A fluted femoral stem is designed to provide diaphyseal rotational stability through multiple longitudinally oriented flutes with varying numbers and positions, depending on the manufacturer. These fluted stems may be a useful reconstructive option in one or more of the following situations:
Proximal femoral cavitary or segmental defects
Abnormal femoral anatomy/femoral deformity
Periprosthetic femoral fracture
Osteopenic proximal femoral bone secondary to stress shielding
Sclerotic bone secondary to prior fracture fixation
ANATOMY
 The proximal femoral anatomy can be quite abnormal in the setting of revision total hip arthroplasty (THA) or complex THA. The proximal femoral bone may be deficient with cavitary or segmental loss and the remaining bone may be osteopenic secondary to stress shielding. The typical bony landmarks used by the orthopaedic surgeon, such as the lesser and greater trochanters, can be abnormal or absent. Higher grades of proximal femoral bone loss can involve the upper femoral diaphysis, which may limit the type of femoral prosthesis that can be used at the time of revision arthroplasty.
The proximal femoral anatomy can be quite abnormal in the setting of revision total hip arthroplasty (THA) or complex THA. The proximal femoral bone may be deficient with cavitary or segmental loss and the remaining bone may be osteopenic secondary to stress shielding. The typical bony landmarks used by the orthopaedic surgeon, such as the lesser and greater trochanters, can be abnormal or absent. Higher grades of proximal femoral bone loss can involve the upper femoral diaphysis, which may limit the type of femoral prosthesis that can be used at the time of revision arthroplasty.
The proximal aspect of the femur is composed of the head, neck, and greater and lesser trochanters.
Important soft tissues include the iliotibial band and muscles that insert into it (tensor fascia lata and gluteus maximus), gluteal muscles (maximus, medius, and minimus), short external rotator muscles (especially when a posterior approach to the revision hip surgery is contemplated), iliopsoas, quadriceps, and hip joint capsule (FIG 1A).
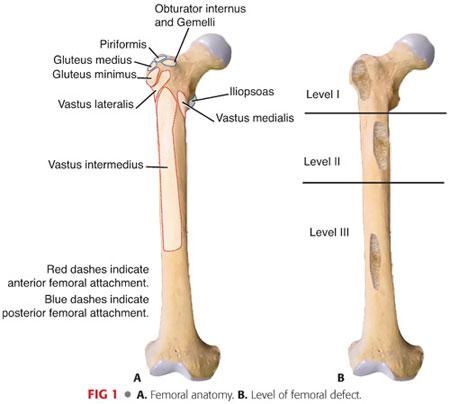
Vascular and neurologic structures include the femoral artery, vein, and nerve; the sciatic nerve; and the lateral cutaneous nerve of the thigh when a more anterior approach is used.
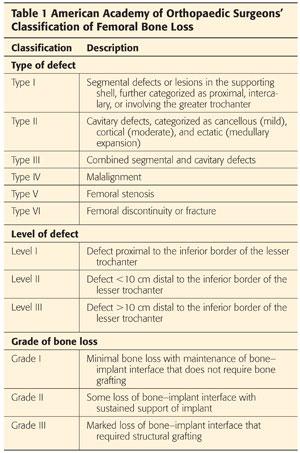
 The American Academy of Orthopaedic Surgeons’ classification of femoral bone loss6 (Table 1; FIG 1B)
The American Academy of Orthopaedic Surgeons’ classification of femoral bone loss6 (Table 1; FIG 1B)
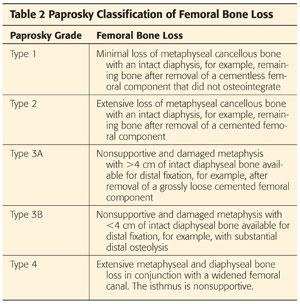
 The Paprosky classification of femoral bone loss19 (Table 2)
The Paprosky classification of femoral bone loss19 (Table 2)
PATHOGENESIS
 Osteolysis, from polyethylene wear particles, results in bone loss and ultimate component loosening, leading to premature THA failure. This has led to the consideration of alternative bearing surfaces including metal-on-metal and ceramic-on-ceramic bearings. Highly cross-linked ultra-high-molecular-weight polyethylene has, in recent times, become the polyethylene of choice for use in THA owing to it’s enhanced wear resistant properties. Ionizing radiation is used to increase the number of cross-links within the polyethylene, leading to a reduction in the amount of wear debris created during cyclical loading of the prosthetic joint.13 A reduction in wear rates is as great as 80% when compared to conventional polyethylene for the 28 and 32 mm head sizes.10
Osteolysis, from polyethylene wear particles, results in bone loss and ultimate component loosening, leading to premature THA failure. This has led to the consideration of alternative bearing surfaces including metal-on-metal and ceramic-on-ceramic bearings. Highly cross-linked ultra-high-molecular-weight polyethylene has, in recent times, become the polyethylene of choice for use in THA owing to it’s enhanced wear resistant properties. Ionizing radiation is used to increase the number of cross-links within the polyethylene, leading to a reduction in the amount of wear debris created during cyclical loading of the prosthetic joint.13 A reduction in wear rates is as great as 80% when compared to conventional polyethylene for the 28 and 32 mm head sizes.10
NATURAL HISTORY
 Uncemented femoral components loosen as a result of progressive osteolysis and bone loss.
