During revision of the femoral component in total knee arthroplasty (TKA), some bone loss from the distal femur is nearly inevitable when the femoral component is removed.
 Distal femoral bone loss can be repaired by bone cement (polymethylmethacrylate), metal augments fixed to the revision femoral component, particulate bone graft or substitutes, bulk allograft to augment one or both femoral condyles, porous metal cones designed for structural support, and complete distal femoral replacement with allograft or metal.
Distal femoral bone loss can be repaired by bone cement (polymethylmethacrylate), metal augments fixed to the revision femoral component, particulate bone graft or substitutes, bulk allograft to augment one or both femoral condyles, porous metal cones designed for structural support, and complete distal femoral replacement with allograft or metal.
ANATOMY
 The anatomy relevant to bone loss during revision TKA are the metaphyseal femur, the medial and lateral epicondyles, and the medial and lateral femoral condyles.
The anatomy relevant to bone loss during revision TKA are the metaphyseal femur, the medial and lateral epicondyles, and the medial and lateral femoral condyles.
 The femoral condyles support the revision femoral component. Accordingly, one goal of distal femoral reconstruction in revision TKA is to restore the condylar anatomy so that it can support a new component.
The femoral condyles support the revision femoral component. Accordingly, one goal of distal femoral reconstruction in revision TKA is to restore the condylar anatomy so that it can support a new component.
PATHOGENESIS
 The pathogenesis of distal femoral bone loss in revision TKA is related to removal of the previous implant and bone cement. Previous implants fixed to bone with cement or by porous ingrowth require dissection for mobilization and extraction; this process incurs a finite amount of bone loss.
The pathogenesis of distal femoral bone loss in revision TKA is related to removal of the previous implant and bone cement. Previous implants fixed to bone with cement or by porous ingrowth require dissection for mobilization and extraction; this process incurs a finite amount of bone loss.
 Even fine osteotomes and saws used to develop a plane between the implant and bone occupy space and cause bone loss from the distal femur. Aggressive extraction of well-fixed femoral components without first developing a plane between exposed metal and bone can result in avulsion of one or both femoral condyles, thereby compounding bone loss.
Even fine osteotomes and saws used to develop a plane between the implant and bone occupy space and cause bone loss from the distal femur. Aggressive extraction of well-fixed femoral components without first developing a plane between exposed metal and bone can result in avulsion of one or both femoral condyles, thereby compounding bone loss.
 Correction of internal rotation of the previous femoral component will result in bone loss both anteriorly and posteriorly as the new component is oriented in the proper rotation.
Correction of internal rotation of the previous femoral component will result in bone loss both anteriorly and posteriorly as the new component is oriented in the proper rotation.
 Osteopenia from stress shielding of the periprosthetic femur, as well as osteolysis related to wear debris particles, can result in cavitary lesions in the distal femur that lead to significant bone loss.
Osteopenia from stress shielding of the periprosthetic femur, as well as osteolysis related to wear debris particles, can result in cavitary lesions in the distal femur that lead to significant bone loss.
NATURAL HISTORY
 Distal femoral bone loss, if severe, can result in the loss of the structural integrity of the femur. When this occurs, the existing femoral component can migrate into the varus or valgus position relative to the femoral shaft.
Distal femoral bone loss, if severe, can result in the loss of the structural integrity of the femur. When this occurs, the existing femoral component can migrate into the varus or valgus position relative to the femoral shaft.
 Surgical treatment is directed at augmenting this bone loss following removal of the previous loose, unstable femoral component.
Surgical treatment is directed at augmenting this bone loss following removal of the previous loose, unstable femoral component.
 If left untreated, continued particulate debris and motion between loose components and bone can lead to persistent symptoms and further bone loss in the distal femur of a failed TKA.
If left untreated, continued particulate debris and motion between loose components and bone can lead to persistent symptoms and further bone loss in the distal femur of a failed TKA.
PATIENT HISTORY AND PHYSICAL FINDINGS
 The diagnosis of a failed TKA with loss of bone in the distal femur is best made by plain radiographs.
The diagnosis of a failed TKA with loss of bone in the distal femur is best made by plain radiographs.
 Findings in any of the following categories can alert the surgeon to the possibility that significant bone loss may be encountered during revision surgery: the time elapsed since the index arthroplasty, the type of implant and fixation used, and any history of diseases such as osteoporosis, advanced age, corticosteroid use, use of cytotoxic drugs, irradiation, rheumatoid arthritis, and periprosthetic femoral fracture.
Findings in any of the following categories can alert the surgeon to the possibility that significant bone loss may be encountered during revision surgery: the time elapsed since the index arthroplasty, the type of implant and fixation used, and any history of diseases such as osteoporosis, advanced age, corticosteroid use, use of cytotoxic drugs, irradiation, rheumatoid arthritis, and periprosthetic femoral fracture.
Patients who have loose femoral components with associated bone loss will present with knee pain, swelling, and instability that is usually worsened by activity.
 Failed TKA femoral components with bone loss will have tenderness to palpation over the distal femur.
Failed TKA femoral components with bone loss will have tenderness to palpation over the distal femur.
An effusion may also be evident on physical examination.
A grossly unstable component may result in ligamentous instability of the knee that is elicited on careful examination.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 High-quality radiographs of the knee can help identify and classify a bone defect in the distal femur. The true lateral view of the knee joint can demonstrate the location and extent of osteolysis and bone loss in the distal femur. Oblique views of the knee often result in obscuring of bony detail by the metal implants. Therefore, a true lateral view of the knee should be obtained in 90 degrees of knee flexion by placing the entire leg, including the knee and ankle joints, flat on the radiograph table.
High-quality radiographs of the knee can help identify and classify a bone defect in the distal femur. The true lateral view of the knee joint can demonstrate the location and extent of osteolysis and bone loss in the distal femur. Oblique views of the knee often result in obscuring of bony detail by the metal implants. Therefore, a true lateral view of the knee should be obtained in 90 degrees of knee flexion by placing the entire leg, including the knee and ankle joints, flat on the radiograph table.
 Other imaging modalities such as computed tomography scans and metal subtraction magnetic resonance imaging scans may prove useful in defining the extent of bone loss in the distal femur, although the efficacy of these imaging studies in routine revision TKA is not established.
Other imaging modalities such as computed tomography scans and metal subtraction magnetic resonance imaging scans may prove useful in defining the extent of bone loss in the distal femur, although the efficacy of these imaging studies in routine revision TKA is not established.
 Intraoperative observation of bone loss after previous component removal and thorough débridement of osteolytic lesions and inflammatory membrane is the best determinant of the nature and extent of bone loss.
Intraoperative observation of bone loss after previous component removal and thorough débridement of osteolytic lesions and inflammatory membrane is the best determinant of the nature and extent of bone loss.
 The surgeon should be prepared for the worst-case scenario because preoperative radiographs will often underestimate the extent of bone loss adjacent to the existing femoral component.
The surgeon should be prepared for the worst-case scenario because preoperative radiographs will often underestimate the extent of bone loss adjacent to the existing femoral component.
Accordingly, allograft bone, a wide range of revision implants, metal augments, porous metal reconstructive cones, and revision equipment should be available.
 The diagnostic workup of femoral bone loss in revision TKA should include a thorough evaluation to exclude the possibility of knee sepsis.
The diagnostic workup of femoral bone loss in revision TKA should include a thorough evaluation to exclude the possibility of knee sepsis.
Preoperative nuclear medicine imaging, laboratory data, knee aspiration, and intraoperative frozen sections of periprosthetic tissues can assist in excluding sepsis.
DIFFERENTIAL DIAGNOSIS
 Deep knee sepsis can result in periprosthetic bone loss and is a relative contraindication to distal femoral reconstruction. Infection must be ruled out before reconstructing the distal femur in anticipation of implanting a revision femoral component.
Deep knee sepsis can result in periprosthetic bone loss and is a relative contraindication to distal femoral reconstruction. Infection must be ruled out before reconstructing the distal femur in anticipation of implanting a revision femoral component.
 Stress shielding of bone adjacent to the femoral component can result in bone loss from the distal femur. When the existing femoral component is extracted, severe loss of bone may be discovered in such cases.
