continue to cause approximately 75% of cases and staphylococci the majority of the remainder. Stasis dermatitis may mimic cellulitis. It does not hurt or cause fever, may be circumferential or centered over the medial malleoli, and is usually bilateral. Allergic contact dermatitis is itchy but not painful. Eosinophilic cellulitis is an exuberant response to an insect bite and can simulate cellulitis but typically has less pain and instead of neutrophilia, there is often an eosinophilia. Erythema migrans (Lyme disease) can also present with a red patch but is typically less painful than cellulitis.
Patients with cellulitis without systemic toxicity can be managed as outpatients. Initial empiric therapy with dicloxacillin or cephalexin for 5 days will usually suffice. If MRSA is strongly suspected because of risk factors, treatment strategies are as outlined for staphylococcal infections at the start of this chapter.
Chronic Recurrent Erysipelas, Chronic Lymphangitis
Erysipelas or cellulitis may be recurrent. Predisposing factors include alcoholism, diabetes, immunodeficiency, tinea pedis, venous stasis, lymphedema with or without lymphangiectasias, prosthetic surgery of the knee, a history of saphenous phlebectomy, lymphadenectomy, or irradiation. Chronic lymphedema is the end result of recurrent bouts of bacterial lymphangitis and obstruction of the major lymphatic channels of the skin. The final result is a permanent hypertrophic fibrosis called elephantiasis nostras. It must be differentiated from lymphangioma, acquired lymphangiectasia, and other causes such as neoplasms or filariasis.
During periods of active lymphangitis, antibiotics in large doses are beneficial, and their use must be continued in smaller maintenance doses, for long periods to achieve their full benefits. Compression therapy to decrease lymphedema may aid in the prevention of recurrence.
Necrotizing Fasciitis
Necrotizing fasciitis is an acute necrotizing infection involving the fascia. It may follow surgery, perforating trauma or may occur de novo. In young children, there are peaks of incidence in the neonatal period and children 1–2 years of age and the fasciitis was more often truncal and caused by one pathogen than in older patients. Within 24–48 hours, redness, pain, and edema quickly progress to central patches of dusky-blue discoloration, with or without serosanguineous blisters ( Fig. 14.19 ). Anesthesia of the involved skin is characteristic. By the fourth or fifth day, these purple areas become gangrenous. Many forms of virulent bacteria have been cultured from necrotizing fasciitis and it may be polymicrobial. Pathogens isolated include microaerophilic β-hemolytic streptococci, hemolytic staphylococcus, coliforms, enterococci, Pseudomonas, and Bacteroides. Both aerobic and anaerobic cultures should always be taken.

Early surgical debridement is an essential component of successful therapy. Laboratory studies may help in assessing the risk of a patient having necrotizing fasciitis. One scoring system gives points for abnormalities in C-reactive protein, white blood cell count, hemoglobin, sodium, creatinine, and glucose. Based on the total score, patients are stratified into low-risk, medium-risk, and high-risk categories. Aside from direct surgical visualization, the most definitive confirmatory test is MRI. At the bedside, the clinician may infiltrate the site with anesthetic, make a 2-cm incision down to the fascia, and probe with the finger. Lack of bleeding, a murky discharge, and lack of resistance to the probing finger are ominous signs. If done, a biopsy should be obtained from normal-appearing tissue near the necrotic zone. Treatment should include early surgical debridement, appropriate IV antibiotics, and supportive care. Intravenous immune globulin (IVIG) has not been shown to improve mortality rates. Mortality rate may be 20% even in the best of circumstances. Poor prognostic factors are age over 50, underlying diabetes or atherosclerosis, delay of more than 7 days in diagnosis and surgical intervention, and infection on or near the trunk rather than the more often involved extremities. Neonatal necrotizing fasciitis most frequently occurs on the abdominal wall and has a higher mortality rate than in adults.
Blistering Distal Dactylitis
Blistering distal dactylitis is characterized by tense superficial blisters occurring on a tender erythematous base over the volar fat pad of the phalanx of a finger or thumb or occasionally a toe ( Fig. 14.20 ). The typical patient is age 2–16 years. Group A β-hemolytic streptococci is most typical although if more purulent Bullous impetigo from S. aureus can have a similar appearance. These organisms may be cultured from blister fluid.