Uncemented femoral components loosen as a result of progressive osteolysis and bone loss.
 Cemented femoral components fail as a result of osteolysis at the cement–bone interface.
Cemented femoral components fail as a result of osteolysis at the cement–bone interface.
 Micromotion resulting from loose components causes further bone loss.
Micromotion resulting from loose components causes further bone loss.
 Cortical thinning may occur.
Cortical thinning may occur.
 Varus deformity of the proximal femur may occur.
Varus deformity of the proximal femur may occur.
 Periprosthetic femoral fracture.
Periprosthetic femoral fracture.
 Eventual failure of the prosthesis.
Eventual failure of the prosthesis.
PATIENT HISTORY AND PHYSICAL FINDINGS
 A complete and focused patient history should be obtained. Specific questions in relation to pain include the following:
A complete and focused patient history should be obtained. Specific questions in relation to pain include the following:
Rest and/or night pain (infection)
Pain with weight bearing (infection, aseptic loosening)
Start-up pain (aseptic loosening)
Severe acute pain (periprosthetic femur fracture, hip dislocation)
 A complete medical and surgical history is necessary to document all information pertaining to the index procedure, including the initial diagnosis, date of surgery, complete operative notes with detailed descriptions of the components used, and the dates of any postoperative complications. All component identifiers (component stickers) should be obtained where possible, as operative reports are sometimes inaccurate.8
A complete medical and surgical history is necessary to document all information pertaining to the index procedure, including the initial diagnosis, date of surgery, complete operative notes with detailed descriptions of the components used, and the dates of any postoperative complications. All component identifiers (component stickers) should be obtained where possible, as operative reports are sometimes inaccurate.8
 Relevant past medical and surgical history should be obtained and appropriate preoperative consultations sought when needed. A decision can also be taken regarding the most suitable form of thromboprophylaxis and whether the patient’s current medications are optimized prior to revision surgery.
Relevant past medical and surgical history should be obtained and appropriate preoperative consultations sought when needed. A decision can also be taken regarding the most suitable form of thromboprophylaxis and whether the patient’s current medications are optimized prior to revision surgery.
 The physical examination should include the following:
The physical examination should include the following:
Gait evaluation. A painful THA will result in a shortened stance phase during the gait cycle. A Trendelenburg gait or abductor lurch may raise concern regarding hip abductor function either from injury to the abductor muscle itself or from altered hip joint biomechanics that can limit the success of revision.
Trendelenburg test is considered positive when the pelvis on the nonstance side moves into a position of relative adduction (the pelvis on the normal side dips toward the floor in the patient with unilateral disease). It may indicate abductor weakness, superior gluteal nerve injury, or a loss of effectiveness of the abductor mechanism (eg, greater trochanteric nonunion).9
Hip abductor strength may indicate abductor weakness, trochanteric bursitis, abductor avulsion, trochanter fracture, or a loose femoral component.
Hip joint range of motion should be pain free. Pain suggests a mechanical dysfunction. A palpable or audible click or clunk may indicate femoral head subluxation.
Leg lengths should be assessed. Progressive true leg length discrepancy suggests implant subsidence.
“Apparent leg length discrepancy may be caused by muscle atrophy, obesity, spinal disease, pelvic obliquity, or asymmetric positioning of the legs, which may be correctable or fixed as in the case of hip adductor contractures.
The skin around the hip should be inspected for previous scars, evidence of poor wound healing, and superficial soft tissue infection.
 A complete neurovascular examination of the limb in question should be performed as well as a complete lower limb peripheral neurologic examination to rule out causes of hip pain that are referred from the spine.21 This examination will also serve as a baseline for comparison with the initial postoperative examination.
A complete neurovascular examination of the limb in question should be performed as well as a complete lower limb peripheral neurologic examination to rule out causes of hip pain that are referred from the spine.21 This examination will also serve as a baseline for comparison with the initial postoperative examination.
Neurovascular Structures
 The sciatic nerve is frequently encased in scar tissue during revision hip surgery. It is located 1 to 2 cm posterior to the posterior rim of the acetabulum and should not be exposed routinely during revision THA if the surgeon is careful with retractor placement and positions the leg appropriately during hip exposure.
The sciatic nerve is frequently encased in scar tissue during revision hip surgery. It is located 1 to 2 cm posterior to the posterior rim of the acetabulum and should not be exposed routinely during revision THA if the surgeon is careful with retractor placement and positions the leg appropriately during hip exposure.
 If the surgeon needs to expose the nerve, it is best identified posterior to the gluteal sling and followed proximally toward the hip joint.
If the surgeon needs to expose the nerve, it is best identified posterior to the gluteal sling and followed proximally toward the hip joint.
Radiographic Examination
 Preoperative radiographic views should include an anteroposterior (AP) pelvis as well as a lateral of the hip in question. The entire implant should be visualized on radiographs. The surgeon should have a low threshold for obtaining full-length femur radiographs. The acetabular and femoral component position and fixation should be assessed and any radiolucent lines about the components or any bony deficits noted. The greater trochanteric anatomy and quality should be reviewed, as this may have a direct effect on the ability to reconstruct the abductor mechanism and limit postoperative instability. Eccentric polyethylene wear and evidence of osteolysis should also be noted. Radiographic evidence of heterotopic ossification should alert the surgeon about the need for a more difficult dissection and approach to the hip joint.
Preoperative radiographic views should include an anteroposterior (AP) pelvis as well as a lateral of the hip in question. The entire implant should be visualized on radiographs. The surgeon should have a low threshold for obtaining full-length femur radiographs. The acetabular and femoral component position and fixation should be assessed and any radiolucent lines about the components or any bony deficits noted. The greater trochanteric anatomy and quality should be reviewed, as this may have a direct effect on the ability to reconstruct the abductor mechanism and limit postoperative instability. Eccentric polyethylene wear and evidence of osteolysis should also be noted. Radiographic evidence of heterotopic ossification should alert the surgeon about the need for a more difficult dissection and approach to the hip joint.
 The amount of competent proximal femoral bone needs to be assessed, as proximal bone deficiencies in this area can be so profound that a fluted stem is not suitable for femoral revision, and a fully porous-coated stem for diaphyseal fixation is preferable.
The amount of competent proximal femoral bone needs to be assessed, as proximal bone deficiencies in this area can be so profound that a fluted stem is not suitable for femoral revision, and a fully porous-coated stem for diaphyseal fixation is preferable.
 Diaphyseal defects in the femur are identified so that during preoperative planning, the proposed femoral component can be templated to bypass the bony defect by at least two cortical diameters.
Diaphyseal defects in the femur are identified so that during preoperative planning, the proposed femoral component can be templated to bypass the bony defect by at least two cortical diameters.
 A series of older radiographs should be reviewed (if available) so that the progression of radiolucencies and femoral component subsidence can be accurately calculated.
A series of older radiographs should be reviewed (if available) so that the progression of radiolucencies and femoral component subsidence can be accurately calculated.
Laboratory Tests
 Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be assessed before any revision procedure to rule out infection. In a series of 202 revision arthroplasties, all patients with deep sepsis had either an ESR above 30 mm or a CRP above 10 mg/L.18
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be assessed before any revision procedure to rule out infection. In a series of 202 revision arthroplasties, all patients with deep sepsis had either an ESR above 30 mm or a CRP above 10 mg/L.18
 Intra-articular preoperative aspiration should also be performed if either the ESR or CRP is elevated. The white cell count and differential of the aspirate should be measured. A portion of the aspirate should also be sent for culture and sensitivity.
Intra-articular preoperative aspiration should also be performed if either the ESR or CRP is elevated. The white cell count and differential of the aspirate should be measured. A portion of the aspirate should also be sent for culture and sensitivity.
 A technetium 99m bone scan hydroxymethylene diphosphonate (HDP) bone scan may demonstrate increased bony metabolism that can represent loosening, increased stress, or infection. However, a technetium bone scan can produce both false-positive and false-negative results and the surgeon should not base the decision to revise solely on this test. A gallium scan and/or an indium-labeled white cell scan can be performed to detect infection in THA. However, these tests are rarely used.
A technetium 99m bone scan hydroxymethylene diphosphonate (HDP) bone scan may demonstrate increased bony metabolism that can represent loosening, increased stress, or infection. However, a technetium bone scan can produce both false-positive and false-negative results and the surgeon should not base the decision to revise solely on this test. A gallium scan and/or an indium-labeled white cell scan can be performed to detect infection in THA. However, these tests are rarely used.
HIP PAIN POST TOTAL HIP ARTHROPLASTY DIFFERENTIAL DIAGNOSIS
 Infection
Infection
 Component loosening (septic or aseptic)
Component loosening (septic or aseptic)
 Periprosthetic fracture
Periprosthetic fracture
 Component impingement
Component impingement
 Psoas tendon irritation
Psoas tendon irritation
 Leg length discrepancy
Leg length discrepancy
 Trunionosis/corrosion
Trunionosis/corrosion
 Greater trochanteric bursitis
Greater trochanteric bursitis
 Lumbosacral pathology
Lumbosacral pathology
NONOPERATIVE MANAGEMENT
 Nonoperative management is usually not an option and is reserved for patients who are deemed to have too high a risk for anesthesia.
Nonoperative management is usually not an option and is reserved for patients who are deemed to have too high a risk for anesthesia.
 Nonoperative measures in specific cases include the following:
Nonoperative measures in specific cases include the following:
Assistive ambulatory devices
Suppressive antibiotics for septic loosening may help control pain or progressive infection in a nonoperative patient.
Hip pain due to bursitis may be improved with nonoperative treatments, including physical therapy, nonsteroidal anti-inflammatory drugs, and injections.
SURGICAL MANAGEMENT
 Revision THA can be performed in a single sitting in the absence of periprosthetic joint infection.
Revision THA can be performed in a single sitting in the absence of periprosthetic joint infection.
 Most fluted femoral stems have a proximal sleeve of varying lengths and configurations that allow for optimal offset and “fit and fill” of the proximal femur. The flutes increase distal fill and resistance to rotational stress.
Most fluted femoral stems have a proximal sleeve of varying lengths and configurations that allow for optimal offset and “fit and fill” of the proximal femur. The flutes increase distal fill and resistance to rotational stress.
Preoperative Planning
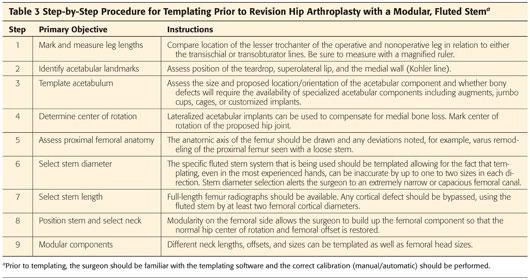
 Templating should be performed using a current AP radiograph of the pelvis and a lateral of the affected hip. Full-length AP and lateral radiographs of the femur should also be considered (Table 3).
Templating should be performed using a current AP radiograph of the pelvis and a lateral of the affected hip. Full-length AP and lateral radiographs of the femur should also be considered (Table 3).
 Additional preoperative planning includes the following:
Additional preoperative planning includes the following:
A pathologist must be available to look at intraoperative frozen sections.
Previous operative reports
Polyethylene liner exchange options
Revision acetabular components (even if the socket looks stable)
Fully porous-coated femoral component if proximal fixation is insufficient for use of a fluted stem
Structural allografts and cables
Positioning
 Following administration of regional anesthesia and insertion of a urinary catheter, the patient should be positioned supine on a gel bump that is centered on the anterior superior iliac spine.
Following administration of regional anesthesia and insertion of a urinary catheter, the patient should be positioned supine on a gel bump that is centered on the anterior superior iliac spine.
 Supine positioning, the authors’ preferred technique, allows the surgeon to accurately assess the patient’s leg lengths and to accurately assess the position of the acetabular component.
Supine positioning, the authors’ preferred technique, allows the surgeon to accurately assess the patient’s leg lengths and to accurately assess the position of the acetabular component.
 The groin and the ipsilateral foot should be isolated with nonsterile drapes, and the entire limb should be prepped. An impervious stockinette can be rolled up to the level of the knee.
The groin and the ipsilateral foot should be isolated with nonsterile drapes, and the entire limb should be prepped. An impervious stockinette can be rolled up to the level of the knee.
Approach
 The approach to the hip in revision surgery should be easily extensile.
The approach to the hip in revision surgery should be easily extensile.
 Direct lateral approach
Direct lateral approach
 Posterior approach (patient needs to be in lateral decubitus position)
Posterior approach (patient needs to be in lateral decubitus position)
Soft Tissue
 The hip abductors require careful preoperative evaluation and intraoperative inspection because they are critical to postoperative hip stability and gait. Prior hip surgery may result in a weakened gluteus medius. There can often be a defect in the abductor tendon encountered in the approach for revision surgery in a patient who previously underwent a direct lateral approach.
The hip abductors require careful preoperative evaluation and intraoperative inspection because they are critical to postoperative hip stability and gait. Prior hip surgery may result in a weakened gluteus medius. There can often be a defect in the abductor tendon encountered in the approach for revision surgery in a patient who previously underwent a direct lateral approach.
 The vastus lateralis may be elevated from its posterior border or split in continuity with the distal extend of the gluteus medius tendon split to give the surgeon access to the femoral shaft for correction of bony deformity, fracture repair, and cable placement.
The vastus lateralis may be elevated from its posterior border or split in continuity with the distal extend of the gluteus medius tendon split to give the surgeon access to the femoral shaft for correction of bony deformity, fracture repair, and cable placement.
 The posterior hip joint capsule and the short external rotators are often adherent when a previous posterior approach was used. These structures can be tagged and preserved for later repair if a posterior approach to the hip is used for the revision surgery.
The posterior hip joint capsule and the short external rotators are often adherent when a previous posterior approach was used. These structures can be tagged and preserved for later repair if a posterior approach to the hip is used for the revision surgery.
 The gluteal sling refers to the insertion of the gluteus maximus on the posterolateral border of the proximal femoral shaft. The 5 cm insertion frequently needs to be partially or completely released to gain exposure of the femur in a posterior approach. This should be repaired to its tendon stump at the end of the case with care taken to avoid suturing the sciatic nerve.
The gluteal sling refers to the insertion of the gluteus maximus on the posterolateral border of the proximal femoral shaft. The 5 cm insertion frequently needs to be partially or completely released to gain exposure of the femur in a posterior approach. This should be repaired to its tendon stump at the end of the case with care taken to avoid suturing the sciatic nerve.
Neurovascular Structures
 The sciatic nerve is frequently found to be encased in scar tissue during revision hip surgery. It is located 1 to 2 cm posterior to the posterior rim of the acetabulum and should not be exposed routinely during revision hip surgery.
The sciatic nerve is frequently found to be encased in scar tissue during revision hip surgery. It is located 1 to 2 cm posterior to the posterior rim of the acetabulum and should not be exposed routinely during revision hip surgery.
 If exposure of the nerve is necessary, it can be identified posterior to the gluteal sling and followed proximally toward the hip joint.
If exposure of the nerve is necessary, it can be identified posterior to the gluteal sling and followed proximally toward the hip joint.
TECHNIQUES
 Routine Revision without Diaphyseal Defect
Routine Revision without Diaphyseal Defect
Carefully assess the proximal femur above the lesser trochanter.
An extended trochanteric osteotomy (ETO) may be used to expose the proximal femur and allows removal of the cement mantle or removal of a fully porous-coated femoral stem.
Reaming and insertion of the different fluted femoral stem systems vary greatly between manufacturers and the surgeon should be aware of the nuances of each system.
Perform straight reaming of the proximal diaphysis until cortical chatter is achieved. Reaming should be done to a depth determined by comparing a line on the reamer to the tip of the greater trochanter1 (TECH FIG 1A).
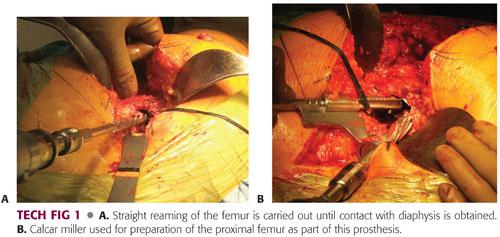
The diameter of the last reamer will determine the size of the implant and reflects the diameter of the distal end of the implant.
Prepare the metaphysis with the conical reamers that correspond with the last straight reamer. Cone reaming should stop whenever contact with structurally sound cortical bone is obtained. Care should be taken in osteoporotic bone not to overream the femoral diaphysis. A fresh reamer battery should be used when nearing the end reaming of so that the reamer does not get trapped in the femoral canal.
If using the S-ROM system, use the calcar miller to prepare the femur for the triangular modular sleeve (TECH FIG 1B).
Implant Placement
After placing the trial sleeve and femoral stem, perform trial reduction in order to assess version, range of motion, and stability. The modular system allows complete freedom to vary anteversion regardless of proximal femoral geometry (TECH FIG 2A–D).
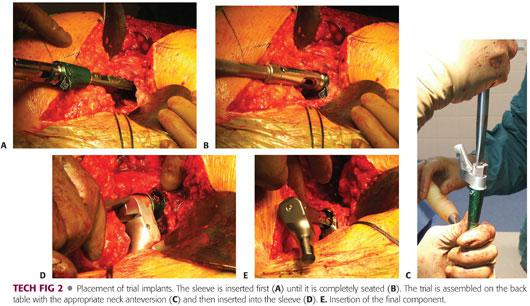
Insert sleeve and finish by inserting femoral stem (TECH FIG 2E).
 Routine Revision with Diaphyseal Defect
Routine Revision with Diaphyseal Defect
Plan to bypass the diaphyseal defect by two cortical diameters with the fluted femoral stem.
A long guidewire can be passed into the medullary canal of the femur to ensure that there are no holes in the cortical bone and to guide the flexible reamer down the canal.
Reamers are used until there is diaphyseal chatter. Reaming is usually 1 to 2 mm greater than the diameter of the final fluted stem, but this varies among different manufacturers.
Conical and miller reaming is performed in the manner described in the previous section.
Place trial implants.
Struts are usually not needed, although they can be used to improve bone stock.
 Extended Trochanteric Osteotomy Needed or Diaphyseal Osteotomy or Open Reduction and Internal Fixation of a Periprosthetic Fracture Distal to a Loose Stem
Extended Trochanteric Osteotomy Needed or Diaphyseal Osteotomy or Open Reduction and Internal Fixation of a Periprosthetic Fracture Distal to a Loose Stem
Remove stem and cement if necessary through the proximal femur or through the fracture site if present.
Perform exposure posterior to the vastus lateralis (or vastus split) to expose the femoral shaft.
The diaphysis can be reamed through the fracture site if present. Use bone clamps to gain control of the proximal segment and reduce the fracture or osteotomy and complete the reaming process. Prophylactic protective cerclage cables can be placed prior to reaming to prevent iatrogenic femoral fracture.
Place the trial implant, crossing the fracture or osteotomy site.
Reduce the hip and assess leg lengths, offset, stability, and soft tissue tension. Rotational stability will not be obtained at the fracture or osteotomy site at this time, so a true stability examination is not possible.
Structural allograft maybe required, and if so, it can be secured using cerclage cables.
Remove trial and place implant. It may be necessary to further ream the distal femur if there is a tight fit to avoid inadvertent fracture (TECH FIG 3).
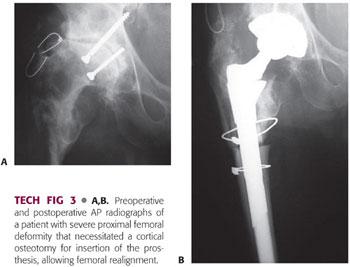
 Use of a Fluted Stem with a Proximal Femoral Allograft
Use of a Fluted Stem with a Proximal Femoral Allograft
Choose a large proximal femoral allograft (critical).
Decide on the level of the graft–host junction and divide the femur at that level. A step cut is not necessary if a bowed and slotted stem is used, as this will give rotational stability.
Ream the distal diaphysis with a straight or flexible reamer until cortical chatter is achieved in the normal way. The last reamer determines the size of the implant.
The allograft is prepared with an appropriate neck cut and reamed. Conical reaming and proximal milling then occur on the proximal femoral allograft.
Make a provisional distal cut of 1 cm on the allograft.
Make a longitudinal cut on the proximal native femur to open it up so the allograft can be inserted within it. Do not remove any soft tissue attachments from the native proximal femur.
Perform a trial reduction by placing the trial within the allograft and inserting the distal portion of the stem into the native femoral diaphysis. Attempt to reduce the hip and assess leg lengths, soft tissue tension, and stability. Remove bone from the distal tip of the allograft to equalize leg lengths. Make sure there is good bony contact at the allograft–host bone junction. The native greater trochanter should be placed in an anatomic position overlying the allograft.
Pass two cables through the lesser trochanter; these will later be used with a claw to secure the native greater trochanter to the allograft.
Downsize the sleeve to allow for a cement mantle and assemble the stem and sleeve on the back table.
Cement the fluted stem and sleeve into the allograft, making sure that all of the cement is wiped off the distal portion of the stem. Keep cement off the distal portion of the allograft to allow complete contact with the host distal femur.
Insert the allograft–stem composite into the native femur. Rotational stability at the graft–host junction should be complete at this time (TECH FIG 4A).
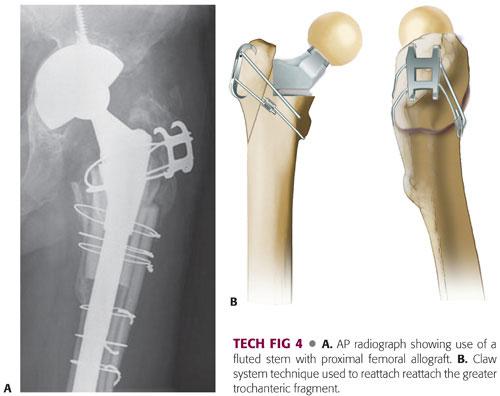
A claw is used to secure the native greater trochanter to the allograft (TECH FIG 4B).
PEARLS AND PITFALLS | |
ETO should be used to access the femoral diaphyseal canal for cement removal or for removal of a well-fixed, extensively porous-coated cementless component. |
|
Complete cement removal is essential. |
|
An extensile approach should be used so that the femur can be directly inspected if cortical perforation has occurred. |
|
Consider an AP and lateral radiograph of the femur at the end of the case to confirm the absence of a fracture distal to the tip of the fluted prosthesis. |
|
Consider the use of larger femoral heads to help prevent instability. |
|
POSTOPERATIVE CARE
 Assisted weight bearing initially with a walking frame, progressing to crutches and eventually a walking stick over the first 6 to 8 weeks for more straightforward revisions.
Assisted weight bearing initially with a walking frame, progressing to crutches and eventually a walking stick over the first 6 to 8 weeks for more straightforward revisions.
 Increasing weight bearing may be more gradual with more complex cases.
Increasing weight bearing may be more gradual with more complex cases.
 Bracing can be used for recurrent instability, but reoperation may be required to increase the level of constraint of the acetabular component.
Bracing can be used for recurrent instability, but reoperation may be required to increase the level of constraint of the acetabular component.
 Standard postoperative protocols should be adhered to, including thromboprophylaxis, antibiotic treatment, early mobilization, and the use of nonsedating analgesia.
Standard postoperative protocols should be adhered to, including thromboprophylaxis, antibiotic treatment, early mobilization, and the use of nonsedating analgesia.
 Any intraoperative tissue and fluid culture results should be obtained.
Any intraoperative tissue and fluid culture results should be obtained.
OUTCOMES
 The majority of studies in the literature on the use of modular femoral stems in revision THA have relatively short follow-up periods.15
The majority of studies in the literature on the use of modular femoral stems in revision THA have relatively short follow-up periods.15
 The use of modular, tapered, fluted, titanium stems in femoral revision with associated bone loss has been shown to facilitate proximal femoral bone reconstitution with improvement in clinical and quality of life measures and survivorship.7,11,12,14,16
The use of modular, tapered, fluted, titanium stems in femoral revision with associated bone loss has been shown to facilitate proximal femoral bone reconstitution with improvement in clinical and quality of life measures and survivorship.7,11,12,14,16
 When used for proximal femoral bone loss or other complex revisions, previous authors have reported excellent radiographic and clinical outcomes with the S-ROM system, including no evidence of osteolysis, a mean postoperative Harris hip score of 82, and 84% patient satisfaction.3,5
When used for proximal femoral bone loss or other complex revisions, previous authors have reported excellent radiographic and clinical outcomes with the S-ROM system, including no evidence of osteolysis, a mean postoperative Harris hip score of 82, and 84% patient satisfaction.3,5
 The S-ROM system has been reported to have a 5–year survival rate of 96%, with a 5% rate of mechanical failure.17,20
The S-ROM system has been reported to have a 5–year survival rate of 96%, with a 5% rate of mechanical failure.17,20
 Inferior outcomes have been associated with the use of large stem diameters with persistent thigh pain associated with stems3 greater than 17 mm.
Inferior outcomes have been associated with the use of large stem diameters with persistent thigh pain associated with stems3 greater than 17 mm.
 Stem diameters greater than 16 mm have been correlated with a significantly increased incidence of stress shielding as well as a lack of bony ingrowth.20
Stem diameters greater than 16 mm have been correlated with a significantly increased incidence of stress shielding as well as a lack of bony ingrowth.20
 Re-revision rates for any reason have been reported from less than 1% to 14%.2,4
Re-revision rates for any reason have been reported from less than 1% to 14%.2,4
COMPLICATIONS
 Infection
Infection
 Loosening of components
Loosening of components
 Dislocation and instability
Dislocation and instability
 Leg length discrepancy
Leg length discrepancy
 Periprosthetic fracture
Periprosthetic fracture
 Osteotomy nonunion
Osteotomy nonunion
 Greater trochanteric bursitis
Greater trochanteric bursitis
 Neurologic deficit
Neurologic deficit
REFERENCES
1. Bolognesi MP, Pietrobon R, Clifford PE, et al. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am 2004;86(12):2720–2725.
2. Bono JV, McCarthy JC, Lee J, et al. Fixation with a modular stem in revision total hip arthroplasty. Instr Course Lect 2000;49:131–139.
3. Chandler HP, Ayres DK, Tan RC, et al. Revision total hip replacement using the S-ROM femoral component. Clin Orthop Relat Res 1995;(319):130–140.
4. Christie MJ, DeBoer DK, Tingstad EM, et al. Clinical experience with a modular noncemented femoral component in revision total hip arthroplasty: 4- to 7-year results. J Arthroplasty 2000;15(7):840–848.
5. Cossetto DJ, McCarthy JC, Bono JV, et al. Minimum four-year radiographic and clinical evaluation of results following femoral revision surgery with the S-ROM modular hip system. Acta Orthop Belg 1996;62(suppl 1):135–147.
6. D’Antonio JA, Capello WN, Borden LS, et al. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res 1989;(243):126–137.
7. Garbuz DS, Toms A, Masri BA, et al. Improved outcome in femoral revision arthroplasty with tapered fluted modular titanium stems. Clin Orthop Relat Res 2006;453:199–202.
8. Goyal N, Diaz-Ledezma C, Tripathi M, et al. Do previous operative reports provide the critical information necessary for revision total hip arthroplasty? J Arthroplasty. 2012;27(6):1023–1026.
9. Hardcastle P, Nade S. The significance of the Trendelenburg test. J Bone Joint Surg Br 1985;67(5):741–746.
10. Hermida JC, Bergula A, Chen P, et al. Comparison of the wear rates of twenty-eight and thirty-two-millimeter femoral heads on cross-linked polyethylene acetabular cups in a wear simulator. J Bone Joint Surg Am 2003;85(12):2325–2331.
11. Kwong LM, Miller AJ, Lubinus P. A modular distal fixation option for proximal bone loss in revision total hip arthroplasty: a 2- to 6-year follow-up study. J Arthroplasty 2003;18(3 suppl 1):94–97.
12. McInnis DP, Horne G, Devane PA. Femoral revision with a fluted, tapered, modular stem seventy patients followed for a mean of 3.9 years. J Arthroplasty 2006;21(3):372–380.
13. Muratoglu OK, Bragdon CR, O’Connor DO, et al. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty 2001;16(2):149–160.
14. Ovesen O, Emmeluth C, Hofbauer C, et al. Revision total hip arthroplasty using a modular tapered stem with distal fixation: good short-term results in 125 revisions. J Arthroplasty 2010;25(3):348–354.
15. Park MS, Lee JH, Park JH, et al. A distal fluted, proximal modular femoral prosthesis in revision hip arthroplasty. J Arthroplasty 2010;25(6):932–938.
16. Rodriguez JA, Fada R, Murphy SB, et al. Two-year to five-year follow-up of femoral defects in femoral revision treated with the link MP modular stem. J Arthroplasty 2009;24(5):751–758.
17. Smith JA, Dunn HK, Manaster BJ. Cementless femoral revision arthroplasty. 2- to 5-year results with a modular titanium alloy stem. J Arthroplasty 1997;12(2):194–201.
18. Spangehl MJ, Younger AS, Masri BA, et al. Diagnosis of infection following total hip arthroplasty. Instr Course Lect. 1998;47:285–295.
19. Valle CJ, Paprosky WG. Classification and an algorithmic approach to the reconstruction of femoral deficiency in revision total hip arthroplasty. J Bone Joint Surg Am 2003;85(suppl 4):1–6.
20. Walter WL, Walter WK, Zicat B. Clinical and radiographic assessment of a modular cementless ingrowth femoral stem system for revision hip arthroplasty. J Arthroplasty 2006;21(2):172–178.
21. Wilkins RH, Bodyia. Lasègue’s sign. Arch Neurol 1969;21(2):219–221.
< div class='tao-gold-member'>