Stress shielding of bone adjacent to the femoral component can result in bone loss from the distal femur. When the existing femoral component is extracted, severe loss of bone may be discovered in such cases.
 Osteopenia of the distal femur from osteoporosis, lytic lesions of bone such as benign bone cysts, neuropathic changes, and malignancy can also result in distal femoral bone loss, thereby complicating femoral reconstruction in TKA.
Osteopenia of the distal femur from osteoporosis, lytic lesions of bone such as benign bone cysts, neuropathic changes, and malignancy can also result in distal femoral bone loss, thereby complicating femoral reconstruction in TKA.
NONOPERATIVE MANAGEMENT
 Nonoperative management of severe distal femoral bone loss in a failed TKA should be reserved for debilitated patients in whom surgery is otherwise contraindicated.
Nonoperative management of severe distal femoral bone loss in a failed TKA should be reserved for debilitated patients in whom surgery is otherwise contraindicated.
 Severe medical comorbidities, integrity of the extensor mechanism, poor condition of the soft tissue, radiation necrosis of adjacent bone, immunosuppression, and metabolic bone disorders should be evaluated carefully to determine whether reconstruction of the distal femur is a reasonable option.
Severe medical comorbidities, integrity of the extensor mechanism, poor condition of the soft tissue, radiation necrosis of adjacent bone, immunosuppression, and metabolic bone disorders should be evaluated carefully to determine whether reconstruction of the distal femur is a reasonable option.
 Where major knee surgery is contraindicated, nonoperative measures such as analgesics, limited ambulation, assistive devices such as a walker or wheelchair, and knee bracing are alternative considerations.
Where major knee surgery is contraindicated, nonoperative measures such as analgesics, limited ambulation, assistive devices such as a walker or wheelchair, and knee bracing are alternative considerations.
 Chronic suppressive antibiotics may be an option in patients with bone loss from severe deep sepsis in whom operative treatment is otherwise contraindicated.
Chronic suppressive antibiotics may be an option in patients with bone loss from severe deep sepsis in whom operative treatment is otherwise contraindicated.
SURGICAL MANAGEMENT
 Distal femoral bone loss can be managed surgically using bone cement (augmented with screws driven into existing bone, if necessary), morselized bone graft, metal augments fixed to the revision implant, porous metal cones designed to reproduce distal femoral anatomy, bulk allograft reconstruction of uncontained defects of the medial or lateral condyles, and bulk allograft replacement of the distal femur with allograft or a special customized prosthesis.
Distal femoral bone loss can be managed surgically using bone cement (augmented with screws driven into existing bone, if necessary), morselized bone graft, metal augments fixed to the revision implant, porous metal cones designed to reproduce distal femoral anatomy, bulk allograft reconstruction of uncontained defects of the medial or lateral condyles, and bulk allograft replacement of the distal femur with allograft or a special customized prosthesis.
Preoperative Planning
 Preoperative planning includes making sure the patient is medically optimized for major elective surgery and excluding the possibility of deep sepsis in the knee. The surgeon should carefully assess existing scars, leg vascularity, nerve function, and the patient’s overall medical condition.
Preoperative planning includes making sure the patient is medically optimized for major elective surgery and excluding the possibility of deep sepsis in the knee. The surgeon should carefully assess existing scars, leg vascularity, nerve function, and the patient’s overall medical condition.
 Pre- and intraoperative assessment of collateral ligament integrity will help the surgeon choose appropriate implants and plan the reconstruction.
Pre- and intraoperative assessment of collateral ligament integrity will help the surgeon choose appropriate implants and plan the reconstruction.
In grossly unstable knees, constrained implants or even rotating hinge revision TKA implants may be indicated.
 The surgeon should prepare for the worst-case scenario so that the correct equipment, implants, and personnel will be available to address any circumstance. However, outside of straightforward TKA revision cases with minimal femoral bone deficiency, such procedures are limited to hospitals that have the necessary equipment and expertise due to the specialized procedures that are needed.
