Abstract
This chapter addresses the subject of breast reconstruction based on free tissue transfer. Preoperatively, the authors stress the importance of the patient factors that will facilitate the selection of the right reconstructive option from the many that are available to the surgeon. The thorough review offered in this chapter discusses the optimum time for reconstruction, diagnostics (CTA, MRA, ultrasound), and the advantages and disadvantages of abdominal-based, free TRAM, muscle-sparing free TRAM, deep inferior epigastric artery perforator, Rubens, gluteal-based, superior and inferior gluteal artery perforator, thigh-based, profunda artery perforator, and transverse fascia lata TFL myocutaenous flaps. Internal mammary and thoracodorsal vessels are also covered. Counsel involving postoperative care and potential outcomes concludes the study.
34 Breast Reconstruction with Free Flaps
34.1 Goals and Objectives
Understand the pros and cons of each type of breast reconstruction based on free tissue transfer.
Understand the advantages, disadvantages, and relative contraindications of each type of flap.
Understand the preoperative planning for the procedures.
Know the evidence-based quality outcomes to maximize patient safety and aesthetics.
34.2 Patient Presentation
Restoring the appearance, shape, and texture of breast after mastectomy has shown to improve quality of life and psychological well-being of patients after breast reconstruction. These procedures are broadly divided into two groups: alloplastic reconstruction using tissue expander and/or implants and autologous reconstruction with pedicled or free tissue transfer. Autologous breast reconstruction offers many advantages over implant based reconstruction. It provides superior feel and appearance to the reconstructed breast, helps restore the breast’s natural shape and ptosis, has lifetime durability requiring fewer revision surgeries, and ages with the patient. This chapter focuses on the microsurgical options available for breast reconstruction.
With the multitude of options available today for breast reconstruction, patient factors come to play an integral part in individualizing the type of reconstruction. A thorough history and physical exam as well as an understanding of patient’s expectations are therefore essential.
Certain patient characteristics affect the decision to proceed with microsurgical breast reconstruction. The factors affecting outcomes can be broadly separated into body habitus, breast shape, comorbidities and the cancer treatment plan. Comorbidities such as coronary artery disease, obesity (body mass index [BMI] > 30), clotting disorders, smoking history, and diabetes, increase the complication rates of free flap surgery. The ideal candidate for microsurgical breast reconstruction is a younger (<65) patient who is otherwise healthy, does not smoke, has no history of clotting abnormality, has adequate donor tissue, and does not require postoperative radiation therapy.
That being said, patients who do not possess these ideal criteria are the vary patients who may benefit from the advantages of free tissue transfer over implants. Generally, if the patient is less than ideal for autologous methods as listed above, they are an even worse candidate for implants. As implants are limited to approximately 800 mL except in rare cases, breasts larger this of this volume are poor candidates for implant reconstruction. As a result of our experience, we take the patient’s comorbidities into account and minimize their effects as best we can and then consider them relative contraindications. Patients who smoke are encouraged to quit prior to semi-elective immediate reconstruction and asked to quit prior to elective or delayed procedures. Patients with large breast size and asymmetry undergo procedures to improve that symmetry such as mastopexy and reduction.
If patient is deemed to be a candidate for microvascular breast reconstruction, the appropriate donor site is selected based on the breast volume and skin requirements, breast size and shape, unilateral versus bilateral reconstruction, patient’s size expectations, and availability of donor sites. The abdomen has become the preferred donor site for most patients if sufficient tissue is available to achieve the desired breast volume. When abdominal tissue is not a suitable option either due to paucity of fat or previous abdominal surgeries precluding sufficient tissue transfer, the gluteal or thigh region are considered.
34.2.1 Timing of Reconstruction
Timing of reconstruction in relation to adjuvant radiation therapy remains a topic of controversy. However, the majority of surgeons agree that microsurgical tissue transfer should be performed at 3 to 6 months after adjuvant radiation therapy is completed. 1 This is because radiation damage can cause significant fibrosis and deformation of reconstructed breast. It is generally recommended to consider delayed immediate reconstruction if suspicious of requiring radiation therapy.
Patients with a BMI over 32 are encouraged to lose weight prior to undergoing abdominally based procedures. Where possible nutrition is maximized, diabetes controlled and hypercoagulable states are minimized. Literature suggests that when possible, adjunctive procedures such as reconstruction should be delayed at least 6 weeks postchemotherapy and Tamoxifen have been shown to increase the risk of flap complications. 2
34.3 Preparation for Surgery
34.3.1 Preoperative Imaging
With development of perforator flaps, the need for assessment of vascular architecture of the flap has become an important part of operative planning. Preoperative imaging has evolved over the past decade to allow for identification and localization of appropriate perforators, especially in abdominally based free flap reconstruction. Modalities used today include Doppler ultrasonography and color duplex sonogram, computed tomographic angiography (CTA), and magnetic resonance angiography (MRA).
34.3.2 Duplex Ultrasound
Doppler ultrasound was one of the first tools used for perforator mapping through the cutaneous territory of the flap. It is also the instrument used by most surgeons intraoperatively and postoperatively to monitor the flap. In case of deep inferior epigastric perforator (DIEP) and transverse rectus abdominis myocutaneous (TRAM) flaps, the majority of dominant perforators have been shown located in the periumbilical area using ultrasound. Color duplex ultrasound can further provide information on the caliber, flow, direction, and velocity of these perforators. In their study Heitland et al showed that TRAM flap has the highest blood flow and velocity followed by DIEP and superior gluteal artery perforator (SGAP) flaps. 3 However, ultrasonography does not provide information on the course and three-dimensional details of the perforator systems. It has been shown to be associated with a high rate of false-positive results and a considerable rate of false-negative results. In their comparative study, Rozen et al demonstrated that CTA was superior to Doppler ultrasound at identifying the course of DIEA and its branching pattern and perforators. Furthermore, it removed the subjective error associated with Doppler ultrasonography. 4 , 5
34.3.3 Computed Tomographic Angiography
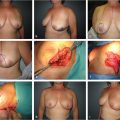
Although not all high volume practices utilize preoperative imaging, specific knowledge anatomy of the blood vessels can help to plan for variables such as adequate donor size, perforator position and recipient adequacy. CTA has become the gold standard for preoperative imaging of perforator flaps. It has been shown to be accurate in assessing the perforator’s caliber and location and their course through the muscle. Most surgeons use CTA for assessment of abdominal flaps and most recently for thigh-based and gluteal flaps. It helps the surgeon make a decision on performing a muscle sparing TRAM versus a DIEP flap, as well as assess the patency of deep and superficial inferior epigastric arteries specially in patients who have had previous abdominal surgeries. Paramedian abdominal scars have been shown to cause the most damage to the DIEA, superficial inferior epigastric artery (SIEA) vessels and the perforators, while laparoscopic incisions cause the least. Casey et al showed that preoperative CTA improved operative efficiency and reduced the incidence of abdominal bulge in DIEP breast reconstruction (Fig. 34‑1). 6 , 7

34.3.4 Magnetic Resonance Angiography
MRA allows reliable visualization of abdominal wall perforators of 1-mm diameter without exposing patients to ionizing radiation or iodinated intravenous contrast. Furthermore, although CTA yields higher spatial resolution, MRA has greater contrast resolution, which allows detection of perforators that may be missed by CTA. Another advantage of MR imaging is that due to absence of ionizing radiation, multiple image acquisitions can be performed after administration of gadolinium-based contrast, resulting in improved ability to obtain images at most optimal times. In this way, patient-related factors can be eliminated.
Gluteal and thigh perforators can also be assessed using MRA imaging. It can help the surgeon identify the location and course of the perforators. 8
34.3.5 Treatment
Abdominal-Based Flaps
Abdominal-based flaps are the method of choice for autologous breast reconstruction. One of the reasons for this preference is that most women who require breast reconstruction are at an age where they tend to accumulate excess tissue in the lower abdomen. The excess tissue provides an ideal donor site with the added benefit of improved abdominal contour resembling that of an abdominoplasty. Abdominal based flaps include those based on the deep inferior epigastric artery system (the free TRAM flap, muscle sparing free TRAMs, DIEP flap), SIEA flap and the external iliac perforator flap.
Compared to a standard pedicled TRAM, the microvascular flaps have been shown to have lower abdominal morbidity and all offer improved flexibility at the time of inset. Counterintuitively, the flap specific morbidity is lower as well. This may be a result of the typically greater investment in preoperative vascularity studies as noted below. Compared to other breast reconstruction free flap option, the abdomen offers the greatest volume of tissue and largest skin paddle. In addition, the abdominally based flaps can be harvested without a position change and can be done concurrently with the mastectomy surgery.
The abdominal free flaps share the disadvantages of a pedicled TRAM versus implant based reconstruction with longer surgical time, longer recovery time and the possibility of abdominal wall weakness, hernia and delayed healing of the donor incision. Relative contraindications are outlined in Table 34‑1.
Although used as first line for breast reconstruction, there are certain contraindications that may preclude use of these flaps. Many surgeons will not offer free flap surgery to active smokers due to significantly higher rate of complications in this group. Furthermore, if the patient has insufficient abdominal tissue to provide the desired breast volume and yet is against prosthetic reconstruction, thigh or gluteal based flaps should be considered. Previous liposuction, obesity, and unfavorable abdominal scars (paramedian, McBurney) are relative contra-indications to use of abdominal based flaps, especially perforator flaps. C-section scar is not a contraindication to use of abdominal tissue. However, preoperative imaging is recommended in any patient with abdominal scar.
Knowledge of the perfusion zones of transverse abdominal flap is important when deciding how much of the flap is to be transferred on a single pedicle. Hartrampf et al first described the perfusion zones of TRAM flap. Four zones were identified 9 :
Ipsilateral to the pedicle, overlying the rectus abdominis muscle (RAM).
Overlying the contralateral RAM.
Lateral to the ipsilateral RAM.
Lateral to the contralateral RAM.
In 2006, the classic Hartrampf zones II and III were shown by Holm et al to be reversed from the standpoint of blood flow. 10 The ipsilateral half of abdominal flap was shown to have an axial pattern of perfusion, whereas the contralateral half had a random pattern. It is this pattern that should be kept in mind when considering how much tissue can be carried for any individual flap. For clarity in discussion of the tissue to be used for reconstruction, the Hartrampf zones continue to be used.
Perfusion studies since then have shown that there is still further variability in the perfusion patterns depending on whether the medial or lateral row perforators are harvested. Wong et al showed that the medial perforators conform to the Hartrampf zones of perfusion while the lateral perforators follow the Holm theory. 11 In other words, the medial rows tend to perfuse across the midline better than the lateral aspect of the abdomen. These facts should also be taken into consideration when designing the flap. Intra-operative fluorescent angiography and near-infrared spectroscopy can further aid in this assessment.
Free TRAM Flap
First described by Holmstrom in 1979, free TRAM involves removal of the full transverse rectus abdominis muscle and the overlying anterior rectus sheath. 12 The obvious disadvantages is the total loss of muscle strength in the area of the flap as well as loss of abdominal wall strength by removal of the overlying fascia. Its use is indicated in patients who require large volume reconstruction (least fat necrosis) or lack suitable perforators or superficial inferior epigastric vessels based on preoperative imaging. It is ideal for those patients that require maximum blood flow such as active smokers, higher BMI patients and those in need of multiple zones of tissue. All other abdominally-based flaps attempt to maintain a portion of the rectus abdominis muscle and fascia.
The advantages of free TRAM flap include the following:
Robust blood supply: Free TRAM has the best vascularity of all abdominal based free flaps and thus has the lowest fat necrosis rates (< 10%). Therefore it is the best option in patients with multiple risk factors or those who require large volume reconstruction.
Compared to pedicle TRAM, free TRAM reconstruction has no pedicle bulge from tunneling.
It allows versatile shaping into a breast mound to increase projection or match contralateral ptosis.
Deep inferior epigastric vessel offers a long pedicle with large artery and two venae comitantes.
Muscle Sparing Free TRAM Flap (MS TRAM)
A true free TRAM is rarely performed today for breast reconstruction. Instead, muscle-sparing TRAM is performed preserving a longitudinal portion of muscle as well as the anterior rectus sheath. Depending on the amount of rectus abdominis muscle preserved, a classification system was developed by Nahabedian et al. 13 The rectus muscle is separated into three longitudinal segments: medial, lateral, and central:
MS-0 → Sacrifice of the full width (partial length).
MS-1 → Preservation of the lateral segment.
MS-2 →Preservation of lateral and medial segments (removal of the central one-third makes denervation likely).
MS-3 →Preservation of entire muscle, sacrificing either no muscle or only a cuff around the perforators (DIEP).
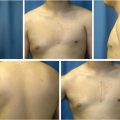
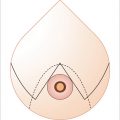
Studies have shown that MS-0 and MS-1 TRAM are associated with greater functional deficit, whereas the difference between MS-2 and DIEP flaps are not as well appreciated (Fig. 34‑2).
Operative Details
The patient is marked in the standing position. A fusiform ellipse is marked from the suprapubic crease to just above the umbilicus, and laterally to the anterior superior iliac spine (ASIS). Once under general anesthesia in supine position, the markings are confirmed to allow primary closure. If there is any doubt as to how much can be taken, elevate the central upper abdominal flap from above the umbilicus to the xiphoid, break the operating room table into a Fowler Position, and then pull the ⟩\rangle flap down to determine the lowest portion of the ellipse. Make the lower abdominal incision. Examine the deep circumflex iliac artery and vein (DCIA/V) laterally and superficial inferior epigastric artery and vein (SIEA/V) medially. If SIEV caliber is more than 1.5 mm, dissect it further until size of the SIEA can be determined. If the size of the artery is adequate, SIEA flap can be considered. In either case, it is safest to dissect both DCIV and SIEV for some length to use them as a lifeboat for venous outflow.
Mark the major perforators, as determined by preoperative imaging and intraoperative Doppler. Elevate the right and left abdominal flaps in the suprafascial plane from lateral to medial direction. Once networks of perforators are identified, incise the anterior rectus sheath surrounding the perforators and extend it caudally. Elevate the fascia off the rectus abdominis muscle and undermine the muscle to visualize the course of deep inferior epigastric vessels. The rectus abdominis muscle is then incised to incorporate all the perforators into the flap and entry point of the pedicle. It is important to preserve the lateral intercostal motor nerves during this dissection to maintain the function of abdominal wall. Once transferred to the breast pocket, the flap is oriented so that the best-perfused portion of the flap (zones I and II) is positioned in the superomedial aspect of the reconstructed breast; this is the least likely site to develop fat necrosis.
Deep Inferior Epigastric Artery Perforator Flap
DIEP flap is an adipocutaneous flap perfused on direct or indirect muscle perforators. Its use is indicated in active patients or women who plan to have a child and therefore require competent abdominal wall. Preoperative CTA or MRA in hand with intraoperative assessment of the perforators allow identification of the eligible abdomen. In general, a single perforating artery or vein of at least 1.5 mm with a palpable pulse is required for a successful perforator flap harvest. Such perforators are typically located in the periumbilical area.
DIEP flap has been shown to offer advantages over the traditional TRAM flap by preserving the rectus muscle. This results in lower donor-site morbidity, specifically risk of abdominal hernia and bulge as well as decreasing postoperative pain and improving recovery time. Perforator flap dissection also increases pedicle length (especially when isolated on a single pedicle), allowing for improved flexibility of flap positioning. On the other hand, due to the need for meticulous dissection, these surgeries are often longer and although the literature varies, older studies demonstrate a higher flap loss rate (5–10% vs. 0.5–1% in free TRAMs). Furthermore, a perforator flap has higher incidence of fat necrosis and venous congestion compared with MS-TRAM as less perforators are included in the flap. More recent studies do not support these findings and show similar complication rates between types of abdominal based free flap breast reconstruction techniques. 14 The improvements may be a result of ever increasing experience among surgeons.
Operative Details
Preoperative markings and initial operative steps are similar to MS-TRAM outlined previously. Once the appropriate perforator(s) are identified, the fascia and muscle are incised as needed to allow perforator dissection; however, no fascia or muscle is harvested. The perforators are dissected by splaying the muscle fibres temporarily and following them down to their origin. The pedicle is identified and elevated off the rectus muscle. In unilateral cases, the contralateral hemi-abdomen is kept intact as a lifeboat until the ipsilateral dissection has been safely completed. The number of perforators necessary to adequately perfuse the flap is largely determined by patient experience. In lieu of this experience, all the perforators can be isolated and individually clamped while assessing the flap for blood flow with either tissue oximetry such as ViOptix or isocyanine green infrared scan. Taking the fewest number of perforators possible will minimize the morbidity of the rectus abdominis muscle. Likewise, taking perforators all from a single row (medial versus lateral) will decrease morbidity as well. Similarly, the SIEV and DCIV are dissected and kept as backup in case they provide the dominant venous outflow to the flap.
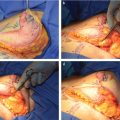
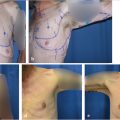
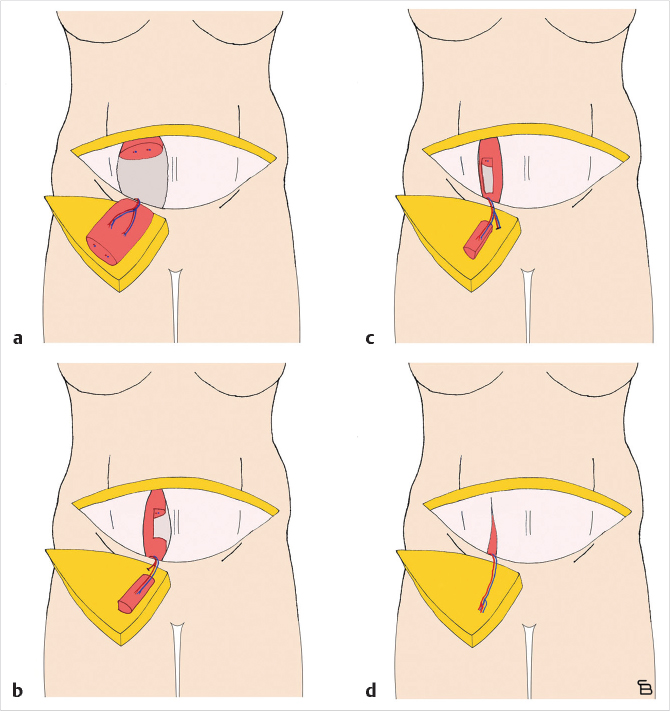
In unilateral cases where the entire abdomen needs to be used to achieve the appropriate volume, a stacked, free TRAM, double-pedicled or bipedicled DIEP/MS-TRAM flap can be harvested. In such cases, both hemi-abdomens are dissected as DIEP or MS TRAM. The secondary pedicle is anastomosed either to a side branch of the primary pedicle or to an independence recipient vessel in the chest (retrograde to internal mammaries or to thoracodorsal system). A classification system was described in 2007 and further refined by Murray et al. based on vessels that are used for crossover anastomosis (Fig. 34‑3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree