Summary
Deoxycholic acid, an adipocytolic agent, is the first injectable therapy approved by the US Food and Drug Administration (FDA) to treat submental fullness (SMF). Excessive SMF contributes to facial aging, which influences social behaviors, confidence, and overall sense of attractiveness. The safety profile and efficacy of synthetic deoxycholic acid (ATX-101) were studied in five phase III double-blinded clinical trials. Injection site reactions like pain, swelling, and bruising are common side effects, managed traditionally with ice and NSAIDs. More serious complications like marginal mandibular nerve injury or dysphagia can occur rarely and are minimized with thorough preprocedure assessment and proper injection technique.
8 Deoxycholic Acid
8.1 Description of Technology/Procedures
8.1.1 Introduction
Excess submental fullness (SMF) and blunting of the cervicomental angle contribute to the aging face, influencing social behaviors, confidence, and overall sense of attractiveness. 1 , 2 SMF is not necessarily due to excess body weight, but rather age-related fat distribution, and decreased skin and soft tissue elasticity. 2 Because SMF is multifactorial, it is difficult to target with diet and exercise routines alone. 2 , 3 Surgical procedures aimed to reduce SMF include liposuction and submentoplasty. However, these invasive methods are associated with long recovery times and the potential for complications including postoperative bleeding, hematoma, pain, and infection. 4 In 2015, the Food and Drug Administration (FDA) approved synthetic deoxycholic acid (ATX-101) as the first injectable therapy to treat SMF. 5 , 6 , 7 In five phase III double-blinded clinical trials conducted in North America and Europe, deoxycholic acid was injected into the submental fat and reduced SMF compared to placebo. 8 , 9 , 10 , 11 , 12 , 13 Patients and physicians reported subjective and objective improvement in SMF following treatment. 8 , 9 , 10 , 11 , 12 , 13 , 14
8.1.2 Mechanism of Action
Deoxycholic acid is a bile acid produced by gut bacteria that emulsifies fat in the intestine. 15 ATX-101 is a synthetic deoxycholic acid formulated for subcutaneous injection that injures fat cell membranes, leading to adipocytolysis or fat cell destruction. 16 After injection into the submental fat, a local inflammatory response clears fragmented cells, recruits fibroblasts, and improves collagen deposition, leading to soft tissue tightening and improvement in the submental angle. 16 , 17 , 18 , 19 , 20 Given the inflammatory mechanism of this subcutaneous injection, patients may develop panniculitis with significant edema and discomfort. 21
8.2 Optimizing Use and Avoiding Complications
8.2.1 Clinical Trials
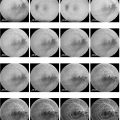
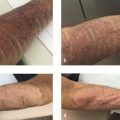
Injection of deoxycholic acid for the treatment of SMF has been studied in multiple phase III clinical trials, including REFINE-1 and REFINE-2 conducted in North America (ClinicalTrials.gov identifiers: NCT01542034, NCT01546142). In those trials, deoxycholic acid resulted in statistically significant and clinically meaningful improvements in submental fat severity based on clinician-assessed scales, patient-reported outcomes, and objective assessments such as magnetic resonance imaging and caliper measurements. Representative results are pictured in Fig. 8.1. Three-year follow-up data from the REFINE trials indicated that most results, namely 25% reduction in submental thickness and unchanged or improved skin laxity in 75% of patients, were maintained. There were no unexpected safety concerns reported. 14
8.2.2 Determination of Ideal Patient
It is important to select the ideal patient for this procedure to minimize complications and optimize the results. On examination, this assessment includes evaluating the submental region from multiple different planes of view. An issue is determining the amount of preplatysmal fat, subplatysmal fat, and skin laxity. 22 , 23 Patients with excessive skin laxity, or strong platysmal bands are not good candidates, as the removal of submental fat in these patients may exacerbate their skin laxity or the prominence of their platysmal bands, leading to less aesthetically undesirable results. 5 , 24 Furthermore, it is important to identify any significant subplatysmal fat component as this should not be treated with deoxycholic acid.
Once a patient is deemed a good candidate for deoxycholic acid, they should be educated on the treatment course.