The surgeon should prepare for the worst-case scenario so that the correct equipment, implants, and personnel will be available to address any circumstance. However, outside of straightforward TKA revision cases with minimal femoral bone deficiency, such procedures are limited to hospitals that have the necessary equipment and expertise due to the specialized procedures that are needed.
 A preoperative planning session with key personnel (ie, surgeon, assistants, and implant manufacturer representative) is invaluable for discussing the problem and considering all possible solutions for efficient execution of the procedure.
A preoperative planning session with key personnel (ie, surgeon, assistants, and implant manufacturer representative) is invaluable for discussing the problem and considering all possible solutions for efficient execution of the procedure.
 Structural allografts to rebuild deficient distal femoral condyles should include several allograft femoral heads.
Structural allografts to rebuild deficient distal femoral condyles should include several allograft femoral heads.
Fresh frozen prepared specimens are usually favored because of their superior mechanical strength.7
 Preoperative radiography and sizing of allograft tissue is desirable but may not be practical or feasible.
Preoperative radiography and sizing of allograft tissue is desirable but may not be practical or feasible.
Instead, intraoperative preparation of the grafts with special equipment such as the Allogrip system (DePuy Synthes, Warsaw, IN) can shape the graft to proper dimensions so that wound closure is not a problem with an oversized graft.4
 A full set of revision TKA instruments is essential, consisting of fine osteotomes to develop the plane between metal and bone, curettes, punches, reamers, Gigli saws, and instruments to clean out the intramedullary femoral canal.
A full set of revision TKA instruments is essential, consisting of fine osteotomes to develop the plane between metal and bone, curettes, punches, reamers, Gigli saws, and instruments to clean out the intramedullary femoral canal.
High-speed burrs attached to a pneumatic power driver can assist in loosening well-fixed implants while preserving bone.
A typical revision TKA instrument set from any major implant manufacturer has these instruments conveniently packaged in a single set.
 Porous tantalum metal cones (and similar cones made of porous titanium metal) are available for proximal tibia reconstruction in revision TKA as well as for distal femoral reconstruction.6 The advantage of this type of metal reconstruction is the rapid healing of structural metal support to living host bone. Initial mechanical stability is achieved by impacting these cones into residual, viable bone of the distal femur. These metal cones offer a custom reconstruction option that permits load bearing and supports cement fixation of the revision implant. Depending on the extent of bone loss, the surgeon can choose the type of metal cone that will best address the deficiency, including replacement of both femoral condyles if necessary. Intermediate-term clinical outcomes with this type of reconstruction have been favorable.
Porous tantalum metal cones (and similar cones made of porous titanium metal) are available for proximal tibia reconstruction in revision TKA as well as for distal femoral reconstruction.6 The advantage of this type of metal reconstruction is the rapid healing of structural metal support to living host bone. Initial mechanical stability is achieved by impacting these cones into residual, viable bone of the distal femur. These metal cones offer a custom reconstruction option that permits load bearing and supports cement fixation of the revision implant. Depending on the extent of bone loss, the surgeon can choose the type of metal cone that will best address the deficiency, including replacement of both femoral condyles if necessary. Intermediate-term clinical outcomes with this type of reconstruction have been favorable.
Positioning
 The patient is placed supine, with a small pad under the ipsilateral buttock to ensure neutral position of the flexed knee. A Stulberg footrest or equivalent device is used to control knee flexion during surgery.
The patient is placed supine, with a small pad under the ipsilateral buttock to ensure neutral position of the flexed knee. A Stulberg footrest or equivalent device is used to control knee flexion during surgery.
 The leg is scrubbed circumferentially and as far proximal as feasible to allow access to the thigh.
The leg is scrubbed circumferentially and as far proximal as feasible to allow access to the thigh.
 In our practice, we do not use a tourniquet during any total knee procedure, but most surgeons exsanguinate the extremity with gravity or an Esmarch bandage before inflating and applying a well-padded tourniquet to the proximal thigh.
In our practice, we do not use a tourniquet during any total knee procedure, but most surgeons exsanguinate the extremity with gravity or an Esmarch bandage before inflating and applying a well-padded tourniquet to the proximal thigh.
The surgeon must remain vigilant of the duration of tourniquet time, particularly if a long and complex reconstruction is anticipated.
