The number of revision total knee arthroplasty (TKA) procedures performed is projected to increase at an annual rate of 19.3%.13
 Femoral bone defects are uncommon in primary TKA but are very common in revision knee surgery.
Femoral bone defects are uncommon in primary TKA but are very common in revision knee surgery.
 Modular femoral augments are useful for moderate-sized bony defects, allowing the surgeon to maximize bone–prosthesis contact while restoring the joint line and/or posterior condylar offset.
Modular femoral augments are useful for moderate-sized bony defects, allowing the surgeon to maximize bone–prosthesis contact while restoring the joint line and/or posterior condylar offset.
 Improvements in design and biomaterials have increased the usefulness and versatility of metal augments in addressing larger bone defects, particularly those that are uncontained.
Improvements in design and biomaterials have increased the usefulness and versatility of metal augments in addressing larger bone defects, particularly those that are uncontained.
 A systematic approach to preoperative planning, intraoperative evaluation, and reconstruction is essential in addressing femoral defects using augments.
A systematic approach to preoperative planning, intraoperative evaluation, and reconstruction is essential in addressing femoral defects using augments.
ANATOMY
 The most common form of bone defect encountered at the time of revision surgery is bone loss from the distal and posterior femur (Table 1).
The most common form of bone defect encountered at the time of revision surgery is bone loss from the distal and posterior femur (Table 1).
 Aside from filling the defect, it is important to restore the femorotibial joint line and posterior condylar offset. Significant alterations in either or both will be detrimental to the function of the prosthesis.
Aside from filling the defect, it is important to restore the femorotibial joint line and posterior condylar offset. Significant alterations in either or both will be detrimental to the function of the prosthesis.
 The joint line typically lies 25 mm distal to the femoral epicondyles and the posterior femoral condyles are offset an average of 25.8 mm from the posterior cortex of the femur.1,2
The joint line typically lies 25 mm distal to the femoral epicondyles and the posterior femoral condyles are offset an average of 25.8 mm from the posterior cortex of the femur.1,2
PATHOGENESIS
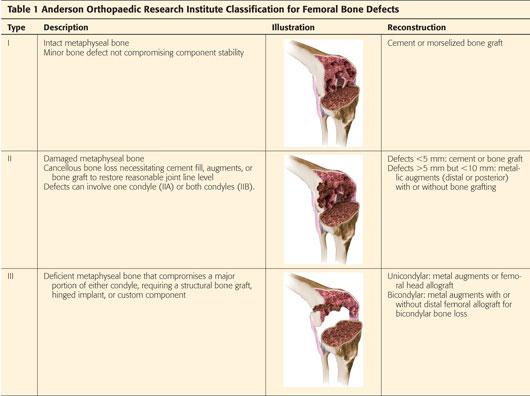
 In unoperated knees, bone loss on the femoral side can be caused by previous osteochondral defects, avascular necrosis, severe valgus or varus deformity, posttraumatic arthritis, or Charcot arthropathy (FIG 1A).
In unoperated knees, bone loss on the femoral side can be caused by previous osteochondral defects, avascular necrosis, severe valgus or varus deformity, posttraumatic arthritis, or Charcot arthropathy (FIG 1A).
 During revision surgery, osteolysis secondary to wear debris and bone loss secondary to removal of well-fixed components or a cement mantle are the most common causes of femoral bone defects (FIG 1B).
During revision surgery, osteolysis secondary to wear debris and bone loss secondary to removal of well-fixed components or a cement mantle are the most common causes of femoral bone defects (FIG 1B).
 Prior trauma resulting in severe angular deformity may rarely require the use of augments for joint reconstruction and restoration of limb alignment.
Prior trauma resulting in severe angular deformity may rarely require the use of augments for joint reconstruction and restoration of limb alignment.
NATURAL HISTORY
 Untreated bone defects in the native knee can lead to progressive joint collapse, ligamentous laxity, or progressive bone loss.
Untreated bone defects in the native knee can lead to progressive joint collapse, ligamentous laxity, or progressive bone loss.
 Osteolytic lesions caused by wear debris can progress and lead to loss of implant support and eventual component loosening.
Osteolytic lesions caused by wear debris can progress and lead to loss of implant support and eventual component loosening.
 Intraoperative mismanagement of defects can lead to suboptimal fixation, significant alterations in knee kinematics, instability, or early implant failure.
Intraoperative mismanagement of defects can lead to suboptimal fixation, significant alterations in knee kinematics, instability, or early implant failure.
PATIENT HISTORY AND PHYSICAL FINDINGS
 A complete history and physical examination must be performed before any revision knee surgery is undertaken. The details of the index arthroplasty with regard to pain relief and the interval to failure should be recorded. In addition, problems during the postoperative period such as falls or operative wound complications should be anticipated.
A complete history and physical examination must be performed before any revision knee surgery is undertaken. The details of the index arthroplasty with regard to pain relief and the interval to failure should be recorded. In addition, problems during the postoperative period such as falls or operative wound complications should be anticipated.
 Patients with loose femoral components often present with painful TKAs. Pain often occurs at start-up, arising from the seated position, and with stair climbing. These patients often may complain of swelling and effusions within the knees.
Patients with loose femoral components often present with painful TKAs. Pain often occurs at start-up, arising from the seated position, and with stair climbing. These patients often may complain of swelling and effusions within the knees.
 An anteroposterior (AP) radiograph of the pelvis and a careful back and hip examination should be performed to rule out coexistent spinal or hip disorder as the cause of the patient’s knee pain.
An anteroposterior (AP) radiograph of the pelvis and a careful back and hip examination should be performed to rule out coexistent spinal or hip disorder as the cause of the patient’s knee pain.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Plain radiographs of the knee, including standing AP, lateral, and Merchant views of the affected knee, should be reviewed. For patients with deformity, the entire length of the affected bone should be visualized.
Plain radiographs of the knee, including standing AP, lateral, and Merchant views of the affected knee, should be reviewed. For patients with deformity, the entire length of the affected bone should be visualized.
 In revision cases, serial radiographs may help assess the progression of osteolysis, radiolucent lines, and implant migration. A knee component is definitely loose when there has been evidence of change of implant position, migration, or subsidence on serial radiographs. An implant is likely loose if there is evidence of progressive radiolucent lines on serial radiographs.
In revision cases, serial radiographs may help assess the progression of osteolysis, radiolucent lines, and implant migration. A knee component is definitely loose when there has been evidence of change of implant position, migration, or subsidence on serial radiographs. An implant is likely loose if there is evidence of progressive radiolucent lines on serial radiographs.
 Computed tomography (CT) scans of the knee permit assessment of component rotation and can help assess the size and location of osteolysis more accurately.7 Recently, magnetic resonance imaging has been shown to be effective in quantifying osteolytic defects.14
Computed tomography (CT) scans of the knee permit assessment of component rotation and can help assess the size and location of osteolysis more accurately.7 Recently, magnetic resonance imaging has been shown to be effective in quantifying osteolytic defects.14
 Blood studies, including a complete blood count with differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), should be obtained to rule out infection.
Blood studies, including a complete blood count with differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), should be obtained to rule out infection.
 Nuclear medicine studies including bone scan (to detect a loose prosthesis) and indium and sulfur colloid scans (to detect the presence of infection) can also sometimes be helpful in the preoperative workup when obvious signs of failure are absent. It is important to remember that these modalities are technique-dependent and a bone scan can continue to show increased activity up to 18 months following index arthroplasty.10
Nuclear medicine studies including bone scan (to detect a loose prosthesis) and indium and sulfur colloid scans (to detect the presence of infection) can also sometimes be helpful in the preoperative workup when obvious signs of failure are absent. It is important to remember that these modalities are technique-dependent and a bone scan can continue to show increased activity up to 18 months following index arthroplasty.10
 Aspiration should be done when there is any suspicion of infection or elevations in serum inflammatory markers (eg, ESR or CRP). The synovial fluid should be examined for the number and differential of white blood cells and cultured for the presence of microorganisms.5
Aspiration should be done when there is any suspicion of infection or elevations in serum inflammatory markers (eg, ESR or CRP). The synovial fluid should be examined for the number and differential of white blood cells and cultured for the presence of microorganisms.5
DIFFERENTIAL DIAGNOSIS
 Infection
Infection
 Arthritis of the hip and spine
Arthritis of the hip and spine
 Flexion instability
Flexion instability
 Patellar maltracking and extensor mechanism dysfunction
Patellar maltracking and extensor mechanism dysfunction
 Tibial component loosening
Tibial component loosening
 Periprosthetic fractures
Periprosthetic fractures
NONOPERATIVE MANAGEMENT
 Unless the patient has obvious signs of component malpositioning, loosening, fracture, or infection, a trial of conservative treatment aimed at strengthening the quadriceps musculature with emphasis on the vastus medialis oblique muscle should be the cornerstone of nonoperative treatment.
Unless the patient has obvious signs of component malpositioning, loosening, fracture, or infection, a trial of conservative treatment aimed at strengthening the quadriceps musculature with emphasis on the vastus medialis oblique muscle should be the cornerstone of nonoperative treatment.
 Patients who have evidence of late osteolysis and polyethylene wear on plain radiographs but no clinical symptoms or pain should be encouraged to obtain serial radiographs annually to check for progression of the lesion(s).
Patients who have evidence of late osteolysis and polyethylene wear on plain radiographs but no clinical symptoms or pain should be encouraged to obtain serial radiographs annually to check for progression of the lesion(s).
 No consensus has been reached regarding when it is appropriate to revise or perform bone grafting in an asymptomatic knee with radiographic evidence of osteolysis. However, revision should occur as soon as symptoms present in order to avoid progression and worsening of bone loss.
No consensus has been reached regarding when it is appropriate to revise or perform bone grafting in an asymptomatic knee with radiographic evidence of osteolysis. However, revision should occur as soon as symptoms present in order to avoid progression and worsening of bone loss.
SURGICAL MANAGEMENT
 A systematic approach is required to reconstruct bone defects successfully during revision surgery.
A systematic approach is required to reconstruct bone defects successfully during revision surgery.
Preoperative Planning
 Thorough preoperative planning is the key to a successful reconstruction.
Thorough preoperative planning is the key to a successful reconstruction.
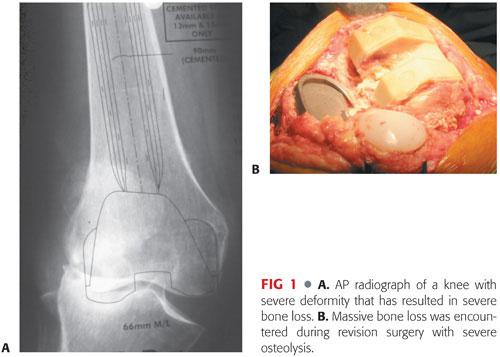
 Review of radiographs and CT scans and careful templating allow anticipation of problems that could be encountered during surgery (FIG 2A,B).
Review of radiographs and CT scans and careful templating allow anticipation of problems that could be encountered during surgery (FIG 2A,B).
 Most bone loss can be addressed by the use of metal augments and a stemmed prosthesis (FIG 2C). There have been no differences in the clinical outcomes of revisions performed using cemented stemmed components compared to uncemented press-fit implants. Diaphyseal engagement of a press-fit stem is required to minimize the risk of component loosening.6
Most bone loss can be addressed by the use of metal augments and a stemmed prosthesis (FIG 2C). There have been no differences in the clinical outcomes of revisions performed using cemented stemmed components compared to uncemented press-fit implants. Diaphyseal engagement of a press-fit stem is required to minimize the risk of component loosening.6
 For larger, uncontained bone lesions, special augments (eg, cones or sleeves), femoral heads, and allografts must be ordered in advance so they are available during reconstruction (see TECH FIG 4B and Video 1).
For larger, uncontained bone lesions, special augments (eg, cones or sleeves), femoral heads, and allografts must be ordered in advance so they are available during reconstruction (see TECH FIG 4B and Video 1).
 In all cases of revision, stemmed and constrained implants (or sometimes a hinged prosthesis) must be considered and made available.
In all cases of revision, stemmed and constrained implants (or sometimes a hinged prosthesis) must be considered and made available.
Positioning
 The standard position for patients undergoing revision TKA is supine.
The standard position for patients undergoing revision TKA is supine.
 Care is taken to drape a wide surgical field in case a more extensile approach is necessary. A sterile tourniquet applied on the surgical field may be helpful.
Care is taken to drape a wide surgical field in case a more extensile approach is necessary. A sterile tourniquet applied on the surgical field may be helpful.
Approach
 The knee is approached via the standard medial parapatellar approach.
The knee is approached via the standard medial parapatellar approach.
 Protection of the patellar tendon during this phase of the operation is crucial.
Protection of the patellar tendon during this phase of the operation is crucial.
 An extensive synovectomy and débridement of the medial and lateral gutters are critical for decompression of the joint.
An extensive synovectomy and débridement of the medial and lateral gutters are critical for decompression of the joint.
 The patella is usually not everted during revision TKA.
The patella is usually not everted during revision TKA.
 In knees with severe ankylosis, techniques such as the quadriceps snip, lateral release, and tibial tubercle osteotomy are helpful for exposure. The surgeon must know the implications of each of these releases and repair or reconstruct them properly at the end of the procedure.
In knees with severe ankylosis, techniques such as the quadriceps snip, lateral release, and tibial tubercle osteotomy are helpful for exposure. The surgeon must know the implications of each of these releases and repair or reconstruct them properly at the end of the procedure.
 The bone defect is addressed by the use of cement (smaller lesions), metal augments, or structural grafts or metaphyseal augments (cones or sleeves) for large uncontained defects. The critical issues are to restore joint line, achieve appropriate alignment, and attain ligamentous balancing. Reconstruction also aims at restoring a stable platform for positioning and fixation of the components.
The bone defect is addressed by the use of cement (smaller lesions), metal augments, or structural grafts or metaphyseal augments (cones or sleeves) for large uncontained defects. The critical issues are to restore joint line, achieve appropriate alignment, and attain ligamentous balancing. Reconstruction also aims at restoring a stable platform for positioning and fixation of the components.
TECHNIQUES
 Metal Augments
Metal Augments
Modular metal augments allow for restoration of distal, posterior, and even metaphyseal femoral defects.
For most systems, the largest femoral augments allow for restoration of 8 to 10 mm of bone defect. The use of cemented stacked augments for filling defects up to 30 mm has been reported.8
Most revision systems have intramedullary systems that allow bone cuts to be made relative to a press-fit intramedullary rod.
The distal femoral cut is freshened to provide a stable platform for the new prosthesis.
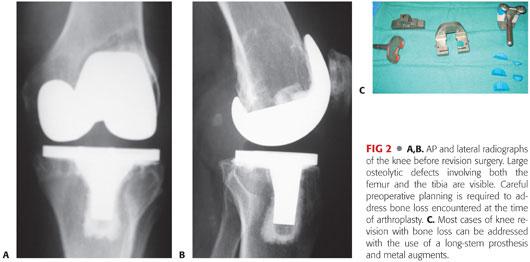
Next, the size of the femoral component is selected. Preoperative templating can give clues to the proper component size. Traditionally, the femoral component is upsized to better fill the flexion gap (TECH FIG 1A).
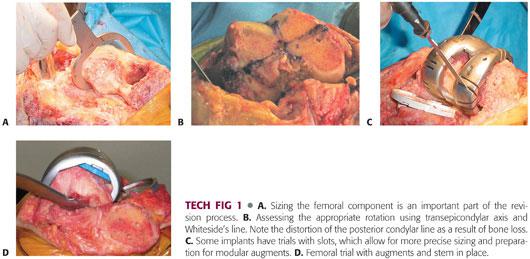
Determining proper femoral component rotation is critical to a successful reconstruction. The femoral component should be set parallel to the transepicondylar axis of the femur (TECH FIG 1B). Other secondary guides include the proximal tibia (parallel) and the femoral intercondylar line (perpendicular).
Malrotation of the femoral component can also exaggerate the severity of bone loss. Typically, a posterolateral augment and distal femoral augments are required to ensure proper restoration of rotation and joint line.
The rest of the reconstruction varies according to the revision knee system being used but should follow a systematic approach. In some systems, the trial components have slots that allow bone cuts to be made for more precise fitting of the modular augment (TECH FIG 1C).
A stemmed trial femoral component with the necessary augments is assembled and inserted. Trialing should focus on the overall stability of the knee in extension and flexion as well as patellofemoral tracking (TECH FIG 1D).
The definitive prosthesis is assembled and cemented into place in the standard fashion.
 Bone Cement
Bone Cement
Bone cement is indicated for use in small, preferably contained, bony defects up to 5 mm in depth.
Its limitations include low modulus of elasticity and that it does not restore bone stock.
Following removal of the existing prosthesis, all surfaces are thoroughly débrided. There is a membrane that often forms between the old bone–cement interface. Areas of bony sclerosis should be débrided with the aid of a high-speed burr to punctate bleeding.
The femur is prepared with the revision instrumentation specific to the implant system, paying attention to joint line restoration, rotation, and restoration of the posterior condylar offset. The use of stemmed implants allows for distribution of joint stresses and is almost always required in cases of revision surgery with bone loss.
The new femoral component is cemented into place separately, and the cement is allowed to harden under direct vision.
 Morselized Autograft or Allograft
Morselized Autograft or Allograft
Indicated for use in larger contained defects, especially in younger patients. The main advantage of this technique is that it allows for restoration of bone stock.
Its limitations are that it cannot be used for uncontained defects and does not restore a stable platform capable of supporting the prosthesis.
Using a curette or a high-speed burr, the host bone is débrided to create a favorable environment for graft incorporation.
Visible defects are packed with morselized autograft or allograft.
A bone tamp may be used to pack the bone chips tightly.
Prepare the femur using the revision instrumentation particular to the system being used.
Once the femur is prepared, insert a stemmed trial femoral component, with or without wedges, onto the femur.
Before final impaction of the trial component, tightly pack the bone chips around the stems and the posterior condyles. Impacting the trial prosthesis into place will effectively shape the new distal femur.
The new stemmed femoral component is cemented separately to minimize the chance of component malposition.
 Structural Allografts (Femoral Hemicondyle)
Structural Allografts (Femoral Hemicondyle)
Structural allografts are indicated for large, uncontained defects involving one femoral condyle. The attachments of the collateral ligaments are preserved by a thin shell of bone following removal of the femoral implant.
The host bone–allograft interface is prepared as previously described.
Gently, using a hemispherical acetabular reamer, the bony defect is reamed to accept a femoral head (TECH FIG 2A).
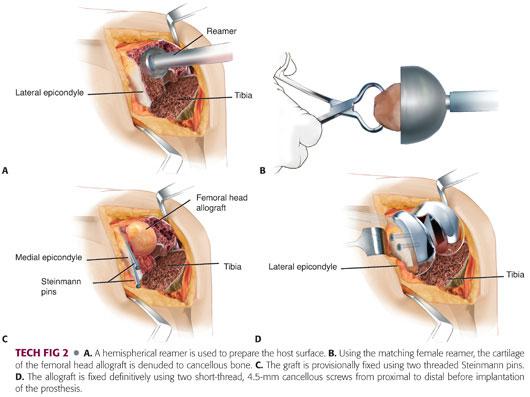
Using the corresponding female resurfacing reamer, a femoral head allograft is reamed to remove all cartilaginous debris (TECH FIG 2B).
The femoral head is coupled to the bony defect and secured using two threaded Steinmann pins inserted from proximal to distal (TECH FIG 2C).
The distal femur is then prepared using the instrumentation particular to the system being used.
The femoral head allograft is fixed to the host bone using 4.5-mm short-thread cancellous screws inserted from proximal to distal (TECH FIG 2D).
Finally, a stemmed femoral component is cemented into place.
 Distal Femoral Allograft
Distal Femoral Allograft
Indicated for massive osteolytic defects involving both distal condyles and distal femoral metaphysis. It allows for restoration of bone stock while preserving the collateral ligament insertions.
In cases in which a distal femoral allograft may be required, preoperative sizing of the host femur and the allograft is crucial.
Comparing radiographs of the allograft to the host femur (may size against an unoperated side if available) improves fit and decreases the chances of mismatch.
The native femur is prepared to accept the allograft by carefully preserving the collateral ligament attachments (TECH FIG 3A).
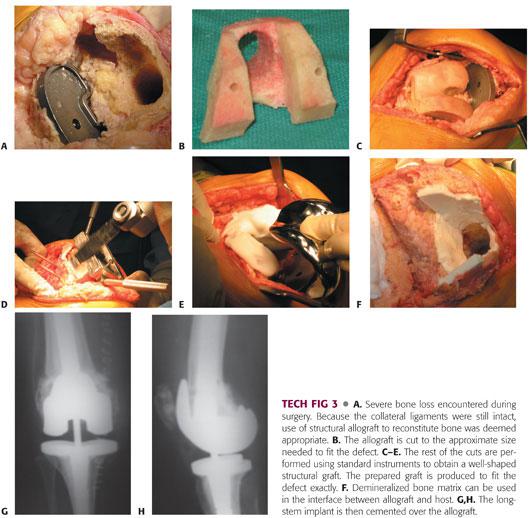
The allograft is then shaped to allow for intussusception (bone within bone) into the native femur. It is important to obtain a secure fit of the allograft during this step (TECH FIG 3B).
Using traditional distal femoral cutting guides, the femur is prepared to accept a stemmed prosthesis (TECH FIG 3C–E).
Demineralized bone matrix is used to line the host–allograft interface. This allows the filling of gaps and also provides a barrier against cement intrusion (TECH FIG 3F).
A stemmed femoral implant is cemented into place (TECH FIG 3G,H).
 Metaphyseal Augments (Cones and Sleeves)
Metaphyseal Augments (Cones and Sleeves)
Indicated for large, uncontained metaphyseal defects when rotation of the femoral component cannot be achieved using cement, morselized grafts, or other structural grafts.
Its advantages over structural allografts include instrumentation, no risk for disease transmission, and osteointegration with no risk for graft resorption.
Following removal of the old femoral component, the host bone is débrided, and the nature, size, and shape of the defect is identified.
Using a high-speed burr (cones) or broaches (sleeves), the femoral defect is prepared to accept the metaphyseal augment. The augment should fit securely into the host bone to maximize the chance for bone ingrowth (TECH FIG 4A,B).
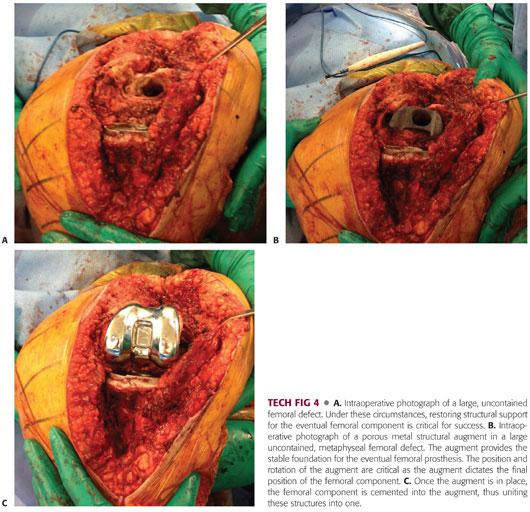
Particular attention should be given to the depth of seating of the augment in relation to the joint line and to the rotation of these augments. If the augment is countersunk, it will not allow for support at the proper level in order for proper joint line restoration. Similarly, if the augment or the sleeve is severely malrotated, it will not allow the revised femoral component to properly restore rotation.
The cone augment is inserted independently prior to femoral component implantation. When a femoral sleeve is used, the augment is united with the femoral component and inserted as part of the final implantation (TECH FIG 4C).
PEARLS AND PITFALLS | |
Preoperative planning |
|
| |
| |
Exposure |
|
| |
Bone loss |
|
| |
| |
| |
Reconstruction |
|
| |
| |
| |
Trial components |
|
| |
| |
Fixation |
|
| |
POSTOPERATIVE CARE
 Early motion is instituted for most patients using a continuous passive motion machine set from 0 to 60 degrees immediately following surgery.
Early motion is instituted for most patients using a continuous passive motion machine set from 0 to 60 degrees immediately following surgery.
 For patients who have had extensive procedures, a well-padded Robert Jones dressing is applied following the operation. Immobilization is maintained for 24 to 48 hours.
For patients who have had extensive procedures, a well-padded Robert Jones dressing is applied following the operation. Immobilization is maintained for 24 to 48 hours.
 Following revision TKA, patients are usually allowed to bear weight as tolerated on the prosthesis.
Following revision TKA, patients are usually allowed to bear weight as tolerated on the prosthesis.
 Alterations of weight-bearing status and limitations on knee flexion are similar to that of other procedures performed at the time of arthroplasty such as tibial tubercle osteotomy, quadriceps snip, or V-Y turndown.
Alterations of weight-bearing status and limitations on knee flexion are similar to that of other procedures performed at the time of arthroplasty such as tibial tubercle osteotomy, quadriceps snip, or V-Y turndown.
OUTCOMES
 Overall, the survivorship of revision femoral components using metal wedges with or without structural augments is 79.4% at 8 years.8
Overall, the survivorship of revision femoral components using metal wedges with or without structural augments is 79.4% at 8 years.8
 For knees with small, contained cavitary defects requiring only cement or morselized graft, the 10-year survivorship approaches that of primary TKA.11
For knees with small, contained cavitary defects requiring only cement or morselized graft, the 10-year survivorship approaches that of primary TKA.11
 Modular femoral augments used for reconstruction of type II defects have an 11-year survival rate of 92%. It is not uncommon to see nonprogressive radiolucent lines surrounding metallic augments.12
Modular femoral augments used for reconstruction of type II defects have an 11-year survival rate of 92%. It is not uncommon to see nonprogressive radiolucent lines surrounding metallic augments.12
 Early results of tantalum femoral cones and porous metal sleeves have shown these devices to achieve reliable fixation and osteointegration in complex revision TKAs.4,9,15
Early results of tantalum femoral cones and porous metal sleeves have shown these devices to achieve reliable fixation and osteointegration in complex revision TKAs.4,9,15
 Femoral revisions augmented with structural allografts have a 10-year survival rate of 75%.3
Femoral revisions augmented with structural allografts have a 10-year survival rate of 75%.3
COMPLICATIONS
 Traditional complications following revision TKA include infection, wound complications, and loosening.
Traditional complications following revision TKA include infection, wound complications, and loosening.
 Patellar maltracking and extensor mechanism dysfunction can also occur, especially if there is component malrotation.
Patellar maltracking and extensor mechanism dysfunction can also occur, especially if there is component malrotation.
 Knee instability can result from an imbalance in the flexion and extension gaps.
Knee instability can result from an imbalance in the flexion and extension gaps.
 Resorption of large structural allografts leading to subsequent implant loosening has been described.
Resorption of large structural allografts leading to subsequent implant loosening has been described.
REFERENCES
1. Banks SA, Harman MK, Bellemans J, et al. Making sense of knee arthroplasty kinematics: news you can use. J Bone Joint Surg Am 2003;85(suppl 4):64–72.
2. Bellemans J, Banks S, Victor J, et al. Fluoroscopic analysis of the kinematics of deep flexion in total knee arthroplasty. Influence of posterior condylar offset. J Bone Joint Surg Br 2002;84:50–53.
3. Clatworthy MG, Ballance J, Brick GW, et al. The use of structural allograft for unconstrained defects in revision total knee replacement. A minimum five-year review. J Bone Joint Surg Am 2001;83:404–411.
4. Daines BK, Dennis DA. Management of bone defects in revision total knee arthroplasty. Instr Course Lect 2013;62:341–348.
5. Della Valle C, Parvizi J, Bauer TW, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am 2011;93(14):1355–1357.
6. Fehring TK, Odum S, Olekson C, et al. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res 2003;(416):217–224.
7. Gonzalez MH, Mekhail AO. The failed total knee arthroplasty: evaluation and etiology. J Am Acad Orthop Surg 2004;12:436–446.
8. Hockman DE, Ammeen D, Engh GA. Augments and allografts in revision total knee arthroplasty: usage and outcome using one modular revision prosthesis. J Arthroplasty 2005;20:35–41.
9. Howard JL, Kudera J, Lewallen DG, et al. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am 2011;93(5):478–484.
10. Kitchener MI, Coats E, Keene G, et al. Assessment of radionuclide arthrography in evaluation of loosening of knee prostheses. Knee 2006;13(3):220–225.
11. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res 2001;(392): 279–282.
12. Patel JV, Masonis JL, Guerin J, et al. The fate of augments to treat type-2 bone defects in revision knee arthroplasty. J Bone Joint Surg Br 2004;86:195–199.
13. Saleh KJ, Rand JA, McQueen DA. Current status of revision total knee replacements: how do we assess results. J Bone Joint Surg Am 2003;85(suppl 1):S18–S20.
14. Vessely MB, Frick MA, Oakes D, et al. Magnetic resonance imaging with metal suppression for evaluation of periprosthetic osteolysis after total knee arthroplasty. J Arthroplasty 2006;21:826–831.
15. Werle JR, Goodman SB, Imrie SN. Revision total knee arthroplasty using large distal femoral augments for severe metaphyseal bone deficiency: a preliminary study. Orthopedics 2002;25:325–327.
< div class='tao-gold-member'>






