Perianal Dermatitis
Clinically, perineal dermatitis presents most often as a superficial, perianal, well-demarcated rim of erythema ( Fig. 14.21 ); fissuring may also be seen. Pain or tenderness, especially prominent on defecation, may lead to fecal retention in affected patients, who are usually between ages 1 and 8. It may not resemble cellulitis, but rather dermatitis. It may also affect the vulvar and penile tissues. Group A streptococci are most often the cause; however, S. aureus may be recovered rarely, and when this occasionally occurs in adults, the usual cause is group B streptococci. The vast majority of infections are caused by streptococci, so a systemic penicillin or erythromycin combined with a topical antiseptic or antibiotic is the treatment of choice. Perianal streptococcal infections can lead to flares of guttate psoriasis especially in young children who are less likely to get streptococcal pharyngitis. The duration should be 14–21 days, depending on clinical response. Posttreatment swabs and urinalysis to monitor for poststreptococcal glomerulonephritis are recommended.

Streptococcal Intertrigo
Infants and young children may develop a fiery-red erythema and maceration in the neck, axillae, or inguinal folds. There are no satellite lesions. It may be painful and have a foul odor. Streptococcal intertrigo can be mistaken for candidal intertrigo but streptococcal infections are generally more painful and macerated and lack the satellite pustules of candida infections. Group A β-hemolytic streptococci are the cause, and topical antibiotics and oral penicillin are curative in streptococcal intertrigo.
Erythema Marginatum
Delayed nonsuppurative sequelae of streptococcal infections include erythema nodosum, poststreptococcal glomerulonephritis, and rheumatic fever. The latter only follows pharyngitis or tonsillitis, but two skin signs are among the diagnostic criteria of rheumatic fever: erythema marginatum and subcutaneous nodules. The remaining major signs making up the revised Jones criteria are carditis, polyarthritis, and chorea. Erythema marginatum appears as a spreading, patchy erythema that migrates peripherally and often forms polycyclic configurations ( Fig. 14.22 ). It is evanescent, appearing for a few hours or days on the trunk or proximal extremities and typically spares the face. Heat may make it more visible, and successive crops may appear over several weeks. It is usually part of the early phase of the disease, coexisting with carditis but usually preceding the arthritis. Children younger than 5 years are more likely to manifest the eruption than older patients. A skin biopsy will show a perivascular and interstitial polymorphonuclear leukocyte predominance. In contrast, the subcutaneous nodules occur over bony prominences and appear as a late manifestation. The lesions of erythema marginatum usually are asymptomatic and resolve spontaneously.

Group B Streptococcal Infection
Streptococcus agalactiae is the major cause of bacterial sepsis and meningitis in neonates. It may cause any type of cellulitis including orbital cellulitis or facial erysipelas in these patients. Up to 25% of healthy adults harbor group B streptococci in their genital or GI tract. A guideline by Money et al. emphasizes prevention of such disastrous infections in the newborn through culture identification of mothers at risk and prophylactic antibiotics before delivery in culture-positive women. Skin and soft tissue infections caused by Strep. agalactiae in adult commonly can lead to invasive disease and bacteremia. S. agalactiae has been reported to cause balanitis, vulvar pain due to fine fissures with minimal erythema, toxic shock–like syndrome, cellulitis, perianal dermatitis, recurrent erysipelas, or blistering dactylitis in adults. Diabetes mellitus, neurologic impairment, cirrhosis, and peripheral vascular disease predispose patients to infection with S. agalactiae. In the postpartum period, abdominal or perineal erysipelas may be caused by this organism.
Streptococcus iniae Infections
Cellulitis of the hands may be caused by the fish pathogen Streptococcus iniae. The bacteria is inoculated when punctures and cuts occur while cleaning freshly killed fish before cooking. Freshwater dolphins and farm-raised fish both can carry the bacteria. Preparation of other raw seafood may also lead to S. iniae infection. Within 24 hours, fever, lymphangitis, and cellulitis without skin necrosis or bulla formation occur. Treatment with penicillin is curative. A similar scenario occurred with a newly described species, Streptococcus hongkongensis sp nov. Amoxicillin-clavulanate was effective in the one reported case.