8.2.3 Assessing Preplatysmal Fat
Prior to injection of deoxycholic acid, the preplatysmal fat must be assessed. Adequate adiposity (as opposed to excessive skin laxity or other causes of SMF) will minimize the risk of injury to surrounding structures such as nerves, salivary glands, and muscle, as well as increase the likelihood for a desirable aesthetic result. Some individuals may have submental fat deep to the platysmal muscle which should not be treated with deoxycholic acid. 25
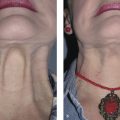
Techniques have been identified to assess preplatysmal fat prior to deoxycholic acid injection. The physician should observe the patient while upright and supine (Table 8.1). If SMF and blunting of the cervicomental angle is due to submental fat, the profile should appear consistent in both positions. In contrast, skin laxity would cause blunting of the cervicomental angle while upright, which could change significantly when the patient is supine (Fig. 8.2). To activate the platysmal muscle and best identify the preplatysmal component of submental fat, the physician can pinch the submental fat while the patient “grimaces” or while swallowing. 26

8.2.4 Proper Injection Technique
In order to decrease the risk of complications, the region of preplatysmal fat should be identified and marked. The preplatysmal fat is bordered superiorly by the mandible, laterally by the sternocleidomastoid muscles, and inferiorly by the hyoid bone (Fig. 8.3, Fig. 8.4). 23 Importantly, a “no treatment zone” must be marked to avoid injury to the marginal mandibular nerve. The “no treatment zone” includes the space from the angle of the mandible to the mentum. 23 Deoxycholic acid should not be injected within a region defined by a 1 to 1.5 cm line below the inferior border of the mandible, or above the inferior border of the mandible. 27
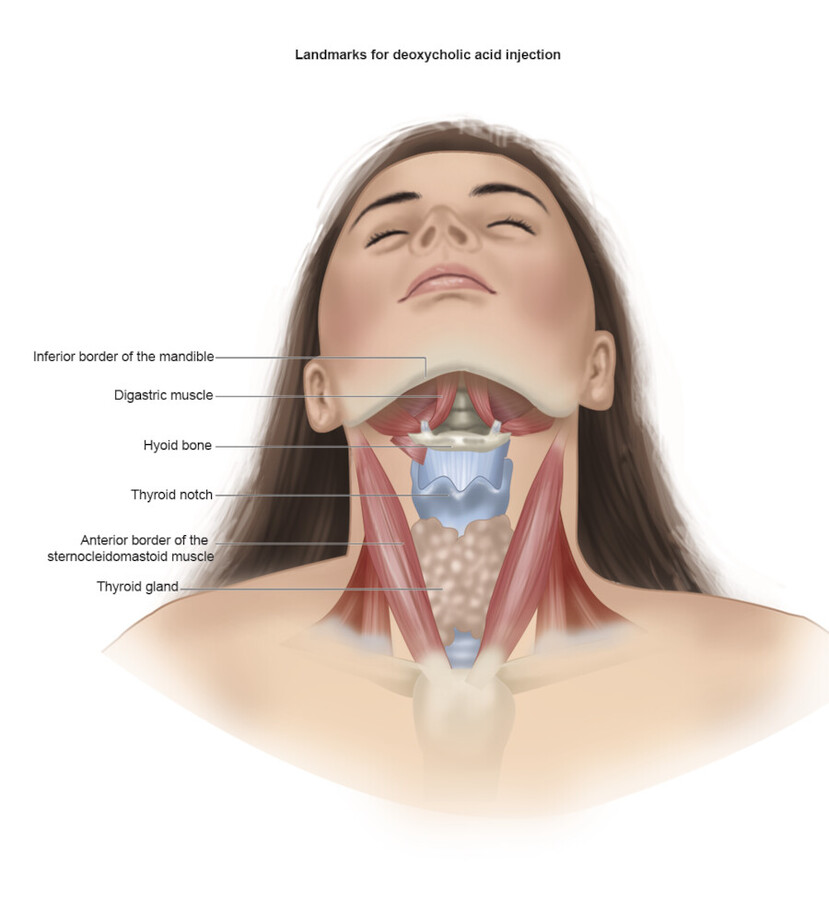
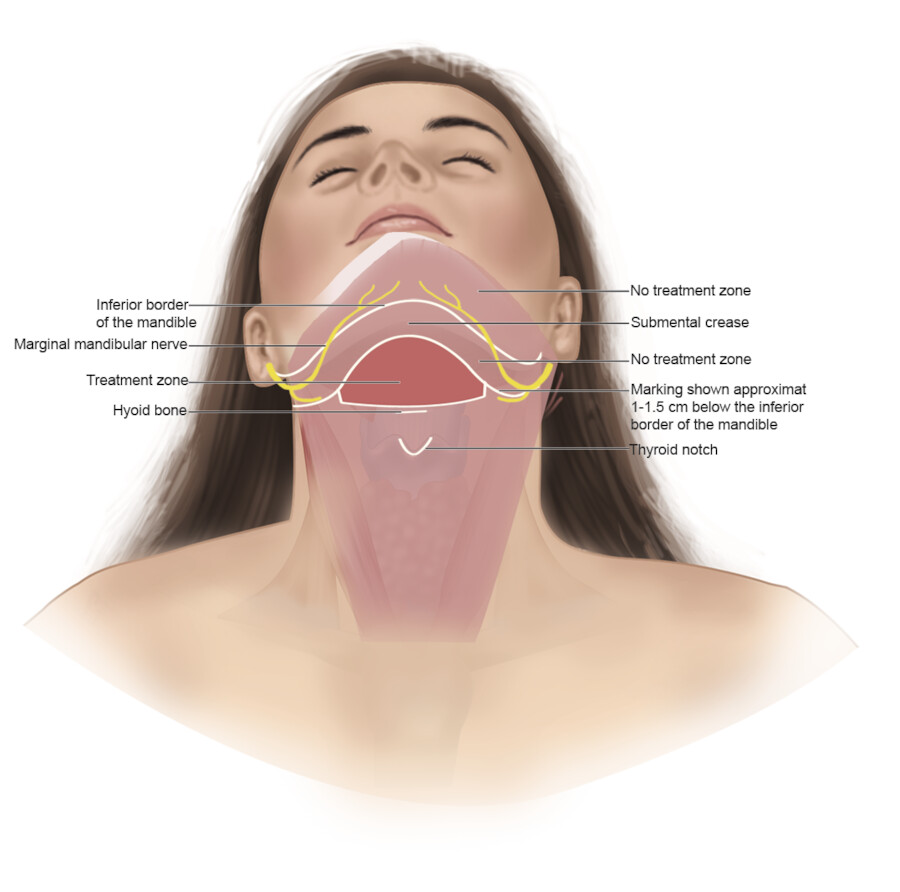
Prior to injection, a temporary grid tattoo or skin markings can help identify the planned treatment area. In the area to be treated, the skin should be mobile and separated from underlying structures. Aliquots of deoxycholic acid are injected, 1 cm apart into each grid space within the planned treatment area. 23 Injections should be administered with the needle perpendicular to the skin. Injection in the mid-subcutaneous space is critical to avoid nerves along the deep surface of the platysma. 30 If there is any resistance noted upon injection, it may indicate infiltration of the muscle (injection too deep) or the dermis (injection too superficial). The physician should withdraw the needle, and try again. Furthermore, before injection, aspiration is recommended to minimize the risk of intra-arterial injection. 31
Multiple treatments may be necessary to achieve a perceptible reduction in submental fat. Sessions should be at least 4 weeks apart, with a maximum of 50 injections (10 mL) per session. 5 In our experience, several physicians use less injection volume and less injections. Many physicians undertreat SMF with an insufficient amount of deoxycholic acid or insufficient number of treatment sessions. However, if injection site firmness/induration occurs after injection, subsequent injections should be delayed.
