Proximal femur replacement is a salvage limb-sparing surgery for nononcologic and oncologic indications that in the past used to be treated with a major amputation.
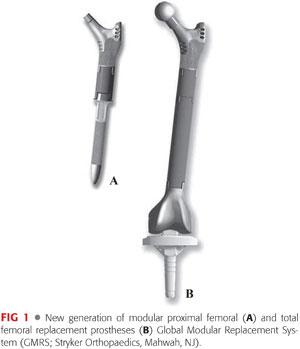
 The magnitude of complexity of revision reconstruction of the femur depends mainly on the quantity and quality of femoral bone. During the past decade, remarkable advances in the field of revision hip reconstruction have been made. One such improvement has been the introduction of second-generation modular prosthetic components (FIG 1), which provide improved ability to restore limb length and achieve optimal soft tissue tension, both of which may reduce the incidence of instability that often follow insertion of a monolithic megaprosthesis. A new generation of megaprostheses also provides a better environment for soft tissue reattachment and the ability to reapproximate the retained host bone to the prosthesis.
The magnitude of complexity of revision reconstruction of the femur depends mainly on the quantity and quality of femoral bone. During the past decade, remarkable advances in the field of revision hip reconstruction have been made. One such improvement has been the introduction of second-generation modular prosthetic components (FIG 1), which provide improved ability to restore limb length and achieve optimal soft tissue tension, both of which may reduce the incidence of instability that often follow insertion of a monolithic megaprosthesis. A new generation of megaprostheses also provides a better environment for soft tissue reattachment and the ability to reapproximate the retained host bone to the prosthesis.
ANATOMY
 Abductors (gluteus medius, gluteus minimus, tensor fascia lata muscles, and iliotibial band) are important stabilizers of the hip and are innervated mainly by the superior gluteal nerve. The nerve exits the pelvis via the suprapiriform portion of the sciatic foramen along with the superior gluteal vessels. Palsy results in abductor lurch, a Trendelenburg gait. The adductors include adductor brevis, adductor longus and gracilis muscles, and the anterior part of the adductor magnus muscle. The external rotators are the piriformis, quadratus femoris, superior gemellus, inferior gemellus, obturator internus, and obturator externus muscles.
Abductors (gluteus medius, gluteus minimus, tensor fascia lata muscles, and iliotibial band) are important stabilizers of the hip and are innervated mainly by the superior gluteal nerve. The nerve exits the pelvis via the suprapiriform portion of the sciatic foramen along with the superior gluteal vessels. Palsy results in abductor lurch, a Trendelenburg gait. The adductors include adductor brevis, adductor longus and gracilis muscles, and the anterior part of the adductor magnus muscle. The external rotators are the piriformis, quadratus femoris, superior gemellus, inferior gemellus, obturator internus, and obturator externus muscles.
PATHOGENESIS
 Femoral bone loss is a constantly rising and predominantly complex and challenging problem in revision arthroplasty. Numerous factors may contribute to the loss of femoral bone stock encountered in revision total hip arthroplasty (THA):
Femoral bone loss is a constantly rising and predominantly complex and challenging problem in revision arthroplasty. Numerous factors may contribute to the loss of femoral bone stock encountered in revision total hip arthroplasty (THA):
Osteolysis secondary to particle debris
Stress shielding with adaptive bone remodeling
Previous infection
The natural processes of aging
Periprosthetic fracture
Multiple reconstructive procedures with insertion and removal of implants, which adversely affect the integrity and function of the abductor muscles
PATIENT HISTORY AND PHYSICAL FINDINGS
 Assessment of history, physical examination, laboratory tests, and radiographic findings lead to correct diagnosis of hip pathology in most patients.
Assessment of history, physical examination, laboratory tests, and radiographic findings lead to correct diagnosis of hip pathology in most patients.
 History should begin with evaluation of the chief complaint. Identifying the location and nature of pain can be helpful for proper diagnosis. Intra-articular and acetabular pathology usually present as groin pain. Thigh pain (especially start-up pain) is more indicative of a loose femoral stem. Patients may present with referred pain in their knees and should be evaluated for hip pathology.
History should begin with evaluation of the chief complaint. Identifying the location and nature of pain can be helpful for proper diagnosis. Intra-articular and acetabular pathology usually present as groin pain. Thigh pain (especially start-up pain) is more indicative of a loose femoral stem. Patients may present with referred pain in their knees and should be evaluated for hip pathology.
 Thorough evaluation of past medical history along with a complete review of systems will help identify any potential factors that may lead to perioperative complications. Patients should be optimized for nutritional status and any underlying medical condition prior to surgery. This should include identification and adequate treatment of sources of potential or concurrent infection. Patients with history of chronic venous stasis ulcers, previous vascular bypass surgery, or absent distal pulses should be evaluated by a vascular surgeon.
Thorough evaluation of past medical history along with a complete review of systems will help identify any potential factors that may lead to perioperative complications. Patients should be optimized for nutritional status and any underlying medical condition prior to surgery. This should include identification and adequate treatment of sources of potential or concurrent infection. Patients with history of chronic venous stasis ulcers, previous vascular bypass surgery, or absent distal pulses should be evaluated by a vascular surgeon.
 The physical examination should begin with analysis of the patient’s gait. Antalgic gait can result from pain in any phase of ambulation with weight bearing and is characterized by a shortened stance phase. A Trendelenburg gait or abductor lurch indicates either paralysis or loss of continuity of the abductor musculature and is identified by shift of the patient’s center of gravity over the affected extremity during the stance phase of gait. Use of ambulatory assistive devices and existence of any deformity or limp should be documented. Previous surgical incisions should be routinely inspected. Planning surgical incision is important in determining the appropriate surgical approach for reconstruction. Although skin flap necrosis after hip surgery is rare, the maximum distance and angle is recommended to avoid this complication. Active and passive range of motion of the hip along with strength of the hip girdle musculature should also be recorded. A positive Trendelenburg test indicates abductor muscle weakness with inability of the patient to stabilize the pelvis during ipsilateral single-leg stance. Leg length should be assessed for apparent or functional discrepancy, which may be due to pelvic obliquity, muscular contracture, or scoliosis. Thomas test is performed by maximal flexion of hip and knee toward the chest and serves to evaluate contralateral hip flexion contracture. Provocative tests serve to localize the origin of the pain. Stinchfield test is positive when groin pain is reproduced with resisted ipsilateral hip flexion at 15 to 30 degrees and is highly suggestive for intra-articular hip pathology. If passive straight-leg raise causes radicular pain along the extremity and below the knee, a lower lumbar disc origin of the pain should be suspected. Physical examination should also include evaluation of neurovascular structures, spine, and abdomen to exclude sources of groin pain other than hip such as neuropathies, vascular claudication, spinal stenosis, or intra-abdominal pathologies.
The physical examination should begin with analysis of the patient’s gait. Antalgic gait can result from pain in any phase of ambulation with weight bearing and is characterized by a shortened stance phase. A Trendelenburg gait or abductor lurch indicates either paralysis or loss of continuity of the abductor musculature and is identified by shift of the patient’s center of gravity over the affected extremity during the stance phase of gait. Use of ambulatory assistive devices and existence of any deformity or limp should be documented. Previous surgical incisions should be routinely inspected. Planning surgical incision is important in determining the appropriate surgical approach for reconstruction. Although skin flap necrosis after hip surgery is rare, the maximum distance and angle is recommended to avoid this complication. Active and passive range of motion of the hip along with strength of the hip girdle musculature should also be recorded. A positive Trendelenburg test indicates abductor muscle weakness with inability of the patient to stabilize the pelvis during ipsilateral single-leg stance. Leg length should be assessed for apparent or functional discrepancy, which may be due to pelvic obliquity, muscular contracture, or scoliosis. Thomas test is performed by maximal flexion of hip and knee toward the chest and serves to evaluate contralateral hip flexion contracture. Provocative tests serve to localize the origin of the pain. Stinchfield test is positive when groin pain is reproduced with resisted ipsilateral hip flexion at 15 to 30 degrees and is highly suggestive for intra-articular hip pathology. If passive straight-leg raise causes radicular pain along the extremity and below the knee, a lower lumbar disc origin of the pain should be suspected. Physical examination should also include evaluation of neurovascular structures, spine, and abdomen to exclude sources of groin pain other than hip such as neuropathies, vascular claudication, spinal stenosis, or intra-abdominal pathologies.
 Erythrocyte sedimentation rate and serum C-reactive protein concentration should be determined. Elevated levels of these markers along with a history of previous infection are indications for joint aspiration and fluid analysis. Negative hip aspiration does not completely rule out infection; if significant risk of infection exists, aspiration should be followed by fresh frozen section evaluation and microbiologic culture of intraoperative tissue samples.
Erythrocyte sedimentation rate and serum C-reactive protein concentration should be determined. Elevated levels of these markers along with a history of previous infection are indications for joint aspiration and fluid analysis. Negative hip aspiration does not completely rule out infection; if significant risk of infection exists, aspiration should be followed by fresh frozen section evaluation and microbiologic culture of intraoperative tissue samples.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Proximal and total femur resections are major surgical procedures that necessitate a detailed preoperative evaluation. Patients with femoral bone deficiency due to malignant tumors should undergo adequate staging workup prior to reconstructive surgery. Most complications can be avoided by anticipating them before surgery and modifying the surgical technique accordingly.
Proximal and total femur resections are major surgical procedures that necessitate a detailed preoperative evaluation. Patients with femoral bone deficiency due to malignant tumors should undergo adequate staging workup prior to reconstructive surgery. Most complications can be avoided by anticipating them before surgery and modifying the surgical technique accordingly.
 Imaging studies aid in determining the extent of bone loss; dimensions of the required prosthesis; proximity of the scarred-in femoral vessels, femoral nerve, and sciatic nerve to the surgical area; extent of soft tissue resection; and reconstruction possibilities.
Imaging studies aid in determining the extent of bone loss; dimensions of the required prosthesis; proximity of the scarred-in femoral vessels, femoral nerve, and sciatic nerve to the surgical area; extent of soft tissue resection; and reconstruction possibilities.
 Plain radiographs of the entire femur are used to evaluate the extent and level of bone destruction. However, they may underestimate bone loss. If needed, computed tomography scanning can be added for further delineation of the femur and acetabulum bone structure.
Plain radiographs of the entire femur are used to evaluate the extent and level of bone destruction. However, they may underestimate bone loss. If needed, computed tomography scanning can be added for further delineation of the femur and acetabulum bone structure.
 Magnetic resonance imaging is used to evaluate the medullary canal and soft tissue around the hip joint.
Magnetic resonance imaging is used to evaluate the medullary canal and soft tissue around the hip joint.
 Three-phase bone scan is essential to determine the presence of metastatic bone disease.
Three-phase bone scan is essential to determine the presence of metastatic bone disease.
 Angiography of the iliofemoral vessels is essential before proximal femoral replacement if distortion of the anatomy due to previous surgeries is suspected.
Angiography of the iliofemoral vessels is essential before proximal femoral replacement if distortion of the anatomy due to previous surgeries is suspected.
DIFFERENTIAL DIAGNOSIS
 Infection including osteomyelitis and periprosthetic joint infection
Infection including osteomyelitis and periprosthetic joint infection
 Primary bone tumors such as multiple myeloma and chondrosarcoma
Primary bone tumors such as multiple myeloma and chondrosarcoma
 Metastatic lesions
Metastatic lesions
 Periprosthetic fracture
Periprosthetic fracture
 Osteolysis
Osteolysis
 Aseptic loosening
Aseptic loosening
 Paget disease
Paget disease
 Metabolic disease
Metabolic disease
CLASSIFICATION
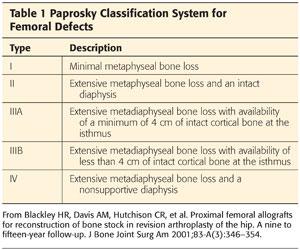
 Multiple classification systems have been developed for femoral bone loss in revision arthroplasty. These include those devised by Mallory,23 the American Academy of Orthopaedic Surgeons,7 Gross et al,11 Saleh et al,39 and Weeden and Paprosky.45 Of these, Weeden and Paprosky’s classification seems to be the most widely used because it is simple and guides the surgeon in how to reconstruct the deficient femur. The basic principle for this classification is that as proximal metaphysis becomes deficient and unsupportive, diaphysis should be considered for reliable fixation of the stem (Table 1).45
Multiple classification systems have been developed for femoral bone loss in revision arthroplasty. These include those devised by Mallory,23 the American Academy of Orthopaedic Surgeons,7 Gross et al,11 Saleh et al,39 and Weeden and Paprosky.45 Of these, Weeden and Paprosky’s classification seems to be the most widely used because it is simple and guides the surgeon in how to reconstruct the deficient femur. The basic principle for this classification is that as proximal metaphysis becomes deficient and unsupportive, diaphysis should be considered for reliable fixation of the stem (Table 1).45
NONOPERATIVE MANAGEMENT
 In most of the conditions discussed in this chapter, surgical intervention is considered the most reasonable option. However, nonsurgical modalities such as braces may be considered if surgery should not be performed on patients with serious underlying medical problems.
In most of the conditions discussed in this chapter, surgical intervention is considered the most reasonable option. However, nonsurgical modalities such as braces may be considered if surgery should not be performed on patients with serious underlying medical problems.
SURGICAL MANAGEMENT
 The objective of revision surgery is to improve function through a biomechanically restored hip by preserving as much of bone and soft tissue as possible, augmenting deficient bone, and creating a stable construct.24
The objective of revision surgery is to improve function through a biomechanically restored hip by preserving as much of bone and soft tissue as possible, augmenting deficient bone, and creating a stable construct.24
 The presence of active superficial or deep infection around the hip is an absolute contraindication for any reconstructive procedure. Relative contraindications can be noncooperative patients with increased risk of dislocation, vascular insufficiency that may impose healing problem, and nonoptimized medical comorbidities with unreasonably high risk for anesthesia.
The presence of active superficial or deep infection around the hip is an absolute contraindication for any reconstructive procedure. Relative contraindications can be noncooperative patients with increased risk of dislocation, vascular insufficiency that may impose healing problem, and nonoptimized medical comorbidities with unreasonably high risk for anesthesia.
 Options available for dealing with severe femoral bone loss are long cemented or press-fit stems,6,17,20,30,31,42,44 allograft impaction,8,13,21,36,37 allograft prosthetic composite (APC),24,35,47 megaprosthesis,34 and resection arthroplasty.40 Age and activity level are important factors in determining the most appropriate reconstructive procedure.
Options available for dealing with severe femoral bone loss are long cemented or press-fit stems,6,17,20,30,31,42,44 allograft impaction,8,13,21,36,37 allograft prosthetic composite (APC),24,35,47 megaprosthesis,34 and resection arthroplasty.40 Age and activity level are important factors in determining the most appropriate reconstructive procedure.
 Allograft impaction is indicated for contained femoral defects in young patients where the diameter of femoral canal is too much expanded and the length of distal intact diaphysis is inadequate to achieve a press-fit for a cementless stem.24–46 This technique is relatively straightforward in concept but time-consuming and technically demanding, with a steep learning curve.14,27,28 Histologic analysis of biopsies taken from 19 patients 1 to 48 months after revision arthroplasty with this technique confirmed its ability to progressively restore bone stock.43 However, its technical complexity and its potential complications limit its use.
Allograft impaction is indicated for contained femoral defects in young patients where the diameter of femoral canal is too much expanded and the length of distal intact diaphysis is inadequate to achieve a press-fit for a cementless stem.24–46 This technique is relatively straightforward in concept but time-consuming and technically demanding, with a steep learning curve.14,27,28 Histologic analysis of biopsies taken from 19 patients 1 to 48 months after revision arthroplasty with this technique confirmed its ability to progressively restore bone stock.43 However, its technical complexity and its potential complications limit its use.
 An important prerequisite for the use of prosthetic femoral replacement and APC is the availability of sufficient distal femoral length (at least 10 cm) for secure fixation of the cemented or uncemented femoral stem. If distal bone is severely deficient, total femoral replacement should be considered.
An important prerequisite for the use of prosthetic femoral replacement and APC is the availability of sufficient distal femoral length (at least 10 cm) for secure fixation of the cemented or uncemented femoral stem. If distal bone is severely deficient, total femoral replacement should be considered.
 An APC consists of cementing a long-stem prosthesis into a proximal femoral allograft.24 Distally, the stem can be press-fit or cemented into native bone. However, if it is to be cemented, extreme caution should be taken not to allow the cement interpose into the graft–bone interface. This procedure is best reserved to restore femoral bone mass in younger, active patients.35 It potentially increases bone stock in the proximal part of the femur and provides sites for soft tissue attachment, including the abductor muscles. However, it is a technically demanding and lengthy procedure that imposes significant physiologic stress on the patient. Moreover, its use is limited due to complications that are not uncommon with this procedure and include infection, junctional nonunion, dislocation, aseptic loosening, graft resorption, and fracture.14,24
An APC consists of cementing a long-stem prosthesis into a proximal femoral allograft.24 Distally, the stem can be press-fit or cemented into native bone. However, if it is to be cemented, extreme caution should be taken not to allow the cement interpose into the graft–bone interface. This procedure is best reserved to restore femoral bone mass in younger, active patients.35 It potentially increases bone stock in the proximal part of the femur and provides sites for soft tissue attachment, including the abductor muscles. However, it is a technically demanding and lengthy procedure that imposes significant physiologic stress on the patient. Moreover, its use is limited due to complications that are not uncommon with this procedure and include infection, junctional nonunion, dislocation, aseptic loosening, graft resorption, and fracture.14,24
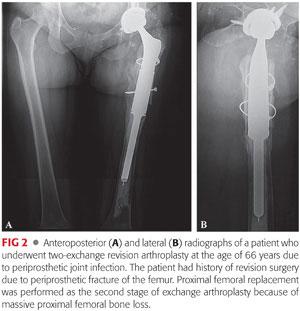
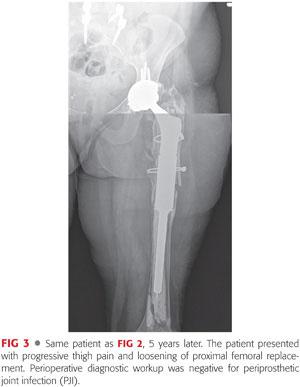
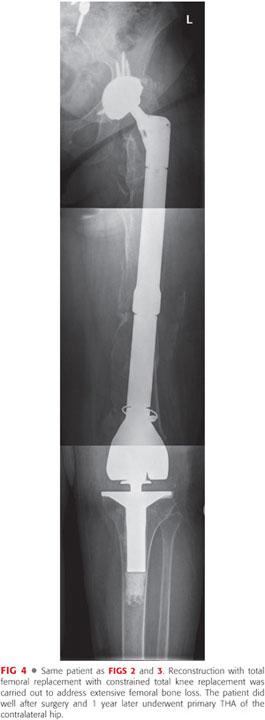
 Proximal and total femoral replacement with a megaprosthesis (FIGS 2 to 4) is probably more available and technically less demanding than an APC for most surgeons. It is a valuable option for extensive circumferential bone loss of femur in elderly patients, particularly if their general medical condition does not allow other lengthier and more complex reconstructive procedures.24,34 This type of prosthesis has been reported to have an unacceptably high failure rate in young patients and is not recommended for this patient population. However, it is indicated in elderly patients for whom immediate mobilization and weight bearing is of utmost importance in their recovery.12,33,41 Potential complications include early loosening due to inadequate fixation to distal femur, stress shielding, fatigue fracture, and instability due to poor condition of the surrounding soft tissue.5,34
Proximal and total femoral replacement with a megaprosthesis (FIGS 2 to 4) is probably more available and technically less demanding than an APC for most surgeons. It is a valuable option for extensive circumferential bone loss of femur in elderly patients, particularly if their general medical condition does not allow other lengthier and more complex reconstructive procedures.24,34 This type of prosthesis has been reported to have an unacceptably high failure rate in young patients and is not recommended for this patient population. However, it is indicated in elderly patients for whom immediate mobilization and weight bearing is of utmost importance in their recovery.12,33,41 Potential complications include early loosening due to inadequate fixation to distal femur, stress shielding, fatigue fracture, and instability due to poor condition of the surrounding soft tissue.5,34
Preoperative Planning
 The importance of preoperative planning in THA in general, and in proximal femur reconstruction in particular, cannot be overstated. These cases can be technically demanding, requiring meticulous attention to detail to achieve success.
The importance of preoperative planning in THA in general, and in proximal femur reconstruction in particular, cannot be overstated. These cases can be technically demanding, requiring meticulous attention to detail to achieve success.
 Proximal femur reconstruction is performed for metaphyseal–diaphyseal lesions that extend below the lesser trochanter, cause extensive cortical destruction, and spare at least 10 cm of the distal femoral diaphysis. Total femur resection is performed for diaphyseal lesions that extend proximally to the lesser trochanter and distally to the distal diaphyseal–metaphyseal junction and cause extensive bone destruction.
Proximal femur reconstruction is performed for metaphyseal–diaphyseal lesions that extend below the lesser trochanter, cause extensive cortical destruction, and spare at least 10 cm of the distal femoral diaphysis. Total femur resection is performed for diaphyseal lesions that extend proximally to the lesser trochanter and distally to the distal diaphyseal–metaphyseal junction and cause extensive bone destruction.
 Preoperative clinical and radiographic (standing films) assessment of limb length should be carried out. Preoperative templating to select the appropriate stem length and diameter is essential. Even with the most accurate preoperative measurements, a variety of prosthesis sizes should be available in the operating room because intraoperative changes in the anticipated size of the prosthesis are common. A representative of the manufacturing company of the prosthesis to be used should be present in the operating room.
Preoperative clinical and radiographic (standing films) assessment of limb length should be carried out. Preoperative templating to select the appropriate stem length and diameter is essential. Even with the most accurate preoperative measurements, a variety of prosthesis sizes should be available in the operating room because intraoperative changes in the anticipated size of the prosthesis are common. A representative of the manufacturing company of the prosthesis to be used should be present in the operating room.
 Intraoperative monitoring of the sciatic and femoral nerves may be required in patients in whom extensive limb lengthening (>4 cm) is anticipated.
Intraoperative monitoring of the sciatic and femoral nerves may be required in patients in whom extensive limb lengthening (>4 cm) is anticipated.
 Problems with removal of existing hardware, specific need for acetabular reconstruction, the potential need for insertion of constrained liners, and determining the absence of previous infection should be anticipated and addressed appropriately. Unexpected situations should always be considered. Even with accurate and detailed preoperative planning, the surgeon should allow for flexibility and consider the possibility of intraoperative modifications.
Problems with removal of existing hardware, specific need for acetabular reconstruction, the potential need for insertion of constrained liners, and determining the absence of previous infection should be anticipated and addressed appropriately. Unexpected situations should always be considered. Even with accurate and detailed preoperative planning, the surgeon should allow for flexibility and consider the possibility of intraoperative modifications.
 The surgical team, including anesthesia personnel, should be experienced. Regional anesthesia is preferred over general anesthesia because it is reportedly associated with lower incidence of perioperative complications. These patients are often elderly and frail, and because of the possibility of large-volume blood loss, invasive monitoring methods such as an arterial line or pulmonary artery catheter are often warranted. Therefore, blood conservation strategies such as preoperative blood donation,32 preoperative administration of erythropoietin,3 and use of cell saver9 should be considered in these patients.
The surgical team, including anesthesia personnel, should be experienced. Regional anesthesia is preferred over general anesthesia because it is reportedly associated with lower incidence of perioperative complications. These patients are often elderly and frail, and because of the possibility of large-volume blood loss, invasive monitoring methods such as an arterial line or pulmonary artery catheter are often warranted. Therefore, blood conservation strategies such as preoperative blood donation,32 preoperative administration of erythropoietin,3 and use of cell saver9 should be considered in these patients.
Positioning
 Place the patient in the lateral decubitus or supine position.
Place the patient in the lateral decubitus or supine position.
 Nonpermeable U-drapes are used to isolate the groin.
Nonpermeable U-drapes are used to isolate the groin.
 The distal third of the extremity is isolated from the field using impermeable drapes. The knee must be included in the operative field, even in patients undergoing proximal femoral replacement.
The distal third of the extremity is isolated from the field using impermeable drapes. The knee must be included in the operative field, even in patients undergoing proximal femoral replacement.
 Extension of the incision and arthrotomy of the knee to address intraoperative problems such as fractures extending distally is not uncommon.
Extension of the incision and arthrotomy of the knee to address intraoperative problems such as fractures extending distally is not uncommon.
 The skin is scrubbed with povidone-iodine solution, alcohol, and DuraPrep (3M, St. Paul, MN) before application of Ioban (3M).
The skin is scrubbed with povidone-iodine solution, alcohol, and DuraPrep (3M, St. Paul, MN) before application of Ioban (3M).
Approach
 We use the direct lateral approach (Hardinge approach) or the posterolateral approach with trochanteric slide osteotomy to gain access to the hip and maintain a low threshold to extend the incision as needed.
We use the direct lateral approach (Hardinge approach) or the posterolateral approach with trochanteric slide osteotomy to gain access to the hip and maintain a low threshold to extend the incision as needed.
TECHNIQUES
 Exposure
Exposure
Meticulous soft tissue handling facilitates tissue healing and minimizes postoperative complications.
Deep tissue specimens for frozen section and culture are obtained in all cases. Thorough débridement is carried out to remove previous metal debris and hardware around the femur, if present.
Extensile surgical approaches that allow for wide visualization should be employed. Modified Hardinge or posterolateral Moore approaches are recommended for proximal femoral replacement. Vastus slide osteotomy, as described by Head et al,15 can be used to mobilize the gluteus minimus and medius, vastus lateralis, and vastus intermedius muscles anteriorly in unison to expose anterior and lateral aspects of the femur (TECH FIG 1).
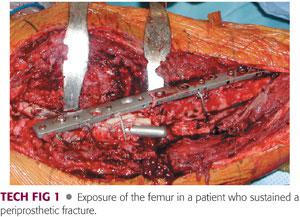
When the posterolateral approach is used for proximal femur resections, the incision can be extended to the anterolateral aspect of the patellar tendon if a total femur resection is required. After the external rotators and their interval with the abductors are identified, they are transected through their tendinous attachments and retracted posteriorly, exposing the hip joint and acetabulum. The vastus lateralis is reflected distally from its origin and the posterior perforating vessels are ligated. The vastus lateralis must be preserved because of its role in soft tissue coverage of the prosthesis; it is advanced proximally and sutured to the abductors. Care is taken not to ligate its main pedicle, which crosses anteriorly and obliquely along the rectus femoris fascia.
 Allograft Prosthetic Composite
Allograft Prosthetic Composite
If the patient’s abductor mechanism remains functional, a sliding trochanteric osteotomy for future incorporation into the allograft is preferred. Distally, a step-cut osteotomy is performed to increase rotational stability of the APC–host bone junction.
The proximal femoral graft should be smaller in diameter than the host bone and larger than the required length.19 Irradiated fresh frozen allografts at −70° C that can be thawed in 5% povidone-iodine solution are preferred. However, use of nonirradiated fresh allografts stored above 0° C have also been reported.37 Osteomy of the neck of allograft is performed 1 cm above the lesser trochanter. Distally, the graft is osteotomized and trimmed to match the receptive surface on the host bone, taking into account that ideally the APC should be telescoped 1 to 2 cm into distal host bone. The graft is reamed and the femoral component is cemented into the graft, ensuring adequate anteversion of the prosthesis. Cerclage cables can be used to secure the composite. Finally, the greater trochanter is attached to the APC through the use of cerclage wires.19
 Proximal Femoral Replacement
Proximal Femoral Replacement
If the femur is intact, an osteotomy to split the proximal femur may be required to facilitate removal of the previous prosthesis or hardware.
A transverse osteotomy is first made at the most proximal area of bone with good circumferential quality. Because the outcome of this procedure is influenced directly by the length of the remaining femur, maximum length of the native femur is maintained at all costs.22,33 We use a longitudinal Wagner type of coronal plane osteotomy to split the proximal femur if the bone quality is poor.
Soft tissue attachments to the proximal femur—particularly the abductor mechanism, if present—should be retained if at all possible. Once the femur is exposed, the distal portion of the canal is prepared through successive broaching. The cancellous bone, when present, is preserved for better cement interdigitation.
Both cemented or noncemented stems are viable options, although cemented stems are believed to provide more predictable and secure fixation.35
After completion of femoral preparation and determination of the size of best-fit broach, trial components are inserted, and the stability of the hip is examined.
A distal cement restrictor is used whenever possible. The restrictor is introduced and advanced distally to allow for at least 2 cm of bone cement at the tip of the stem.
The cement is pressurized and the final component implanted, with care taken to ensure that the porous-coated portion of the stem is placed directly and firmly against the diaphyseal bone with no interpositioning cement.
The prosthesis can be assembled and then cemented distally or, alternatively, the stem can be cemented and the body assembled onto it.
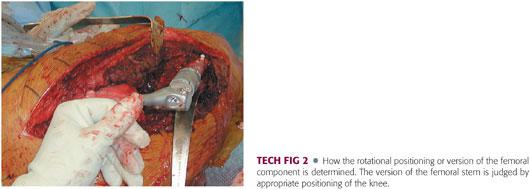
Extreme care must be exercised to prevent rotational malpositioning (TECH FIG 2). To mark the rotation, we use a sharp osteotome to scratch the distal femoral cortex once the trial component is appropriately positioned. The rotation of the component cannot be changed once the distal stem is cemented in place.
 Total Femur Replacement
Total Femur Replacement
Indications for total femoral replacement are rare and generally include conditions in which stem fixation is precluded due to inadequate length (<10 cm) or quality of distal femoral bone.
Once exposure of the femur is completed using a lateral vastus reflecting approach, the entire femur is split longitudinally in the coronal plane. Again, even if bone is of extremely poor quality, as much of it with its soft tissue attachment is retained as possible.
Total femoral replacement includes an arthrotomy of the knee to allow prosthetic replacement of the knee. The subvastus approach is extended to include a lateral or medial arthrotomy of the knee and eversion of the patella.
The amount of tibial bone resected is kept to a minimum, but it must be of adequate thickness to allow implantation of the components and insertion of polyethylene without elevating the joint line. The tibia is prepared in the same manner as for total knee arthroplasty. Once appropriate tibial component size is determined, preparation of the tibia followed by insertion of the trial component is carried out.
A full-length trial femur is assembled, ensuring that appropriate limb length is restored. Unless constrained liners are to be used, we prefer to use a large femoral head size to improve arc of motion and minimize instability.
The tibial polyethylene is usually between 15 and 20 mm thick, but it may be necessary to adjust the thickness to obtain appropriate length of the extremity and restore the joint line.
A linked articulated knee design is necessary because of loss of the stabilizing ligamentous structures. Once the prosthesis is assembled, a trial reduction is carried out to test for stability.
We usually do not resurface the patella unless severe wear of the articular cartilage is noted.
 Intraoperative Determination of Length of Femoral Component
Intraoperative Determination of Length of Femoral Component
There are two methods for intraoperative determination of the length of the femoral component. In any case, tension of soft tissue is the main determinant of the appropriate length of the femoral prosthesis.33
The first method is to apply traction to the limb with measurement from the cup to the host bone osteotomy site (for proximal femoral replacement).
The second and preferred method is to place a Steinmann pin in the iliac crest to measure a fixed point on the femur before dislocation.
With the long-stem trial prosthesis in place, proper leg length can be accurately restored. For patients with total femur replacement, radiographs of the opposite, normal femur may be obtained preoperatively and used for accurate templating for length.
The length of the prosthesis usually equals the length of the resected bone, although in many patients, the integrity of the bone has been breached and the anatomy markedly altered.
Ultimately, the femoral prosthesis length depends on the soft tissue tension around the hip. Balancing tension, restoring limb length, and avoiding excessive tension on the sciatic nerve are of utmost importance if complications are to be avoided.
 Acetabular Reconstruction
Acetabular Reconstruction
The acetabulum is exposed at the beginning of the operation and examined carefully. If a previous acetabular component is in place, the stability and positioning of the component are scrutinized. If the component is appropriately placed and stable, the liner is exchanged. If a previous acetabular component is not in place, a new component is inserted in a press-fit manner with or without screw fixation.
The type of acetabular liner is determined after reconstruction of the femur has been completed because it may be necessary to use constrained liners in patients with poor soft tissue tension and a high probability of instability. The constrained liners can be either snap-fit or cemented into the shell, depending on the type of the acetabular component implanted. In our experience, constrained liners are required in approximately half of patients receiving a megaprosthesis. Absolute indication for the use of a constrained liner is for patients with properly positioned components and equal or near-equal leg length who have intraoperative instability secondary to soft tissue deficiency.
More complex acetabular reconstruction, such as the use of an antiprotrusio cage, is occasionally needed.
 Closure
Closure
The femur, however poor in quality, is maintained and wrapped around the megaprosthesis at the conclusion of implantation.
The muscle–tendon attachments are preserved whenever possible.
The soft tissues—especially the abductors, if present—are meticulously secured to the prosthesis (TECH FIG 3).
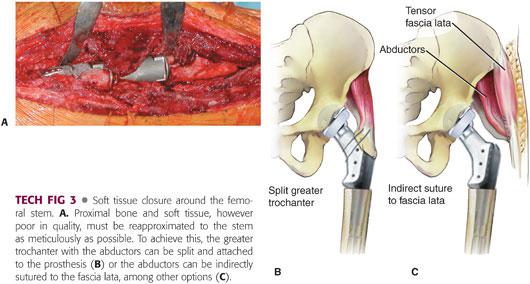
Multiple loops of nonabsorbable sutures are passed around the trochanter remnant and the attached soft tissue.
The leg is brought to abduction and the trochanter firmly fixed onto the proximal portion of the prosthesis by passing the sutures through the holes in the prosthesis or around the proximal body and the deep tissues.
We occasionally suture the abductors to the vastus lateralis, the tensor fascia lata, or the host greater trochanter, if available.
Two surgical drains are inserted before the wound is closed in layers using interrupted absorbable sutures. Meticulous skin closure, with excision of hypertrophic prior scar if necessary, is carried out to minimize postoperative wound drainage.
PEARLS AND PITFALLS | |
Preoperative |
|
| |
| |
| |
| |
Intraoperative |
|
| |
| |
| |
POSTOPERATIVE CARE
 Intravenous prophylactic antibiotics are given and maintained until final cultures are obtained. Thromboembolic prophylaxis also is administered for 6 weeks.
Intravenous prophylactic antibiotics are given and maintained until final cultures are obtained. Thromboembolic prophylaxis also is administered for 6 weeks.
 Patients are allowed to commence protected weight bearing on postoperative day 1. We recommend use of an abduction orthosis for all patients and protected weight bearing for 12 weeks until adequate soft tissue healing occurs. Patients are usually able to ambulate with the use of a walking aid during this time. In patients who undergo the APC procedure, protected weight bearing for several months should be maintained and serial x-rays should be taken until radiographic evidence of union is observed.
Patients are allowed to commence protected weight bearing on postoperative day 1. We recommend use of an abduction orthosis for all patients and protected weight bearing for 12 weeks until adequate soft tissue healing occurs. Patients are usually able to ambulate with the use of a walking aid during this time. In patients who undergo the APC procedure, protected weight bearing for several months should be maintained and serial x-rays should be taken until radiographic evidence of union is observed.
 Daily physical therapy for assistance with ambulation and range-of-motion exercise for the knee are recommended. Patients receiving total femur replacement may require the use of continuous passive motion machines for rehabilitation of the knee replacement.
Daily physical therapy for assistance with ambulation and range-of-motion exercise for the knee are recommended. Patients receiving total femur replacement may require the use of continuous passive motion machines for rehabilitation of the knee replacement.
OUTCOMES
 APC: According to various studies, APC can provide durable long-term results. In a meta-analysis regarding the outcome of APC, 16 studies with a minimum follow-up of 2 years and mean follow-up of 8 years were included. The pooled success rate was reported to be 81%.37 Two different studies reported a 10-year survival rate of 69%1 and a 15-year survival rate of 82%.38 Moreover, based on the analysis by Babis et al,1 survivorship is significantly affected by baseline bone defects and the number of previous surgeries.
APC: According to various studies, APC can provide durable long-term results. In a meta-analysis regarding the outcome of APC, 16 studies with a minimum follow-up of 2 years and mean follow-up of 8 years were included. The pooled success rate was reported to be 81%.37 Two different studies reported a 10-year survival rate of 69%1 and a 15-year survival rate of 82%.38 Moreover, based on the analysis by Babis et al,1 survivorship is significantly affected by baseline bone defects and the number of previous surgeries.
 The first experience with use of a megaprosthesis for reconstruction of the proximal femur in nonneoplastic conditions was reported in 1981.41 Although all 21 patients in that cohort had significant pain relief, there were two failures. One patient required acetabular component revision and the second needed revision of the femoral component for recurrent instability.
The first experience with use of a megaprosthesis for reconstruction of the proximal femur in nonneoplastic conditions was reported in 1981.41 Although all 21 patients in that cohort had significant pain relief, there were two failures. One patient required acetabular component revision and the second needed revision of the femoral component for recurrent instability.
 Two studies have been published regarding proximal femoral replacement in nonneoplastic conditions. Malkani et al22 reported the outcome of 50 consecutive prosthetic femoral replacements in 49 patients treated for nonneoplastic conditions. All patients had massive proximal bone loss and some had multiple failed attempts with other reconstructive procedures. The mean follow-up was 11 years. The mean preoperative Harris hip score of 43 ± 13 points improved significantly to 80 ± 10 points at 1 year and to 76 ± 16 points at the latest follow-up. Before surgery, 86% of the patients had moderate to severe pain. Pain relief was achieved in 88% of patients at 1 year and 73% of patients at the latest follow-up. However, there was some deterioration in all parameters over time. Detailed radiographic analysis revealed an increase in the incidence of progressive radiolucent lines on the femoral and acetabular sides. Progressive radiolucency was seen in 37% of the acetabular components and 30% of the femoral components. Aseptic loosening was the main reason for revision surgery. Using revision as an end point, overall survivorship in this series was 64% at 12 years. The most common complication was dislocation, with an overall rate of 22%.
Two studies have been published regarding proximal femoral replacement in nonneoplastic conditions. Malkani et al22 reported the outcome of 50 consecutive prosthetic femoral replacements in 49 patients treated for nonneoplastic conditions. All patients had massive proximal bone loss and some had multiple failed attempts with other reconstructive procedures. The mean follow-up was 11 years. The mean preoperative Harris hip score of 43 ± 13 points improved significantly to 80 ± 10 points at 1 year and to 76 ± 16 points at the latest follow-up. Before surgery, 86% of the patients had moderate to severe pain. Pain relief was achieved in 88% of patients at 1 year and 73% of patients at the latest follow-up. However, there was some deterioration in all parameters over time. Detailed radiographic analysis revealed an increase in the incidence of progressive radiolucent lines on the femoral and acetabular sides. Progressive radiolucency was seen in 37% of the acetabular components and 30% of the femoral components. Aseptic loosening was the main reason for revision surgery. Using revision as an end point, overall survivorship in this series was 64% at 12 years. The most common complication was dislocation, with an overall rate of 22%.
 In another study, Parvizi et al34 reported on 48 patients from two institutions (mean age 73.8 years) who underwent placement of a modular megaprosthesis with or without bone grafting for the following nonneoplastic indications: periprosthetic fracture (20 patients), reimplantation because of a deep infection (13 patients), failed arthroplasty (13 patients), nonunion of an intertrochanteric fracture (1 patient), radiation-induced osteonecrosis with a subtrochanteric fracture (1 patient). Three died before the minimum 2-year follow-up interval had elapsed and 2 additional patients were lost to follow-up. The mean duration of follow-up for the remaining study group of 43 patients was 36.5 months. At the time of follow-up, there was a significant improvement in function as measured with the Harris hip score. The major complications were instability (8 patients), failure of the acetabular component (4 patients), and infection (1 patient). Of the 8 patients with instability, 6 required reoperation because of dislocation and 2 with subluxation required no further intervention. With revision used as the end point, the survivorship of the implant was 87% at 1 year and 72% at 5 years.
In another study, Parvizi et al34 reported on 48 patients from two institutions (mean age 73.8 years) who underwent placement of a modular megaprosthesis with or without bone grafting for the following nonneoplastic indications: periprosthetic fracture (20 patients), reimplantation because of a deep infection (13 patients), failed arthroplasty (13 patients), nonunion of an intertrochanteric fracture (1 patient), radiation-induced osteonecrosis with a subtrochanteric fracture (1 patient). Three died before the minimum 2-year follow-up interval had elapsed and 2 additional patients were lost to follow-up. The mean duration of follow-up for the remaining study group of 43 patients was 36.5 months. At the time of follow-up, there was a significant improvement in function as measured with the Harris hip score. The major complications were instability (8 patients), failure of the acetabular component (4 patients), and infection (1 patient). Of the 8 patients with instability, 6 required reoperation because of dislocation and 2 with subluxation required no further intervention. With revision used as the end point, the survivorship of the implant was 87% at 1 year and 72% at 5 years.
 The results of 11 patients undergoing total femur replacement at the Mayo Clinic were recently evaluated. Six of these patients had total femur reconstructions performed for multiple failed ipsilateral total knee arthroplasty and THA. Five patients, 4 of whom had pathologic fractures, underwent total femur replacement as limb salvage for musculoskeletal malignancy. Of the 6 patients who had total femoral replacement for failed arthroplasties, hip instability in 2 necessitated conversion to a constrained acetabular liner. Of 2 patients with previous infections, 1 developed recurrent infection despite staged total femoral reimplantation, and 1 had an elevated sedimentation rate on chronic antibiotic suppression but no evidence of clinical infection. All patients ambulated with either a walker or a cane. Of the 5 patients who had total femoral replacement for treatment of tumor, 1 developed hip and knee pain within 3 years, had wear of the knee hinge bushings, and sought disability. One patient developed wound dehiscence and postoperative sepsis and died. Two patients ambulated with a cane and 3 did not have the routine use of any gait aides.
The results of 11 patients undergoing total femur replacement at the Mayo Clinic were recently evaluated. Six of these patients had total femur reconstructions performed for multiple failed ipsilateral total knee arthroplasty and THA. Five patients, 4 of whom had pathologic fractures, underwent total femur replacement as limb salvage for musculoskeletal malignancy. Of the 6 patients who had total femoral replacement for failed arthroplasties, hip instability in 2 necessitated conversion to a constrained acetabular liner. Of 2 patients with previous infections, 1 developed recurrent infection despite staged total femoral reimplantation, and 1 had an elevated sedimentation rate on chronic antibiotic suppression but no evidence of clinical infection. All patients ambulated with either a walker or a cane. Of the 5 patients who had total femoral replacement for treatment of tumor, 1 developed hip and knee pain within 3 years, had wear of the knee hinge bushings, and sought disability. One patient developed wound dehiscence and postoperative sepsis and died. Two patients ambulated with a cane and 3 did not have the routine use of any gait aides.
 Blackley et al5 reported the outcomes of revision THA with use of a proximal femoral replacement in a cohort of patients who had a Vancouver type B3 periprosthetic fracture. A modular femoral replacement with proximal porous coating was used in all cases. Twenty-one patients (mean age 78.3 years; range 52 to 90 years) were included in the cohort. At follow-up (mean 3.2 years), all but one of the patients were able to walk and had minimal to no pain. Complications included persistent wound drainage that was treated with incision and drainage (two patients), dislocation (two hips), refracture of the femur distal to the stem (one patient), and acetabular cage failure (one hip).
Blackley et al5 reported the outcomes of revision THA with use of a proximal femoral replacement in a cohort of patients who had a Vancouver type B3 periprosthetic fracture. A modular femoral replacement with proximal porous coating was used in all cases. Twenty-one patients (mean age 78.3 years; range 52 to 90 years) were included in the cohort. At follow-up (mean 3.2 years), all but one of the patients were able to walk and had minimal to no pain. Complications included persistent wound drainage that was treated with incision and drainage (two patients), dislocation (two hips), refracture of the femur distal to the stem (one patient), and acetabular cage failure (one hip).
 Zehr et al47 conducted a comparative study among 33 consecutive patients who required reconstructive surgery following resection of neoplasms of the proximal femur. Functional outcome and survivorship of the allograft prosthesis composite were not found to be significantly different from proximal femoral replacement, and the authors concluded that both procedures do equally well.
Zehr et al47 conducted a comparative study among 33 consecutive patients who required reconstructive surgery following resection of neoplasms of the proximal femur. Functional outcome and survivorship of the allograft prosthesis composite were not found to be significantly different from proximal femoral replacement, and the authors concluded that both procedures do equally well.
COMPLICATIONS
 Instability, infection, aseptic loosening, and fracture (prosthetic component, allograft, or host bone) are major common complications after proximal femoral reconstruction.
Instability, infection, aseptic loosening, and fracture (prosthetic component, allograft, or host bone) are major common complications after proximal femoral reconstruction.
 Allograft prosthesis composites are associated with the risk of disease transmission, graft resorption, and nonunion. Graft resorption can occur in various degrees and does not commonly lead to failure of the construct.38 Symptomatic junctional nonunion may require osteosynthesis and/or bone grafting.
Allograft prosthesis composites are associated with the risk of disease transmission, graft resorption, and nonunion. Graft resorption can occur in various degrees and does not commonly lead to failure of the construct.38 Symptomatic junctional nonunion may require osteosynthesis and/or bone grafting.
 The major complications encountered following the use of a megaprosthesis are early dislocation and aseptic loosening. The cause of instability in this group of patients is multifactorial. First, these patients often have had multiple previous reconstructive procedures that have led to compromised abductors around the hip. Furthermore, the inability to achieve a secure repair of the residual soft tissues to the prosthesis predisposes these patients to instability.10 The problem is further exacerbated in patients in whom the proper leg length and appropriate soft tissue tension is not achieved. The rate of dislocation with megaprostheses has been reported to be between 20% and 30% in previous studies.12,16,22 Bickels et al4 reported an exceptionally lower rate of dislocation of 1.7% in their series with mean follow-up of 6.5 years and attributed this low rate to acetabular preservation, Dacron-type capsulorrhaphy, and elaborate reconstruction of the iliopsoas, gluteus medius, gluteus maximus, and vastus lateralis tendons. We have implemented changes in our practice to minimize instability. These include the use of constrained cups in selective cases, routine use of a postoperative abduction brace, and augmentation of the proximal bone with the use of strut allograft, which imparts more rigidity for soft tissue attachment. It is conceivable that the problem of soft tissue to metal attachment may be better addressed in the future with the use of trabecular metals such as tantalum, which has excellent potential for soft tissue ongrowth. The use of a modular prosthesis has been a better strategy for dealing with this problem. The proximal femoral bone, however poor in quality, should be retained and reapproximated to the prosthesis to minimize dislocation. In addition, all efforts should be made to achieve equal limb lengths and to obtain acceptable soft tissue tension. Another important factor in the prevention of instability is the use of larger femoral heads in less active, elderly patients if instability is encountered intraoperatively.
The major complications encountered following the use of a megaprosthesis are early dislocation and aseptic loosening. The cause of instability in this group of patients is multifactorial. First, these patients often have had multiple previous reconstructive procedures that have led to compromised abductors around the hip. Furthermore, the inability to achieve a secure repair of the residual soft tissues to the prosthesis predisposes these patients to instability.10 The problem is further exacerbated in patients in whom the proper leg length and appropriate soft tissue tension is not achieved. The rate of dislocation with megaprostheses has been reported to be between 20% and 30% in previous studies.12,16,22 Bickels et al4 reported an exceptionally lower rate of dislocation of 1.7% in their series with mean follow-up of 6.5 years and attributed this low rate to acetabular preservation, Dacron-type capsulorrhaphy, and elaborate reconstruction of the iliopsoas, gluteus medius, gluteus maximus, and vastus lateralis tendons. We have implemented changes in our practice to minimize instability. These include the use of constrained cups in selective cases, routine use of a postoperative abduction brace, and augmentation of the proximal bone with the use of strut allograft, which imparts more rigidity for soft tissue attachment. It is conceivable that the problem of soft tissue to metal attachment may be better addressed in the future with the use of trabecular metals such as tantalum, which has excellent potential for soft tissue ongrowth. The use of a modular prosthesis has been a better strategy for dealing with this problem. The proximal femoral bone, however poor in quality, should be retained and reapproximated to the prosthesis to minimize dislocation. In addition, all efforts should be made to achieve equal limb lengths and to obtain acceptable soft tissue tension. Another important factor in the prevention of instability is the use of larger femoral heads in less active, elderly patients if instability is encountered intraoperatively.
 The other common complication of megaprosthesis reconstruction is acetabular and femoral radiolucency, which has a relatively high reported incidence.12,18,22,47 The reason for this complication lies in the biomechanical aspect of this reconstructive procedure. Diaphyseal cement fixation predisposes the bone-cement-prosthesis unit to high torsional and compressive stresses, leading to early loosening. Cemented long-stem revision implants are known to have limited success and currently are recommended only for elderly and sedentary patients.26 As expected, the incidence of radiolucency after the use of press-fit or proximally or extensively coated ingrowth stems is markedly lower than that with a megaprosthesis.2,29 The incidence of radiolucency after megaprosthesis reconstruction at our institution has declined somewhat. This may be the result of improved cementing techniques, namely, the use of pulse lavage and plugging of the canal for better cement interdigitation. However, the more likely explanation for the reduction in the incidence of radiolucency is that we have narrowed the indications for the use of megaprosthesis to elderly and sedentary patients who place lower demands on the prosthesis.
The other common complication of megaprosthesis reconstruction is acetabular and femoral radiolucency, which has a relatively high reported incidence.12,18,22,47 The reason for this complication lies in the biomechanical aspect of this reconstructive procedure. Diaphyseal cement fixation predisposes the bone-cement-prosthesis unit to high torsional and compressive stresses, leading to early loosening. Cemented long-stem revision implants are known to have limited success and currently are recommended only for elderly and sedentary patients.26 As expected, the incidence of radiolucency after the use of press-fit or proximally or extensively coated ingrowth stems is markedly lower than that with a megaprosthesis.2,29 The incidence of radiolucency after megaprosthesis reconstruction at our institution has declined somewhat. This may be the result of improved cementing techniques, namely, the use of pulse lavage and plugging of the canal for better cement interdigitation. However, the more likely explanation for the reduction in the incidence of radiolucency is that we have narrowed the indications for the use of megaprosthesis to elderly and sedentary patients who place lower demands on the prosthesis.
REFERENCES
1. Babis GC, Sakellariou VI, O’Connor MI, et al. Proximal femoral allograft-prosthesis composites in revision hip replacement: a 12-year follow-up study. J Bone Joint Surg Br 2010;92(3):349–355.
2. Berry DJ, Harmsen WS, Ilstrup D, et al. Survivorship of uncemented proximally porous-coated femoral components. Clin Orthop Relat Res 1995;(319):168–177.
3. Bezwada HP, Nazarian DG, Henry DH, et al. Preoperative use of recombinant human erythropoietin before total joint arthroplasty. J Bone Joint Surg Am 2003;85-A(9):1795–1800.
4. Bickels J, Meller I, Henshaw RM, et al. Reconstruction of hip stability after proximal and total femur resections. Clin Orthop Relat Res 2000;(375):218–230.
5. Blackley HR, Davis AM, Hutchison CR, et al. Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip. A nine to fifteen-year follow-up. J Bone Joint Surg Am 2001;83-A(3):346–354.