Bachmeyer C, et al: Relapsing erysipelas of the buttock due to Streptococcus agalactiae in an immunocompetent woman. Clin Exp Dermatol 2009; 34: 267.
Buckland GT 3rd, et al: Persistent periorbital and facial lymphedema associated with group A beta-hemolytic streptococcal infection. Ophthalm Plast Reconstr Surg 2007; 23: 161.
Carvalho SM, et al: Rheumatic fever. Rev Bras Rheumatol 2012; 52: 241.
Del Giudice P, et al: Severe relapsing erysipelas associated with chronic Streptococcus agalactiae vaginal colonization. Clin Infect Dis 2006; 43: e67.
Diaz JH: Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med 2014; 21: 207.
El Bouch R, et al: A case of recurrent toxin-mediated perineal erythema. Arch Dis Child 2013; 98: 776.
Fretzayas A, et al: MRSA blistering distal dactylitis and review of reported cases. Pediatr Dermatol 2011; 28: 433.
Glatz M, et al: Erysipelas of the thigh and gluteal region. Dermatology 2012; 225: 277.
Gunderson CG, et al: A systematic review of bacteremias in cellulitis and erysipelas. J Infect 2012; 64: 148.
Hakkarainen TW, et al: Necrotizing soft tissue infections. Curr Probl Surg 2014; 51: 344.
Hirschmann JV, et al: Lower limb cellulitis and its mimics. J Am Acad Dermatol 2012; 67: 163. e1.
Jamal N, et al: Necrotizing fasciitis. Pediatr Emerg Care 2011; 27: 1195.
Kadri SS, et al: Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with vasopressor-dependent shock. 2016; 64: 877.
Kahlke V, et al: Perianal streptococcal dermatitis in adults. Colorectal Dis 2013; 15: 602.
Kilburn SA, et al: Interventions for cellulitis and erysipelas. Cochrane Database Syst Rev 2010; 6: CD004299.
Krasagakis K, et al: Local complications of erysipelas. Clin Exper Dermatol 2010; 36: 351.
Kutsuna S, et al: Scarlet fever in an adult. Intern Med 2014; 53: 167.
Lau SK, et al: Streptococcus hongkongensis sp. nov., isolated from a patient with an infected puncture wound and from a marine flatfish. Int J Syst Evol Microbiol 2013; 63: 2570.
Laucerotto L, et al: Necrotizing fasciitis. J Trauma 2012; 72: 560.
Mirowski GW, et al: Cutaneous vulvar streptococcal infection. J Low Genit Tract Dis 2012; 16: 281.
Mittal MK, et al: Group B streptococcal cellulitis in infancy. Pediatr Emerg Care 2007; 23: 324.
Money D, et al: The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can 2013; 35: 939.
Picard D, et al: Risk factors for abscess formation in patients with superficial cellulitis (erysipelas) of the leg. Br J Dermatol 2012; 168: 859.
Raff AB, Kroshinsky D: Cellulitis. JAMA 2016; 316: 325.
Ralph AP, et al: Group A streptococcal diseases and their global burden. Curr Top Microbiol Immunol 2013; 368: 1.
Ruppen C, et al: Osteoarticular and skin and soft-tissue infections caused by Streptococcus agalactiae in elderly patients are frequently associated with bacteremia. Diagn Microbiol Infect Dis 2018; 90: 55.
Silverman RA, et al: Streptococcal intertrigo of the cervical folds in a five-month-old infant. Pediatr Infect Dis J 2012; 31: 872.
Singer AJ, et al: Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med 2014; 370: 1039.
Sun JR, et al: Invasive infection with Streptococcus iniae in Taiwan. J Med Microbiol 2007; 56: 1246.
Thomas K, et al: Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg. Br J Dermatol 2012; 166: 169.
Thomas KS, et al: Penicillin to prevent recurrent leg cellulitis. N Engl J Med 2013; 368: 1695.
Wasserzug O, et al: A cluster of ecthyma outbreaks caused by a single clone of invasive and highly infective Streptococcus pyogenes. Clin Infect Dis 2009; 48: 1213.
Zundel S, et al: Diagnosis and treatment of pediatric necrotizing fasciitis. Eur J Pediatr Surg 2017; 27: 127.