8.2.5 Common Complications
The most common reported complications (> 5–10% of patients) have been pain (53.3–84.6% of patients), hematoma (70.0–72.9% of patients), edema/swelling (37.4–67.8% of patients), anesthesia (47.9–66.9% of patients), erythema (6.5–40.5% of patients), induration or nodule (10.0–28.3% of patients), pruritus (8.6–16.3% of patients), and paresthesias (0–14.7% of patients). These adverse events were more common in the treatment groups than placebo groups. 8 , 9 , 10 , 11 , 12 , 13 Most injection site reactions were short-lived (< 30 d), but some reactions were longer lasting: anesthesia (occurring up to a median of 62 d) and nodule formation (occurring up to a median of 101 d). 13 When adverse events were stratified by mild SMF versus severe SMF, bruising, edema, anesthesia, swelling, pruritus, nodule, and erythema occurred at a higher rate in patients with more severe SMF. 13 Since FDA-approval of deoxycholic acid injections for SMF, few publications have addressed approaches to minimizing injection site reactions. Premedication with ibuprofen or pretreatment injection with epinephrine and buffered lidocaine can reduce pain and bruising. Following injection of each grid site in the treatment area, ice should be applied to reduce swelling. 29 , 32 , 33
8.2.6 Complications: Nerve injury
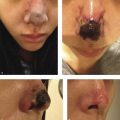
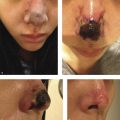
In clinical trials of deoxycholic acid injection, temporary nerve injury events occurred in 0.9 to 4.3% of subjects, and resolved without sequelae within a range of 4 to 115 days. 9 , 12 , 13 Marginal mandibular nerve injury and “pseudomarginal mandibular nerve injury” have been reported. The course of this nerve varies. It can lie anteriorly above the inferior border of the mandible. In approximately one in five patients, however, the nerve courses 1 to 2 cm below the inferior border of the mandible. 30 In cadaver studies, deoxycholic acid causes myelin sheath damage when exposed to the marginal mandibular nerve. 34 Compromise of the marginal mandibular nerve leads to reduced function of the depressor muscles of the lip and can result in an asymmetric smile (Fig. 8.5).
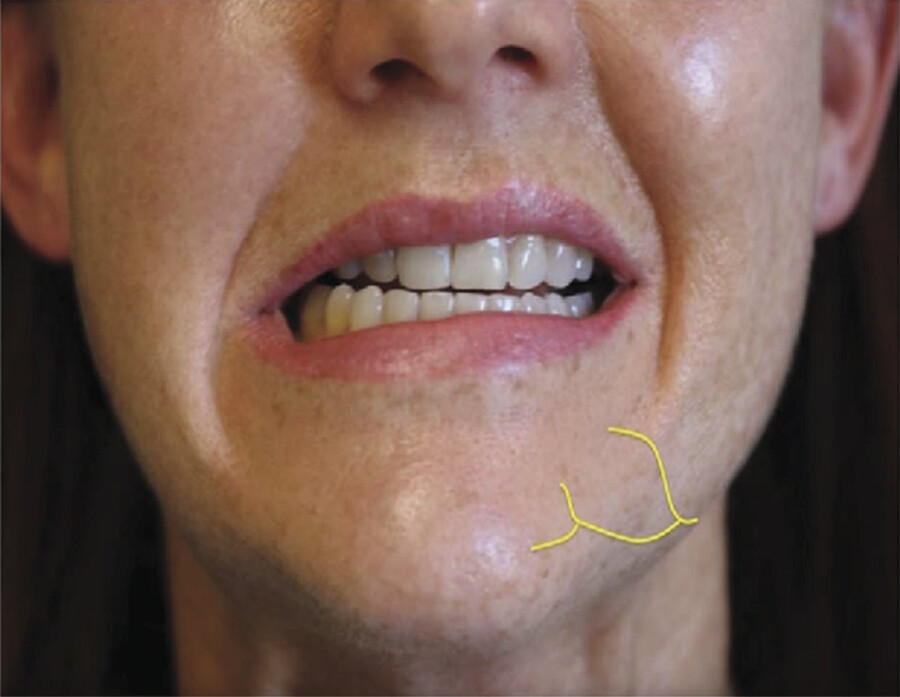
Compromise of the mentalis results in deficits of lip eversion. 30 “Pseudomarginal mandibular nerve injury” was originally reported in rhytidectomy literature to describe deficits resulting from injury to the cervical branch of the facial nerve. 35 Pseudomarginal mandibular nerve injury results in a clinically asymmetric smile, similar to a true marginal mandibular nerve injury. However, in pseudomarginal mandibular nerve injury, lip eversion remains intact. 30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree