Bone loss and indications for the use of metallic augments in revision total knee arthroplasty (TKA) usually are guided by classification of the bony defect and the intraoperative findings.
 Probably the most widely used, the Anderson Orthopaedic Research Institute (AORI) bone defect classification system divides bone loss of the distal femur or the proximal tibia into three types, based on the radiographic status of the metaphyseal bone.5,6
Probably the most widely used, the Anderson Orthopaedic Research Institute (AORI) bone defect classification system divides bone loss of the distal femur or the proximal tibia into three types, based on the radiographic status of the metaphyseal bone.5,6
Proximal tibial metaphyseal defects are graded as type I (TI), type II (TII), or type III (TIII).
TI defects of the proximal tibia have intact metaphyseal bone with no component subsidence or loss of the primarily reconstructed joint line.
• Minor defects may be present that will not compromise the stability of the tibial component; primary type reconstruction components may typically be used without augments.
TII defects of the proximal tibia have damaged metaphyseal bone with component subsidence or joint line alteration due to loss of metaphyseal bone.
• Bone loss in TII defects can involve either the lateral or, more commonly, the medial tibial plateau as well as the entire proximal tibia.
• Defect reconstruction with cement, metal augments, or bone grafting is required and revision stemmed components usually are required.
• Collateral ligament origins and insertions are preserved in TII-type defects.
TIII defects of the proximal tibia have deficiency of the proximal metaphyseal bone that involves a major segment of the proximal tibia.
• This type of defect may involve the tibial tubercle, with resulting patellar tendon detachment and loss of extensor mechanism function.
• The medial collateral ligament also may be detached or functionally incompetent as a result of bony deficiency.
• The broad insertion of the medial collateral ligament on the proximal medial metaphysis of the tibia renders incompetence or frank loss of attachment due to tibial bone loss less likely as compared to femoral condylar bone loss where the origin of the medial collateral has a much smaller area of attachment to the medial epicondyle.
 In summary, proximal tibial bone defects are classified as intact (TI), damaged (TII), and deficient (TIII).
In summary, proximal tibial bone defects are classified as intact (TI), damaged (TII), and deficient (TIII).
By definition, the use of metallic augments is restricted to TII or TIII defects. TIII defects with larger bone voids typically require reconstruction with bulk allograft or the newer highly porous metal metaphyseal augments.
ANATOMY
 Although native knee anatomy is relevant in primary TKA, this chapter will focus on the pertinent anatomy that is relevant in the more complex revision TKA requiring augments.
Although native knee anatomy is relevant in primary TKA, this chapter will focus on the pertinent anatomy that is relevant in the more complex revision TKA requiring augments.
 The tip of the fibular head is approximately 1 cm below the surface of the lateral tibial plateau2 and is the most commonly used bony reference for joint line restoration in the revision TKA setting.
The tip of the fibular head is approximately 1 cm below the surface of the lateral tibial plateau2 and is the most commonly used bony reference for joint line restoration in the revision TKA setting.
Improved outcomes are noted if the joint line is elevated less than 8 to 10 mm.9,12
 The tibial tubercle is 25 to 40 mm below the joint surface, and the average insertion point of the patellar tendon is 29 mm distal to the tibial plateau.
The tibial tubercle is 25 to 40 mm below the joint surface, and the average insertion point of the patellar tendon is 29 mm distal to the tibial plateau.
The distal pole of the patella averages 15 mm (range, 12 to 16 mm) above the joint surface.
 Neurovascular injury during primary and revision TKA is rare.
Neurovascular injury during primary and revision TKA is rare.
The popliteal neurovascular bundle is 3 to 12 mm posterior to the tibia articular surface when the leg is extended and 6 to 15 mm posteriorly when the knee is flexed to 90 degrees.18
At the level of the tibial resection, the distance is approximately 2 cm posterior to the cut surface and the popliteal artery and vein are anterior to the tibial nerve at this level.14
Most revisions do not put the tibial artery trifurcation at risk unless more than 30 mm of proximal tibia is resected.
Most neurovascular injuries during primary and revision TKA result from tourniquet use in the patient with peripheral vascular disease.
 The proximal tibial anatomy of the TKA requiring revision TKA is highly variable due to the various mechanisms of TKA failure and their effect on the native bone stock through overt loss or remodeling.17
The proximal tibial anatomy of the TKA requiring revision TKA is highly variable due to the various mechanisms of TKA failure and their effect on the native bone stock through overt loss or remodeling.17
PATHOGENESIS
 Proximal tibial bone loss in primary TKA failure can be attributed to the following factors: implant malalignment primarily or due to bony collapse, aseptic loosening with implant migration, osteolysis due to particulate wear debris, bone loss from excessive motion of cement spacers used for staged treatment of chronic infection, or intraoperative bone loss during implant removal.8
Proximal tibial bone loss in primary TKA failure can be attributed to the following factors: implant malalignment primarily or due to bony collapse, aseptic loosening with implant migration, osteolysis due to particulate wear debris, bone loss from excessive motion of cement spacers used for staged treatment of chronic infection, or intraoperative bone loss during implant removal.8
 If osteolysis is present, its severity is affected by implant design and the quality of polyethylene as well as host response to particulate debris and the quality of the host bone.
If osteolysis is present, its severity is affected by implant design and the quality of polyethylene as well as host response to particulate debris and the quality of the host bone.
 Osteolysis of the proximal tibial bone is a result of the histiocytic and macrophage response to polyethylene particulate debris from wear at the bearing interface as well as “backside” wear.4
Osteolysis of the proximal tibial bone is a result of the histiocytic and macrophage response to polyethylene particulate debris from wear at the bearing interface as well as “backside” wear.4
Osteolytic lesions are either focal or expansile, depending on the submicron particle burden as well as the host response to the particles and will typically follow the paths of least resistance around tibial component of the implant.6
Tibial component aseptic loosening may occur with failure of the interface fixation and is typically noted when a radiolucent line is circumferential on radiographs. Loosening may be at the cement–bone interface of a cemented tibial component or at the implant–bone interface of a cementless design.
• Aseptic loosening may also occur at the prosthesis–cement interface in a process called debonding. Both expansile osteolysis and component loosening can lead to tibial component subsidence, usually into a varus position, which creates the most typical scenario requiring a metal augment.
 Tibial bone loss may also occur in periprosthetic infection either due to the erosive infectious process in chronic infection or component removal in acute or chronic sepsis.
Tibial bone loss may also occur in periprosthetic infection either due to the erosive infectious process in chronic infection or component removal in acute or chronic sepsis.
 Most chronic prosthetic infections are treated with two-stage revision arthroplasty of the knee in which either a static antibiotic spacer or a dynamic articulated type of spacer is used for the first stage of the revision.
Most chronic prosthetic infections are treated with two-stage revision arthroplasty of the knee in which either a static antibiotic spacer or a dynamic articulated type of spacer is used for the first stage of the revision.
Explantation of a well-fixed primary component leads to potentially significant bone loss when it is removed from an osteopenic tibial metaphysis.
 The antibiotic cement spacers used in the joint between the revision stages may contribute to bone loss through relative motion during the interval stage before reimplantation.
The antibiotic cement spacers used in the joint between the revision stages may contribute to bone loss through relative motion during the interval stage before reimplantation.
 A comparison study of static and articulated spacers showed greater bone loss with static spacers than with articulated spacers.7
A comparison study of static and articulated spacers showed greater bone loss with static spacers than with articulated spacers.7
 The AORI classification also applies to bone loss, either present or anticipated, at the second stage of the staged revision knee arthroplasty.
The AORI classification also applies to bone loss, either present or anticipated, at the second stage of the staged revision knee arthroplasty.
NATURAL HISTORY
 The osteolytic process typically is clinically silent until the prosthesis loosens. However, some patients may present with a painful synovitis due to polyethylene wear before actually prosthetic loosening.
The osteolytic process typically is clinically silent until the prosthesis loosens. However, some patients may present with a painful synovitis due to polyethylene wear before actually prosthetic loosening.
 A loose prosthesis causes “start-up pain” as well as cyclic pain with loading, provided that fracture or gross displacement of the component has not occurred.
A loose prosthesis causes “start-up pain” as well as cyclic pain with loading, provided that fracture or gross displacement of the component has not occurred.
The pain may decrease with weight bearing as the component re-sits itself and motion at the bone implant interface decreases, termed start-up pain.
 Expansile osteolysis and component subsidence or proximal metaphyseal fracture my result in gross lower extremity deformity, usually varus, and occasionally may also present with hyperextension if the component subsides into an anteriorly sloped position.
Expansile osteolysis and component subsidence or proximal metaphyseal fracture my result in gross lower extremity deformity, usually varus, and occasionally may also present with hyperextension if the component subsides into an anteriorly sloped position.
 Radiographic examinations of the implanted knee that is suspected of loosening should be performed at regular intervals to detect the presence or progression of osteolysis.
Radiographic examinations of the implanted knee that is suspected of loosening should be performed at regular intervals to detect the presence or progression of osteolysis.
 Septic failure of TKA is noted in the perioperative period in 0.5% of patients.
Septic failure of TKA is noted in the perioperative period in 0.5% of patients.
Delayed hematogenous infection occurs in approximately 1% to 2% of TKAs over the lifetime of the implant.
Differentiation between septic and aseptic loosening of the tibial component is of paramount importance because the management of each type is different, and a misdiagnosis can result in loss of limb.
PATIENT HISTORY AND PHYSICAL FINDINGS
 The patient history and physical findings are centered around differentiating between septic and aseptic loosening, the amount of bony destruction and the ligamentous insufficiency present, and the resultant instability of the knee.
The patient history and physical findings are centered around differentiating between septic and aseptic loosening, the amount of bony destruction and the ligamentous insufficiency present, and the resultant instability of the knee.
 Patients with symptomatic osteolysis or loosening of the tibial component usually describe gradual onset of pain that is worse with start-up type of activities such as arising from the bed or getting out of a chair or a vehicle.
Patients with symptomatic osteolysis or loosening of the tibial component usually describe gradual onset of pain that is worse with start-up type of activities such as arising from the bed or getting out of a chair or a vehicle.
They also commonly describe increased pain with the first steps of ambulation, which may decrease as the component settles into the remaining bony mantle of the proximal tibia.
There is less or no pain with rest.
 The infected TKA will cause pain at rest, and patients who have deep infection may describe a temporal pain pattern with increasing or steady pain from the day of surgery to the day of clinical evaluation with no remittance.
The infected TKA will cause pain at rest, and patients who have deep infection may describe a temporal pain pattern with increasing or steady pain from the day of surgery to the day of clinical evaluation with no remittance.
Late hematogenous infection presents with sudden onset of pain, loss of motion, and stiffness in a previously well-functioning prosthesis.
 Complete neurovascular examination of the affected extremity should be carried out, along with focal examination of the knee.
Complete neurovascular examination of the affected extremity should be carried out, along with focal examination of the knee.
 Visual inspection for edema and erythema is pertinent in the face of infection.
Visual inspection for edema and erythema is pertinent in the face of infection.
 Range of motion and patellar tracking should be evaluated along with ligamentous stability and overall limb alignment. A difference between active and passive extension demonstrates extension lag versus flexion contracture.
Range of motion and patellar tracking should be evaluated along with ligamentous stability and overall limb alignment. A difference between active and passive extension demonstrates extension lag versus flexion contracture.
 Specific attention should be directed toward evaluating the functional status of the medial collateral ligament as well as overall stability of the knee.
Specific attention should be directed toward evaluating the functional status of the medial collateral ligament as well as overall stability of the knee.
Varus and valgus stress tests in extension, in midflexion, and at 90 degrees of flexion are useful to evaluate the collateral ligaments for instability. If present, instability may be graded according to laxity.
Excessive anterior tibial translation with the knee in 90-degree flexion, tenderness of the pes anserine hamstring insertion, and effusion are consistent with flexion instability.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Plain film radiographic views of the affected knee are required and should include weight-bearing anteroposterior (AP), lateral, and patellofemoral views.
Plain film radiographic views of the affected knee are required and should include weight-bearing anteroposterior (AP), lateral, and patellofemoral views.
 It is important to obtain tangential views to properly align the bone implant interface with the beam of the radiograph to assess radiolucent lines and prosthesis fixation status.
It is important to obtain tangential views to properly align the bone implant interface with the beam of the radiograph to assess radiolucent lines and prosthesis fixation status.
 If plain radiographs are thought to underrepresent the true volume of osteolysis, consideration may be given to computed tomography (CT) or magnetic resonance imaging (MRI) with metal suppression.19
If plain radiographs are thought to underrepresent the true volume of osteolysis, consideration may be given to computed tomography (CT) or magnetic resonance imaging (MRI) with metal suppression.19
Conversely, if there is doubt regarding actual loosening of the component, technetium 99m bone scanning may indicate possible looseness of the tibial component.
 In cases in which infection is suspected, the bone scan can be compared to an indium 111–tagged white blood cell scan.
In cases in which infection is suspected, the bone scan can be compared to an indium 111–tagged white blood cell scan.
 Aspiration of the knee joint and analysis for synovial white cell count, neutrophil differential, and culture of a definitive organism is the ideal confirmation of an infected TKA in the face of elevated serum markers for infection.
Aspiration of the knee joint and analysis for synovial white cell count, neutrophil differential, and culture of a definitive organism is the ideal confirmation of an infected TKA in the face of elevated serum markers for infection.
DIFFERENTIAL DIAGNOSIS
 Osteolysis
Osteolysis
 Infection
Infection
 Tibial component loosening or debonding
Tibial component loosening or debonding
 Tibial component subsidence
Tibial component subsidence
 Periprosthetic proximal tibial fracture
Periprosthetic proximal tibial fracture
NONOPERATIVE MANAGEMENT
 Nonoperative management of a loose tibial component due to osteolysis with or without subsidence and fracture should be considered only if the revision procedure will place the patient’s survival in jeopardy or make their quality of life worse.
Nonoperative management of a loose tibial component due to osteolysis with or without subsidence and fracture should be considered only if the revision procedure will place the patient’s survival in jeopardy or make their quality of life worse.
 The same caution applies to patients with indolent infections. Virulent organisms can cause bacteremia and sepsis. Emergent surgical explantation of the components may be required to prevent septic shock and death.
The same caution applies to patients with indolent infections. Virulent organisms can cause bacteremia and sepsis. Emergent surgical explantation of the components may be required to prevent septic shock and death.
SURGICAL MANAGEMENT
Preoperative Planning
 Evaluating the component position and classifying the present bone loss on plain films is the cornerstone of preoperative planning.
Evaluating the component position and classifying the present bone loss on plain films is the cornerstone of preoperative planning.
Metaphyseal bone quality should be evaluated and intraoperative bone loss should be anticipated.
Templating should be carried out with the focus on reestablishing the tibial platform with metaphyseal augmentation if needed, balancing the flexion and extension space, and restoring the joint line when able. The broad insertion of the medial collateral ligament presents an issue only for severe bone defect unless it is found to be incompetent on the preoperative physical examination. Close attention should be given to the planned level of resection and its relation to the insertion of the extensor mechanism.
Wedge augments should be avoided due to a higher incidence of radiolucent lines and failures due to the shear stresses imparted to the fixation interface. Because cement performs ideally in compression, rather than shear, defects should be shaped to accept a block augment when possible.
 The condition of the patient’s skin should be carefully evaluated in the physical examination.
The condition of the patient’s skin should be carefully evaluated in the physical examination.
All previous skin incision and their age should be noted.
An anterior midline incision is preferred, but it may be necessary to use the most lateral incision to protect the patient from skin necrosis in areas between incisions. A 7-cm skin bridge should be maintained between all incisions if at all possible.
 The existing implant in the knee should be identified, and the operative reports of the previous surgery should be obtained prior to surgery when possible.
The existing implant in the knee should be identified, and the operative reports of the previous surgery should be obtained prior to surgery when possible.
Positioning
 A well-padded, nonsterile tourniquet is placed as proximally as possible on the operative lower extremity. All bony prominences are well padded.
A well-padded, nonsterile tourniquet is placed as proximally as possible on the operative lower extremity. All bony prominences are well padded.
 A bump may be placed under the hip and pelvis on the operative extremity side, depending on the surgeon’s preference.
A bump may be placed under the hip and pelvis on the operative extremity side, depending on the surgeon’s preference.
 A beanbag, horizontal post, or leg-holding device such as an Alvarado leg positioner may be used to hold the leg in a flexed position during the procedure if desired by the surgeon.
A beanbag, horizontal post, or leg-holding device such as an Alvarado leg positioner may be used to hold the leg in a flexed position during the procedure if desired by the surgeon.
TECHNIQUES
 Approach
Approach
A standard midline incision with a medial parapatellar arthrotomy is ideal, taking care in the revision setting to create full-thickness flaps.
 Component Explanation
Component Explanation
Once the joint is opened, scar tissue is resected as necessary for adequate exposure. Specifically, the medial and lateral gutters should be restored with care to avoid injuring the collateral ligament origins.20 The suprapatellar pouch also should be restored via resection of scar tissue under the quadriceps tendon and from the anterior aspect of the distal femur just proximal to the femoral component.
The subperiosteal flap is elevated off the proximal and anteromedial aspect of the tibia to allow exposure to the entire tibial component and proximal tibial bone.
The tibial insert is removed from the tray to allow adequate visualization.
Particular attention is paid to the extensor mechanism, primarily the patellar tendon, during exposure. External rotation of the tibia with the knee in flexion will facilitate exposure of the tibia and protect the extensor mechanism by minimizing the tension on the patellar tendon insertion.
Careful release of the posterior capsule will also facilitate proximal tibial exposure and allow safe placement of a posterior tibial retractor to bring the tibial metaphyseal surface forward.
The tibial tray is then explanted using the techniques outlined in Chapter 43, if necessary, and a careful assessment of the tibial bone deficiency is performed.
Stemmed tibial revision components are recommended when using augments to provide additional bony stabilization of the implant, thereby shifting some of the load from the damaged or deficient metaphyseal tibia to the diaphysis.
 Medial or Lateral Block or Step Augment
Medial or Lateral Block or Step Augment
Intramedullary reaming is followed by placement of an intramedullary alignment cutting guide, and a minimal transverse proximal tibial “skim” resection is taken after the cutting guide is pinned in place.
A block augment cutting guide can either be attached separately or will be incorporated into the intramedullary alignment cutting guide that exists in most revision knee systems. Particular attention must be paid to tibial component rotation so that the sagittal block cut will align with the final implant in the correct rotation (TECH FIG 1A,B).
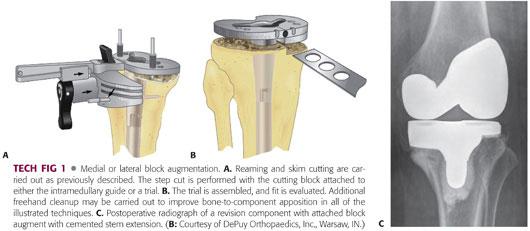
Most surgeons will avoid wedge or sloped augments; it has been shown that block augments are superior to wedges biomechanically in creating an overall more stable and rigid tibial construct.3
Once the proximal tibial surface is adequately prepared, a trial stemmed component that reflects the intramedullary stem to be used with attached augments is trialed.
When adequate bony support is achieved, the joint surface is restored and flexion and extension gaps are balanced. The component to be implanted then is constructed to match the trial and appropriately cemented into place (TECH FIG 1C).
 Metaphyseal Cone (Sleeve) Augmentation
Metaphyseal Cone (Sleeve) Augmentation
Intramedullary reaming should be carried out to the depth of the stem available in the revision system in use to place the tibial tray at the appropriate level. Most metaphyseal sleeves are used with cementless stem extensions and therefore an intimate fit of the reamer and subsequent stem into the diaphysis is desired.
Once the appropriate-sized diaphyseal engaging stem is selected, the proximal tibia is sequentially broached with the appropriate-sized trial stem attached to the broach to provide proper alignment (TECH FIG 2A).
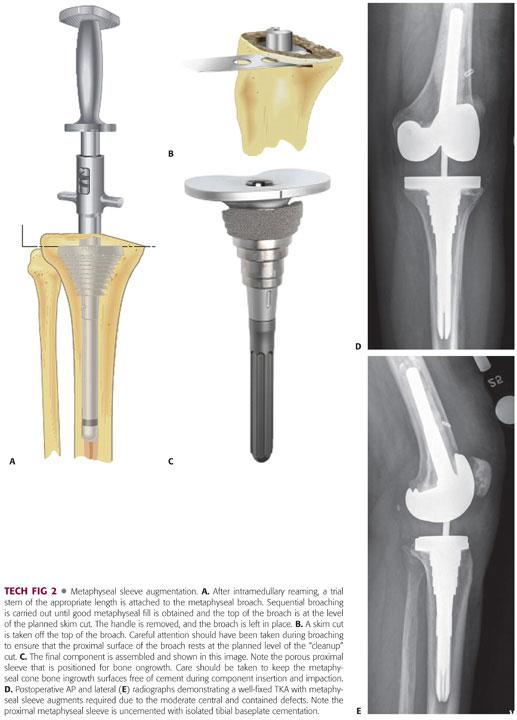
There is some rotational freedom between the metaphyseal sleeve and the tibial tray, so that the surgeon can focus on optimizing metaphyseal fixation. However, the surgeon should be familiar with the degree of rotational freedom within the system to ensure optimal tibial rotation.
Broaching is carried out until rotational and axial stability is obtained, which usually occurs when the majority of the metaphyseal defect is filled with the sleeve augment.
Some systems then use the proximal surface of the broach as the cutting guide. This necessitates placing the broach at the level determined by preoperative planning and intraoperative assessment. Ideally, this position resects 2 mm or less of the proximal tibial metaphysis (TECH FIG 2B).
Once the proximal tibia is resected and broached, a trial metaphyseal sleeve is placed and rotation is marked on the anterior tibia, a properly sized trial tibial tray and stem are assembled and placed through the cone, lack of excessive rotational disparity between the two is verified, and tibial tray rotation is marked.
Final assembly of the tibial components is done to match the trial, and the tibial cone is impacted onto the Morse taper of the revision tibial baseplate, with care taken to match the trial model cone rotation on the trial stem (TECH FIG 2C).
Cement is applied to the assembled component selectively at the tibial baseplate undersurface only as most cones allow for bony ingrowth with a porous surface coating, and the diaphyseal stem is press-fit and baseplate-cemented to the proximal tibia. The metaphyseal sleeve is allowed to contact the bone to facilitate osseointegration of the porous coating (TECH FIG 2D,E).
 Porous Tantalum Metaphyseal Augments
Porous Tantalum Metaphyseal Augments
After a very minimal freshening cut perpendicular to the anatomic axis of the tibia, the cavitary defect of the proximal tibia is curetted clean, and all membrane is removed.
The size and shape trabecular metal augment that most closely fits the defect is selected, and a high-speed burr is used to remove minimal amounts of bone to allow for a tight press-fit of the augment.
The augment is impacted into place. In cases in which the augment does not fully contact the surrounding bone, crushed cancellous allograft croutons can be combined with demineralized bone matrix to fill the peripheral void (TECH FIG 3A).
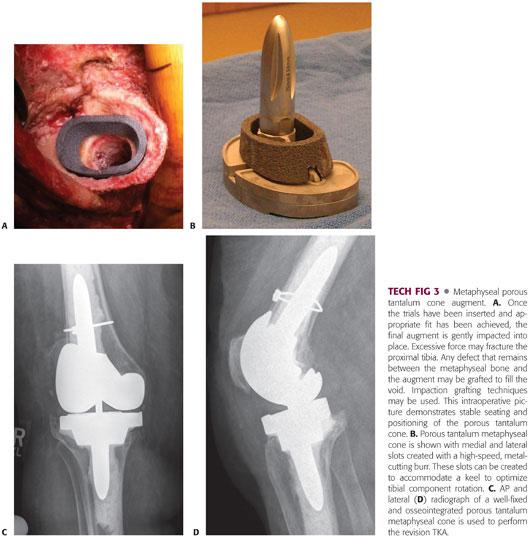
To minimize the chance of intraoperative periprosthetic fracture, the surgeon should be careful of overly aggressive impaction of the final implant. Tibial metaphyseal bone in the revision setting is typically sclerotic, damaged, mechanically weak, and prone to inadvertent fracture. The frictional coefficient of the actual porous tantalum implant will create greater resistance to insertion and subsequent stability.
If an offset stem is selected to allow for good tibial coverage, or if the augment is placed off-center of the diaphysis to allow for best void fill, then a high-speed metal-cutting burr can be used to trim the augment centrally. The metal-cutting burr can also be used to cut slots for the tibial tray and optimized rotation.
Once clearance is obtained for the augment, the trial tibial stem with baseplate is inserted into the tibial diaphysis through the trabecular metal augment to verify fit (TECH FIG 3B).
When adequate bony support is achieved, the joint surface is restored, and flexion and extension gaps are balanced, the component to be implanted is constructed to match the trial and appropriately cemented into place (TECH FIG 3C,D).
The proximal portion of the tibial stem is cemented to the trabecular metal augment, and the stem is either press-fit in the tibial diaphysis or cemented per the preoperative.
Newer porous tantalum cones were developed that are smaller in size and can be implanted in the diaphysis or metaphysis. If proximal tibial defects are moderate and contained with an intact peripheral rim and supporting cortical bone but insufficient central metaphysis for rotational control of the tibial implant, the diaphyseal cone can be implanted into the metaphysis for rotational stability.
PEARLS AND PITFALLS | |
Block augment overhang |
|
Joint line elevation |
|
Tibial stem to tibial tray mismatch |
|
Internal rotation of the tibial component |
|
Extensor mechanism/tibial tubercle |
|
POSTOPERATIVE CARE
 Postoperative care is directed by the intraoperative findings and the stability of the newly implanted component.
Postoperative care is directed by the intraoperative findings and the stability of the newly implanted component.
 If a proximally cemented stemmed component is seated on cortical bone with all defects contained after use of an augment, then immediate full weight bearing may be allowed.
If a proximally cemented stemmed component is seated on cortical bone with all defects contained after use of an augment, then immediate full weight bearing may be allowed.
 Range-of-motion exercises also may begin immediately if the skin over the anterior knee is in good condition postoperatively and the incision has been closed without tension.
Range-of-motion exercises also may begin immediately if the skin over the anterior knee is in good condition postoperatively and the incision has been closed without tension.
 When the tibial component is not fully supported directly by native metaphyseal bone, then toe-touch weight bearing should be initiated until incorporation of any allograft that was used in conjunction with augmentation.
When the tibial component is not fully supported directly by native metaphyseal bone, then toe-touch weight bearing should be initiated until incorporation of any allograft that was used in conjunction with augmentation.
 When ongrowth cones or porous tantalum metal augmentation is used with less than full bone support, consideration should be given to delaying full weight bearing until ingrowth occurs.
When ongrowth cones or porous tantalum metal augmentation is used with less than full bone support, consideration should be given to delaying full weight bearing until ingrowth occurs.
 In cases in which partial weight bearing is initiated postoperatively, progression to full weight bearing can take place at 6 weeks postoperatively.
In cases in which partial weight bearing is initiated postoperatively, progression to full weight bearing can take place at 6 weeks postoperatively.
OUTCOMES
 Several studies have reported successful midterm results with modular metal augments in revision knee arthroplasty.10,15,16 Patel et al15 reported the 5- to 10-year results of 102 revision knee arthroplasties in patients with TII defects treated with augments and stems which were studied prospectively. Average follow-up was 7 years, and nonprogressive radiolucent lines were observed around the augment in 14% of knees but were not associated with decreased survivorship or increased failure of the implants. The overall survivorship of the components was 92% at 11 years.15
Several studies have reported successful midterm results with modular metal augments in revision knee arthroplasty.10,15,16 Patel et al15 reported the 5- to 10-year results of 102 revision knee arthroplasties in patients with TII defects treated with augments and stems which were studied prospectively. Average follow-up was 7 years, and nonprogressive radiolucent lines were observed around the augment in 14% of knees but were not associated with decreased survivorship or increased failure of the implants. The overall survivorship of the components was 92% at 11 years.15
 Rand16 prospectively studied 41 consecutive revision TKAs with modular augmentation. Modular augments were used for the distal femur alone in 2 knees, posterior condyles of the femur alone in 16, and both distally and posteriorly in 12 knees. Tibial augmentation was used in 13 knees. At a mean of 3 years follow-up, 96% of the knees demonstrated good to excellent results and there were no cases of aseptic loosening.16
Rand16 prospectively studied 41 consecutive revision TKAs with modular augmentation. Modular augments were used for the distal femur alone in 2 knees, posterior condyles of the femur alone in 16, and both distally and posteriorly in 12 knees. Tibial augmentation was used in 13 knees. At a mean of 3 years follow-up, 96% of the knees demonstrated good to excellent results and there were no cases of aseptic loosening.16
 Early outcomes with highly porous metaphyseal cones used in large tibial defects for revision TKA have been reported by multiple authors.11,13 Meneghini et al13 reported a series of 15 revision knee arthroplasties that were performed with a porous metal metaphyseal tibial cone and were followed for a minimum of 2 years. All tibial cones were found to be osseointegrated radiographically and clinically at final follow-up with no reported failures in this initial series.
Early outcomes with highly porous metaphyseal cones used in large tibial defects for revision TKA have been reported by multiple authors.11,13 Meneghini et al13 reported a series of 15 revision knee arthroplasties that were performed with a porous metal metaphyseal tibial cone and were followed for a minimum of 2 years. All tibial cones were found to be osseointegrated radiographically and clinically at final follow-up with no reported failures in this initial series.
 In a series of 16 revision TKAs with severe tibial defects, Long and Scuderi11 reported good results with osseointegration of the porous tantalum cone in 14 of 16 cases at a minimum 2-year follow-up. Two metaphyseal cones required removal for recurrent sepsis and were found to be well-fixed at surgery.11 These early results appear equivalent to those obtained with bulk allograft, custom implants, or large modular metal augments at the same time interval. Further clinical and radiographic follow-up will provide insight into the long-term durability of these highly porous augments.
In a series of 16 revision TKAs with severe tibial defects, Long and Scuderi11 reported good results with osseointegration of the porous tantalum cone in 14 of 16 cases at a minimum 2-year follow-up. Two metaphyseal cones required removal for recurrent sepsis and were found to be well-fixed at surgery.11 These early results appear equivalent to those obtained with bulk allograft, custom implants, or large modular metal augments at the same time interval. Further clinical and radiographic follow-up will provide insight into the long-term durability of these highly porous augments.
 Recently, short-term results of porous metal titanium metaphyseal sleeves have been reported in revision TKA.1,2 Barnett et al2 reported on 36 revision TKAs using stepped metaphyseal sleeves at a mean of 38 months. At final follow-up, all metaphyseal sleeves demonstrated radiographic osseointegration without loosening or migration.2
Recently, short-term results of porous metal titanium metaphyseal sleeves have been reported in revision TKA.1,2 Barnett et al2 reported on 36 revision TKAs using stepped metaphyseal sleeves at a mean of 38 months. At final follow-up, all metaphyseal sleeves demonstrated radiographic osseointegration without loosening or migration.2
COMPLICATIONS
 Complications of revision TKA with metallic tibial augmentation can be divided into two categories: early and delayed.
Complications of revision TKA with metallic tibial augmentation can be divided into two categories: early and delayed.
 Perioperative or early complications can include intraoperative damage to neurovascular structures, extensor mechanism and collateral ligamentous disruption, and early postoperative infection.
Perioperative or early complications can include intraoperative damage to neurovascular structures, extensor mechanism and collateral ligamentous disruption, and early postoperative infection.
 Delayed complications most commonly include osteolysis, aseptic loosening, and late septic prosthetic arthropathy.
Delayed complications most commonly include osteolysis, aseptic loosening, and late septic prosthetic arthropathy.
REFERENCES
1. Alexander GE, Bernasek TL, Crank RL, et al. Cementless metaphyseal sleeves used for large tibial defects in revision total knee arthroplasty. J Arthroplasty 2013;28(4):604–607.
2. Barnett SL, Mayer RR, Gondusky JS, et al. Use of stepped porous titanium metaphyseal sleeves for tibial defects in revision total knee arthroplasty: short term results. J Arthroplasty 2014;29(6):1219–1224.
3. Chen F, Krackow KA. Management of tibial defects in total knee arthroplasty. A biomechanical study. Clin Orthop Relat Res 1994;(305): 249–257.
4. Collier MB, Engh CA Jr, McAuley JP, et al. Osteolysis after total knee arthroplasty: influence of tibial baseplate surface finish and sterilization of polyethylene insert. Findings at five to ten years postoperatively. J Bone Joint Surg Am 2005;87(12):2702–2708.
5. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect 1999;48:167–175.
6. Engh GA, Ammeen DJ. Classification and preoperative radiographic evaluation: knee. Orthop Clin North Am 1998;29(2):205–217.
7. Fehring TK, Odum S, Calton TF, et al. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res 2000;(380):9–16.
8. Fehring TK, Odum S, Griffin WL, et al. Early failures in total knee arthroplasty. Clin Orthop Relat Res 2001;(392):315–318.
9. Figgie HE III, Goldberg VM, Heiple KG, et al. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am 1986;68(7):1035–1040.
10. Haas SB, Insall JN, Montgomery W III, et al. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am 1995;77(11):1700–1707.
11. Long WJ, Scuderi GR. Porous tantalum cones for large metaphyseal tibial defects in revision total knee arthroplasty: a minimum 2-year follow-up. J Arthroplasty 2009;24(7):1086–1092.
12. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am 1977;59(1):77–79.
13. Meneghini RM, Lewallen DG, Hanssen AD. Use of porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement. J Bone Joint Surg Am 2008;90(1):78–84.
14. Ninomiya JT, Dean JC, Goldberg VM. Injury to the popliteal artery and its anatomic location in total knee arthroplasty. J Arthroplasty 1999;14(7):803–809.
15. Patel JV, Masonis JL, Guerin J, et al. The fate of augments to treat type-2 bone defects in revision knee arthroplasty. J Bone Joint Surg Br 2004;86(2):195–199.
16. Rand JA. Modularity in total knee arthroplasty. Acta Orthop Belg 1996;62(suppl 1):180–186.
17. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg 2001;9(4):253–257.
18. Smith PN, Gelinas J, Kennedy K, et al. Popliteal vessels in knee surgery. A magnetic resonance imaging study. Clin Orthop Relat Res 1999;(367):158–164.
19. Vessely MB, Frick MA, Oakes D, et al. Magnetic resonance imaging with metal suppression for evaluation of periprosthetic osteolysis after total knee arthroplasty. J Arthroplasty 2006;21(6):826–831.
20. Younger AS, Duncan CP, Masri BA. Surgical exposures in revision total knee arthroplasty. J Am Acad Orthop Surg 1998;6(1):55–64.
< div class='tao-gold-member'>