Miscellaneous Gram-Positive Skin Infections
Erysipeloid of Rosenbach
The most frequent form of erysipeloid is a sharply marginated and often polygonal patch of purplish erythema ( Fig. 14.23 ). The first symptom is pain at the site of inoculation, followed by swelling and erythema. The erythema slowly spreads to produce a sharply defined, slightly elevated zone that extends peripherally as the central portion fades away. If the finger is involved, the swelling and tenseness make movement difficult. Vesicles frequently occur.

Another characteristic of the disease is its migratory nature; new purplish red patches appear at nearby areas. If the infection originally involved one finger, eventually all the fingers and the dorsum of the hand, the palm, or both may become infected, with the erythema appearing and disappearing; or extension may take place by continuity. The disease involutes without desquamation or suppuration. A diffuse or generalized eruption in regions remote from the site of inoculation may occur, with fever and arthritic symptoms. Rarely, septicemia may eventuate in endocarditis, with prolonged fever and constitutional symptoms.
The infection is caused by Erysipelothrix rhusiopathiae. E. rhusiopathiae is present on dead matter of animal origin. Swine are more frequently infected than any other animal. Turkeys are also often infected, and the disease may arise from handling contaminated dressed turkeys. It is also present in the slime of saltwater fish, on crabs, and on other shellfish. The disease is widespread along the entire Atlantic seacoast among commercial fishermen. The infection also occurs among veterinarians and in the meatpacking industry, principally from handling pork products. E. rhusiopathiae is a rod-shaped, nonmotile, gram-positive organism that tends to form long-branching filaments. The organism is cultured best on media fortified with serum, at room temperature.
Treatment
The majority of the mild cases of erysipeloid run a self-limited course of about 3 weeks. In some patients, after a short period of apparent cure, the eruption reappears either in the same area or, more likely, in an adjacent, previously uninvolved area. Penicillin, 1 g/day for 5–10 days, or ampicillin, 500 mg four times daily, is the best treatment for localized disease. If penicillin cannot be used, imipenem or piperacillin-tazobactam are effective. For systemic forms, 12–20 million units/day of IV penicillin for up to 6 weeks may be necessary.
Veraldi S, et al: Erysipeloid. Clin Exp Dermatol 2009; 34: 859.
Werner K, et al: Erysipeloid ( Erysipelothrix rhusiopathiae infection) acquired from a dead kakapo. Arch Dermatol 2011; 147: 1456.
Pneumococcal Cellulitis
Cellulitis may be caused by Streptococcus pneumoniae. Children present with facial or periorbital cellulitis, which may manifest a violaceous hue or bullae. Most patients under 36 months of age are previously healthy. Fever, leukocytosis, and septicemia are almost universal. Response to treatment with penicillin or, in resistant cases, vancomycin is excellent. Most responsible strains are included in the pneumococcal vaccine, so this condition has become rare, as has Haemophilus influenzae cellulitis. Chronically ill or immunosuppressed adults may develop pneumococcal cellulitis or other soft tissue infections, such as abscesses or pyomyositis. In patients with diabetes or substance abuse, extremity involvement is the rule, whereas in those with SLE, nephritic syndrome, hematologic disorders, or HIV disease, the head, neck, and upper torso are typically affected. Skin involvement may also be seen as a surgical wound infection. Because septicemia, tissue necrosis, and suppurative complications are common, aggressive management is crucial, with surgical drainage and IV antibiotics directed at the susceptibility of the cultured organism.
Garcia-Lechuz JM, et al: Streptococcus pneumoniae skin and soft tissue infections. Eur J Clin Microbiol Infect Dis 2007; 26: 247.
Khan T, Martin DH: Streptococcus pneumoniae soft tissue infections in human immunodeficiency virus. Am J Med Sci 2011; 342: 235.
Anthrax
Cutaneous anthrax is uncommon in much of the world; human infection generally results from contact with infected animals or the handling of hides or other animal products from stock that has died from splenic fever. Cattlemen, woolsorters, tanners, butchers, and workers in the goat-hair industry are most liable to infection. Human-to-human transmission has occurred from contact with dressings from lesions. The spores of Bacillus anthracis persist and may be aerosolized, so it is a bioterrorism threat.
Anthrax is an acute infectious disease characterized by a rapidly necrosing, painless eschar with associated edema and suppurative regional adenitis ( Fig. 14.24 ). Four forms of the disease occur in humans: cutaneous, accounting for 95% of cases worldwide and almost all U.S. cases; inhalational, known as woolsorter’s disease; gastrointestinal, the first case of which occurred in the United States in 2010; and injectional, more than 50 cases of which occurred in the United Kingdom and Germany. It is a complication of IV drug use, primarily in heroin addicts.

The first clinical manifestation of the cutaneous form is an inflammatory papule, which begins about 3–7 days after inoculation, usually on an exposed site. The inflammation develops rapidly, and a bulla surrounded by intense edema and infiltration forms within another 24–36 hours. It then ruptures spontaneously, and a black eschar is visible, surrounded by vesicles situated on a red, hot, swollen, and indurated area. The lesion is neither tender nor painful. This is of diagnostic importance. Pustules are almost never present. The regional lymph glands become tender and enlarged and frequently suppurate.
In patients with severe disease, the inflammatory signs increase; there is extensive edematous swelling, and other bullae and necrotic lesions develop, accompanied by a high temperature and prostration, terminating in death in a few days or weeks. This may occur in up to 20% of untreated patients. In mild cases, the constitutional symptoms are sometimes slight; the gangrenous skin sloughs, and the resulting ulcer heals.
Internally, inhalational anthrax and GI infection are manifested as necrotic hemorrhagic lesions followed by bacteremia, always ending in death. Patients with injectional disease present with fever and swelling of an extremity.
The disease is produced by Bacillus anthracis, a large, square-ended, rod-shaped gram-positive organism that occurs singly or in pairs in smears from the blood or in material from the local lesion, or in long chains on artificial media, where it tends to form spores. The bacillus possesses three virulence factors: a polyglutamate acid capsule inhibiting phagocytosis; an edema toxin, composed of edema factor and a transport protein termed protective antigen; and lethal toxin, composed of lethal factor plus protective antigen.
A biopsy should be obtained. This allows for immunohistochemical and PCR studies, as well as routine histology and tissue Gram stain. Microscopically, there is loss of the epidermis at the site of the ulcer, with surrounding spongiosis and intraepidermal vesicles. Leukocytes are abundant in the epidermis. The dermis is edematous and infiltrated with abundant erythrocytes and neutrophils. Vasodilation is marked. The causative organisms are numerous and are easily seen, especially with Gram stain.
The diagnosis is made by demonstration of the causative agent in smears and cultures of the local material. The characteristic gangrenous lesion, surrounded by vesiculation, intense swelling and redness, lack of pain, and the patient’s occupation are accessory factors. PCR identification is readily available due to its bioterrorism threat. Staphylococcal carbuncle is the most easily confused entity, but here tenderness is prominent.
Early diagnosis and prompt treatment with ciprofloxacin (500 mg) or doxycycline (100 mg), twice daily for 60 days, are curative in the cutaneous form when there are no systemic symptoms, lesions are not on the head or neck and are without significant edema, and the patient is not a child younger than 2 years. In these latter conditions, more aggressive IV therapy is required, as outlined in the CDC management guidelines available at the CDC website. Asymptomatic exposed individuals should be given prophylactic treatment with a 6-week course of doxycycline or ciprofloxacin. A vaccine is available.
Aquino LL, et al: Cutaneous manifestations of category A bioweapons. J Am Acad Dermatol 2011; 65: 1213.
Booth M, et al: Confirmed Bacillus anthracis infection among persons who inject drugs, Scotland, 2009-2010. Emerg Infect Dis 2014; 20: 1452.
Denk A, et al: Cutaneous anthrax. Cutan Ocul Toxicol 2016; 35: 177.
Doganay M, Demiraslan H: Human anthrax as a re-emerging disease. Recent Pat Antiinfect Drug Discov 2015; 10: 10.
Listeriosis
Listeria monocytogenes is a gram-positive bacillus with rounded ends that may be isolated from soil, water, animals, and asymptomatic individuals. Human infection probably occurs through the GI tract; in the majority of patients, however, the portal of entry is unknown. Infections in humans usually produce meningitis or encephalitis with monocytosis. Risk factors include alcoholism, advanced age, pregnancy, and immunosuppression.
Cutaneous listeriosis is a rare disease. Veterinarians may contract cutaneous listeriosis from an aborting cow. The organism in the skin lesions is identical to that isolated from the fetus. The eruption consists of erythematous tender papules and pustules scattered over the hands and arms. There may be axillary lymphadenopathy, fever, malaise, and headache.
Neonates are also at risk. Endocarditis, meningitis, and encephalitis caused by Listeria may be accompanied by petechiae, pustules, and papules in the skin.
Cases of listeriosis may easily be missed on bacteriologic examination, because the organism produces few colonies on original culture and may be dismissed as a streptococcus or as a contaminant diphtheroid because of the similarity in gram-stained specimens. Serologic tests help to make the diagnosis.
Listeria monocytogenes is sensitive to most antibiotics. Ampicillin combined with gentamicin is the treatment of choice, and TMP-SMX is an effective alternate.
Godshall CE, et al: Cutaneous listeriosis. J Clin Microbiol 2013; 51: 3591.
Zelenik K, et al: Cutaneous listeriosis in a veterinarian with evidence of zoonotic transmission. Zoonoses Public Health 2014; 61: 238.
Cutaneous Diphtheria
Cutaneous diphtheria is common in tropical areas. Most of the U.S. cases are in nonimmunized migrant farmworker families and in elderly alcoholics. Travelers to developing countries may also import disease.
Skin lesions are caused by infection with Corynebacterium diphtheria or ulcerans, usually in the form of ulcerations. The ulcer is punched out and has hard, rolled, elevated edges with a pale-blue tinge. Often, the lesion is covered with a leathery, grayish membrane. Regional lymph nodes may be affected. Other types of skin involvement include eczematous, impetiginous, vesicular, and pustular lesions. Postdiphtherial paralysis and potentially fatal cardiac complications may occur. These are mediated by a potent exotoxin, which stops protein production at the ribosome level.
Treatment consists of intramuscular (IM) injections of diphtheria antitoxin, 20,000–40,000 U, after a conjunctival test has been performed to rule out hypersensitivity to horse serum. Erythromycin, 2 g/day, is the drug of choice, unless large proportions of resistant organism are known in the area. In severe cases, IV penicillin G, 600,000 U/day for 14 days, is indicated. Rifampin, 600 mg/day for 7 days, will eliminate the C. diphtheria carrier state. The reservoir for C. ulcerans is thought to be animals.
Abdul Rahim NR, et al: Toxigenic cutaneous diphtheria in a returned traveler. Commun Dis Intell Q Rep 2014; 38: E298.
Lowe CF, et al: Cutaneous diphtheria in the urban poor populations of Vancouver, British Columbia. J Clin Microbiol 2011: 49: 2664.
Moore LSP, et al: Corynebacterium ulcerans cutaneous diphtheria. Lancet Infect Dis 2015; 15: 1100.
Corynebacterium jeikeium Sepsis
Corynebacterium jeikeium colonizes the skin of healthy individuals, with the highest concentration being in the axillary and perineal areas. Hospitalized patients are more heavily colonized. Patients with granulocytopenia, indwelling catheters, prosthetic devices, exposure to multiple antibiotics, and valvular defects are at highest risk for the development of sepsis or endocarditis. A papular eruption, cellulitis, subcutaneous abscesses, tissue necrosis, hemorrhagic pustules, and palpable purpura may be seen on the skin. Vancomycin is the drug of choice. Mortality rate is greater than 30% in those with leukopenia.
Olson JM, et al: Cutaneous manifestations of Corynebacterium jeikeium sepsis. Int J Dermatol 2009; 48: 886.
Erythrasma
Erythrasma is characterized by sharply delineated, dry, brown, slightly scaling patches occurring in the intertriginous areas ( Fig. 14.25 ), especially the axillae, the genitocrural crease, and the webs between the fourth and fifth toes and less often the third and fourth toes. There may also be patches in the intergluteal cleft, perianal skin, and inframammary area. The vulvar mucosa can be affected by thick, desquamating, yellowish hyperkeratosis. Rarely, widespread eruptions with lamellated plaques occur. The lesions are asymptomatic except in the groin, where there may be some itching and burning. Patients with extensive erythrasma have been found to have diabetes mellitus or other debilitating diseases.