Approach
 If several previous scars are present, choose the one closest to the midline that will allow extensile exposure proximally or distally. The most laterally based incision is the wisest as it preserves blood supply to the overlying skin. For most revision TKA that involves distal femoral reconstruction, a standard medial parapatellar arthrotomy will adequately expose the distal femur.
If several previous scars are present, choose the one closest to the midline that will allow extensile exposure proximally or distally. The most laterally based incision is the wisest as it preserves blood supply to the overlying skin. For most revision TKA that involves distal femoral reconstruction, a standard medial parapatellar arthrotomy will adequately expose the distal femur.
 Exposure can be facilitated by avoiding patella eversion in knee flexion. After the patellar component is addressed in knee extension, the patella can be pushed into the lateral gutter and retracted safely.
Exposure can be facilitated by avoiding patella eversion in knee flexion. After the patellar component is addressed in knee extension, the patella can be pushed into the lateral gutter and retracted safely.
In our experience, exposure of the distal femur is improved if patella eversion is avoided.
 To understand the extent of bone loss, inflammatory membranous tissue overlying the femoral bone after removal of the previous component must be dissected away. The electrocautery knife works well for this purpose. Previous membrane and granulomas must be débrided thoroughly to appreciate the amount of distal femur available for reconstruction.
To understand the extent of bone loss, inflammatory membranous tissue overlying the femoral bone after removal of the previous component must be dissected away. The electrocautery knife works well for this purpose. Previous membrane and granulomas must be débrided thoroughly to appreciate the amount of distal femur available for reconstruction.
 Anteriorly, a proximal quadricepsplasty,3,9 tibial tubercle osteotomy,2 or other specialized dissection may be needed to mobilize the extensor mechanism safely and expose the distal femoral cortex.
Anteriorly, a proximal quadricepsplasty,3,9 tibial tubercle osteotomy,2 or other specialized dissection may be needed to mobilize the extensor mechanism safely and expose the distal femoral cortex.
The exposure chosen depends on the difficulty in exposing the distal femur and the extent of débridement and preparation for bone reconstruction required.
TECHNIQUES
 Morselized Allograft or Bone Cement
Morselized Allograft or Bone Cement
Small, contained defects in the femoral condyles can be filled with bone cement or morselized autograft or allograft.10 These grafts are not load bearing and are suitable only for focal cystic lesions that are surrounded by intact structural bone (TECH FIG 1).

A burr or curette is effective in cleaning out such defects.
To optimize allograft–host bone contact, excise the inflammatory membrane, remove previous metal and cement particles, and create a viable and healthy host bone bed.
Morselize an allograft femoral head using small acetabular reamers. Pack graft into the defects and cement the revision implant in place.
Alternatively, bone cement or a synthetic bone graft substitute filler can be used to pack small, contained defects between the revision implant and host bone.
 Metal Augments on Revision Femoral Component
Metal Augments on Revision Femoral Component
After excision of previous cement and inflammatory granulomas, remove the minimum amount of bone from the distal and posterior femoral condyles to expose viable host bone (TECH FIG 2A,C).

Small defects in the distal and posterior femoral condyles can be reconstructed by using metal augments on the revision femoral component8 (TECH FIG 2B,D).
Determine the joint line by examining the position of the epicondyles, the existing femoral component, position of the patella relative to the femur, contralateral knee radiographs, and the contour of the posterior femoral condyles projected laterally.
A combination of these variables makes it possible to accurately estimate and recreate the joint line.
Removal of additional bone from the distal femur will move the joint line more proximally.
Impact the trial femoral component on the distal femur and measure the defects between metal and host bone distally, anteriorly, and posteriorly after determining proper external rotation of the component.
Reinsert the trial implant after installing trial augments of the appropriate thickness and check the fit.
Augments in revision TKA systems come in a variety of thicknesses to accommodate femoral bone loss from the anterior cortex, posterior condyles, and distal femoral condyles.
The goal of revision component augmentation is stable contact between metal and host bone without resorting to a custom implant.
If metal augments are used on the revision femoral component, an intramedullary rod extension should be attached to the revision femoral component to achieve initial implant stability.8
 Bulk Femoral Head Reconstruction of Condylar Defects
Bulk Femoral Head Reconstruction of Condylar Defects
If one or both femoral condyles are not amenable to reconstruction with metal augments, structural deficiencies can be addressed with allograft tissue (TECH FIG 3A,B).

The condylar defect is reamed with small diameter male acetabular reamers from the Allogrip system (TECH FIG 3C).
Matched diameter female reamers are used to prepare the convex surface of the allograft femoral head (TECH FIG 3D).
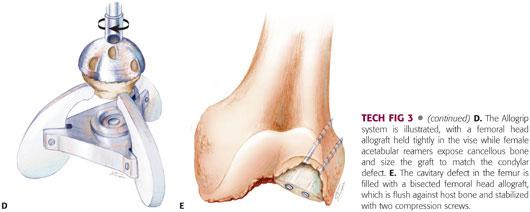
The allograft femoral head is placed in the reamed defect. Either a whole or a half femoral head allograft will fill the cavitary defect where it is attached to host bone with cancellous screws (TECH FIG 3E).
Then the allograft–host bone composite is cut to match the size of the revision femoral component.
Metal augments may be needed for residual bone defects that remain after femoral head allograft reconstruction of the femoral condyles or to recreate the joint line.
The technique of using metal augments on the revision femoral component is described earlier.
Attention must be maintained throughout to ensure recreation of the joint line and the appropriate external rotation of the femoral component needed for patella stability.
 Bulk Allograft Replacement of the Distal Femur
Bulk Allograft Replacement of the Distal Femur
For extensive loss of the distal femur, reconstruction with bulk allograft replacement of deficient bone is a proven option.1
If extensive bone loss leaves only an intact cortical shell in the distal femur, an undersized distal femur bulk allograft matched to the operative side can be stabilized within the host cortical shell (TECH FIG 4A).
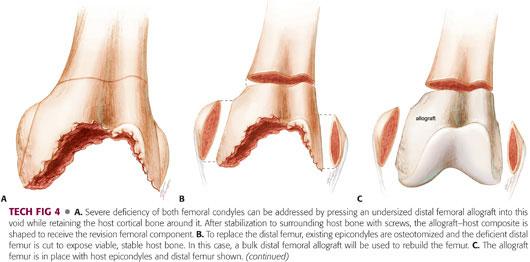

The proximal end of the graft should rest against viable host bone, with mechanical stability and maximum host bone–allograft contact to promote healing.
Conservative resection of the distal femur to match the end of the allograft will accomplish contact between host bone and allograft.
Once stabilized, the allograft distal femur within the host cortex is sized and cut to match the revision femoral component.
Collateral ligaments, if present, are preserved on the outer shell of host cortical bone.
An alternative to this technique is osteotomy of the epicondyles and replacement of deficient bone by a bulk femoral allograft (TECH FIG 4B,C).
The graft–host bone junction can be cut in a step cut configuration to ensure rotational stability.
In all cases of allograft reconstruction of the distal femur, offloading of the graft and rotational stability should be ensured by using an intramedullary rod attached to the revision femoral component.
If necessary, the rod can be cemented into the allograft and host bone for stability, although extrusion of cement at the host bone–allograft junction must be avoided to allow healing.
After implantation of the revision component (TECH FIG 4D), the epicondyles should be attached to the allograft with cancellous screws augmented with washers. To accomplish this step, the epicondyles on the bulk allograft must be cut off and removed.
Before final implantation, check the soft tissue envelope to make sure inadvertent oversizing has not occurred.
 Replacement of Distal Femur with a Tumor Reconstruction Prosthesis
Replacement of Distal Femur with a Tumor Reconstruction Prosthesis
For severe loss of the metaphyseal and diaphyseal femur (such as in tumor resection), modular implants designed for limb salvage may be the only option for reconstruction.5
This option is reserved for cases in which the extent of bone loss precludes reconstruction with bulk allograft.
Template radiographs and have appropriate reconstruction systems with varying modular lengths available to rebuild the deficient femur.
Perform an osteotomy of the femur to expose viable bone that is suitable for weight bearing.
Prepare the femur retrograde for cementing, using techniques similar to those for cementing a femoral implant in total hip replacement surgery.
Use trial and error to reproduce the appropriate limb length, soft tissue tension, and implant rotation. This is most easily accomplished by reconstructing the tibial side first, so that all trial reductions can be assessed by changing the femoral side only, thereby simplifying the procedure.
When correct rotation and length are determined, mark the host bone and implant to reproduce this rotation, and cement the implant into the distal femur to the appropriate depth and in the desired rotation.
Uncemented fixation into the distal femur may be an option with some reconstruction systems.
Assemble the knee articulation (these designs usually rely on a rotating hinge articulation with multidirectional constraint built into the articulation).
In severe cases, or if the proximal femur is unsuitable for mechanical fixation with an intramedullary rod, the entire femur can be bypassed with metal.
In cases of complete femoral replacement, a rotating hinge knee reconstruction is done at the distal end and a constrained hip replacement at the proximal end.
PEARLS AND PITFALLS | |
Preparations |
|
Implants |
|
Grafts |
|
Sizing |
|
Experience and resources |
|
Operative time |
|
POSTOPERATIVE CARE
 The goal of distal femoral reconstruction is to achieve initial mechanical stability. Accordingly, the surgeon should aim for weight bearing as soon as possible after surgery.
The goal of distal femoral reconstruction is to achieve initial mechanical stability. Accordingly, the surgeon should aim for weight bearing as soon as possible after surgery.
 If allograft reconstruction of the femur is necessary, healing to host bone occurs over a prolonged time. Therefore, protected weight bearing will be required for an extended period of time.
If allograft reconstruction of the femur is necessary, healing to host bone occurs over a prolonged time. Therefore, protected weight bearing will be required for an extended period of time.
 Assistive devices such as a cane, walker, or crutches should be prescribed for all patients who undergo revision TKA with distal femoral reconstruction to protect them against accidental falls or twists on the reconstructed knee and to allow healing of graft tissue.
Assistive devices such as a cane, walker, or crutches should be prescribed for all patients who undergo revision TKA with distal femoral reconstruction to protect them against accidental falls or twists on the reconstructed knee and to allow healing of graft tissue.
 Range of motion should be assessed intraoperatively following distal femur reconstruction. Usually, range of motion will depend on the quality of the soft tissues and integrity of the extensor mechanism, assuming that mechanical stability of the reconstruction has been achieved. If knee range of movement must be limited for a period of time, a knee brace that allows movement only through a prescribed range of motion may be necessary.
Range of motion should be assessed intraoperatively following distal femur reconstruction. Usually, range of motion will depend on the quality of the soft tissues and integrity of the extensor mechanism, assuming that mechanical stability of the reconstruction has been achieved. If knee range of movement must be limited for a period of time, a knee brace that allows movement only through a prescribed range of motion may be necessary.
 Straight-leg raises, isometric exercises, and ankle and calf rehabilitation should be possible soon after all distal femoral reconstructions.
Straight-leg raises, isometric exercises, and ankle and calf rehabilitation should be possible soon after all distal femoral reconstructions.
 A multimodal deep venous thrombosis prevention regimen should be instituted after surgery and the patient monitored appropriately.
A multimodal deep venous thrombosis prevention regimen should be instituted after surgery and the patient monitored appropriately.
OUTCOMES
 Radiographs at regular intervals and patient interviews will allow assessment of outcomes. Radiographs should be assessed for stability of the reconstruction and for healing of bone at the allograft–host bone junction.
Radiographs at regular intervals and patient interviews will allow assessment of outcomes. Radiographs should be assessed for stability of the reconstruction and for healing of bone at the allograft–host bone junction.
 Bulk allografts heal to living host bone, and allograft bone away from this healed junction remains nonviable over the long term. In load-sharing configurations, where the allograft is supported by host bone or by metal implants, the long-term outcomes are excellent.
Bulk allografts heal to living host bone, and allograft bone away from this healed junction remains nonviable over the long term. In load-sharing configurations, where the allograft is supported by host bone or by metal implants, the long-term outcomes are excellent.