6. Böhm P, Bischel O. The use of tapered stems for femoral revision surgery. Clin Orthop Relat Res 2004;(420):148–159.
7. D’Antonio J, McCarthy JC, Bargar WL, et al. Classification of femoral abnormalities in total hip arthroplasty. Clin Orthop Relat Res 1993;(296):133–139.
8. Duncan CP, Masterson EL, Masri BA. Impaction allografting with cement for the management of femoral bone loss. Orthop Clin North Am 1998;29(2):297–305.
9. Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus 2011;9(2):139–147.
10. Giurea A, Paternostro T, Heinz-Peer G, et al. Function of reinserted abductor muscles after femoral replacement. J Bone Joint Surg Br 1998;80(2):284–287.
11. Gross AE, Hutchison CR, Alexeeff M, et al. Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip. Clin Orthop Relat Res 1995;(319):151–158.
12. Haentjens P, De Boeck H, Opdecam P. Proximal femoral replacement prosthesis for salvage of failed hip arthroplasty: complications in a 2-11 year follow-up study in 19 elderly patients. Acta Orthop Scand 1996;67(1):37–42.
13. Halliday BR, English HW, Timperley AJ, et al. Femoral impaction grafting with cement in revision total hip replacement. Evolution of the technique and results. J Bone Joint Surg Br 2003;85(6):809–817.
14. Hartman CW, Garvin KL. Femoral fixation in revision total hip arthroplasty. J Bone Joint Surg Am 2011;93(24):2311–2322.
15. Head WC, Mallory TH, Berklacich FM, et al. Extensile exposure of the hip for revision arthroplasty. J Arthroplasty 1987;2(4):265–273.
16. Ilyas I, Pant R, Kurar A, et al. Modular megaprosthesis for proximal femoral tumors. Int Orthop 2002;26(3):170–173.
17. Isacson J, Stark A, Wallensten R. The Wagner revision prosthesis consistently restores femoral bone structure. Int Orthop 2000;24(3):139–142.
18. Johnsson R, Carlsson A, Kisch K, et al. Function following mega total hip arthroplasty compared with conventional total hip arthroplasty and healthy matched controls. Clin Orthop Relat Res 1985;(192):159–167.
19. Kellett CF, Boscainos PJ, Maury AC, et al. Proximal femoral allograft treatment of Vancouver type-B3 periprosthetic femoral fractures after total hip arthroplasty. Surgical technique. J Bone Joint Surg Am 2007;89(suppl 2, pt 1):68–79.
20. Kwong LM, Miller AJ, Lubinus P. A modular distal fixation option for proximal bone loss in revision total hip arthroplasty: a 2- to 6-year follow-up study. J Arthroplasty 2003;18(3 suppl 1):94–97.
21. Mahoney CR, Fehringer EV, Kopjar B, et al. Femoral revision with impaction grafting and a collarless, polished, tapered stem. Clin Orthop Relat Res 2005;(432):181–187.
22. Malkani AL, Settecerri JJ, Sim FH, et al. Long-term results of proximal femoral replacement for non-neoplastic disorders. J Bone Joint Surg Br 1995;77(3):351–356.
23. Mallory TH. Preparation of the proximal femur in cementless total hip revision. Clin Orthop Relat Res 1988;(235):47–60.
24. Mayle RE Jr, Paprosky WG. Massive bone loss: allograft-prosthetic composites and beyond. J Bone Joint Surg Br 2012;94(11 suppl A):61–64. doi:10.1302/0301-620X.94B11.30791.
25. Meding JB, Ritter MA, Keating EM, et al. Impaction bone-grafting before insertion of a femoral stem with cement in revision total hip arthroplasty. A minimum two-year follow-up study. J Bone Joint Surg Am 1997;79(12):1834–1841.
26. Morris HG, Capanna R, Del Ben M, et al. Prosthetic reconstruction of the proximal femur after resection for bone tumors. J Arthroplasty 1995;10(3):293–299.
27. Oakes DA, Cabanela ME. Impaction bone grafting for revision hip arthroplasty: biology and clinical applications. J Am Acad Orthop Surg 2006;14(11):620–628.
28. Ornstein E, Atroshi I, Franzén H, et al. Early complications after one hundred and forty-four consecutive hip revisions with impacted morselized allograft bone and cement. J Bone Joint Surg Am 2002;84-A(8):1323–1328.
29. Paprosky WG. Distal fixation with fully coated stems in femoral revision: a 16-year follow-up. Orthopedics 1998;21(9):993–995.
30. Paprosky WG, Aribindi R. Hip replacement: treatment of femoral bone loss using distal bypass fixation. Instr Course Lect 2000;49:119–130.
31. Paprosky WG, Greidanus NV, Antoniou J. Minimum 10-year-results of extensively porous-coated stems in revision hip arthroplasty. Clin Orthop Relat Res 1999;(369):230–242.
32. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg 2011;24(1):25–31.
33. Parvizi J, Sim FH. Proximal femoral replacements with megaprostheses. Clin Orthop Relat Res 2004;(420):169–175.
34. Parvizi J, Tarity TD, Slenker N, et al. Proximal femoral replacement in patients with non-neoplastic conditions. J Bone Joint Surg Am 2007;89(5):1036–1043.
35. Parvizi J, Vegari DN. Periprosthetic proximal femur fractures: current concepts. J Orthop Trauma 2011;25(suppl 2):S77–S81.
36. Pekkarinen J, Alho A, Lepistö J, et al. Impaction bone grafting in revision hip surgery. A high incidence of complications. J Bone Joint Surg Br 2000;82(1):103–107.
37. Rogers BA, Sternheim A, De Iorio M, et al. Proximal femoral allograft in revision hip surgery with severe femoral bone loss: a systematic review and meta-analysis. J Arthroplasty 2012;27(6):829–836.e1.
38. Safir O, Kellett CF, Flint M, et al. Revision of the deficient proximal femur with a proximal femoral allograft. Clin Orthop Relat Res 2009;467(1):206–212.
39. Saleh KJ, Holtzman J, Gafni A, et al. Reliability and intraoperative validity of preoperative assessment of standardized plain radiographs in predicting bone loss at revision hip surgery. J Bone Joint Surg Am 2001;83-A(7):1040–1046.
40. Sharma H, De Leeuw J, Rowley DI. Girdlestone resection arthroplasty following failed surgical procedures. Int Orthop 2005;29(2):92–95.
41. Sim FH, Chao EY. Hip salvage by proximal femoral replacement. J Bone Joint Surg Am 1981;63(8):1228–1239.
42. Sporer SM, Paprosky WG. Femoral fixation in the face of considerable bone loss: the use of modular stems. Clin Orthop Relat Res 2004;(429):227–231.
43. Ullmark G, Obrant KJ. Histology of impacted bone-graft incorporation. J Arthroplasty 2002;17(2):150–157.
44. Wagner H. Revision prosthesis for the hip joint in severe bone loss [in German]. Orthopade. 1987;16(4):295–300.
45. Weeden SH, Paprosky WG. Minimal 11-year follow-up of extensively porous-coated stems in femoral revision total hip arthroplasty. J Arthroplasty 2002;17(4 suppl 1):134–137.
46. Wraighte PJ, Howard PW. Femoral impaction bone allografting with an Exeter cemented collarless, polished, tapered stem in revision hip replacement: a mean follow-up of 10.5 years. J Bone Joint Surg Br 2008;90(8):1000–1004.
47. Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res 1996;(322):207–223.
< div class='tao-gold-member'>