 If allograft bone is used in load-bearing configurations, late failure of the nonviable bone from repetitive loading is predictable.
If allograft bone is used in load-bearing configurations, late failure of the nonviable bone from repetitive loading is predictable.
Allograft bone cannot remodel in response to stress; therefore, intramedullary stems that bypass the graft completely and transfer loads to living host bone are essential during the reconstruction.
 In some complex reconstructions involving distal femur replacements with bulk allograft or limb salvage implants, the patient should be counseled to use protected weight bearing for a prolonged time, such as 6 months or longer.
In some complex reconstructions involving distal femur replacements with bulk allograft or limb salvage implants, the patient should be counseled to use protected weight bearing for a prolonged time, such as 6 months or longer.
COMPLICATIONS
 Postoperative infection is a devastating complication following complex distal femoral reconstruction with allograft bone. Early diagnosis and aggressive wound débridement may salvage the situation in some instances, but removal of all allograft, cement, and implants in preparation for a staged reconstruction is usually necessary.
Postoperative infection is a devastating complication following complex distal femoral reconstruction with allograft bone. Early diagnosis and aggressive wound débridement may salvage the situation in some instances, but removal of all allograft, cement, and implants in preparation for a staged reconstruction is usually necessary.
 Late deep infections with a virulent organism in a knee with massive bone loss and allograft reconstruction of deficient host bone may necessitate a limb amputation.
Late deep infections with a virulent organism in a knee with massive bone loss and allograft reconstruction of deficient host bone may necessitate a limb amputation.
 Mechanical failure of distal femoral reconstructions usually occurs if the surgeon fails to achieve initial mechanical stability. Repeat surgery is necessary to rebuild the femur and achieve rotational and axial stability to permit protected weight bearing after the procedure.
Mechanical failure of distal femoral reconstructions usually occurs if the surgeon fails to achieve initial mechanical stability. Repeat surgery is necessary to rebuild the femur and achieve rotational and axial stability to permit protected weight bearing after the procedure.
 Because anticoagulation for prophylaxis against deep venous thrombosis is necessary after distal femoral reconstruction in revision TKA, the surgeon should monitor the patient for postoperative bleeding.
Because anticoagulation for prophylaxis against deep venous thrombosis is necessary after distal femoral reconstruction in revision TKA, the surgeon should monitor the patient for postoperative bleeding.
 If a tense hematoma develops or new wound drainage is encountered, aggressive surgical decompression should be considered early to avoid the risk of infection.
If a tense hematoma develops or new wound drainage is encountered, aggressive surgical decompression should be considered early to avoid the risk of infection.
REFERENCES
1. Bezwada HP, Shah AR, Zambito K, et al. Distal femoral allograft reconstruction for massive osteolytic bone loss in revision total knee arthroplasty. J Arthroplasty 2006;21:242–248.
2. Clarke HD. Tibial tubercle osteotomy. J Knee Surg 2003;16:58–61.
3. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res 2006;446:59–68.
4. Engh GA, Herzwurm PJ, Parks NL. Treatment of major defects of bone with bulk allografts and stemmed components during total knee arthroplasty. J Bone Joint Surg Am 1997;79A:1030–1039.
5. Harrison RJ Jr, Thacker MM, Pitcher JD, et al. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop Relat Res 2006;446:113–120.
6. Levine BR, Sporer S, Poggie RA, et al. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006;27:4671–4681.
7. Pelker RR, Friedlaender GE. Biomechanical aspects of bone autografts and allografts. Orthop Clin North Am 1987;18:235–239.
8. Radnay CS, Scuderi GR. Management of bone loss: augments, cones, offset stems. Clin Orthop Relat Res 2006;446:83–92.
9. Trousdale RT, Hanssen AD, Rand JA, et al. V-Y quadricepsplasty in total knee arthroplasty. Clin Orthop Relat Res 1993;286:48–55.
10. van Loon CJ, de Waal Malefijt MC, Verdonschot N, et al. Morsellized bone grafting compensates for femoral bone loss in revision total knee arthroplasty. An experimental study. Biomaterials 1999;20:85–89.
< div class='tao-gold-member'>









