Key points
- •
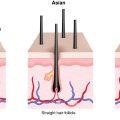
Androgenetic alopecia is the most common cause of hair loss and arises from androgen-dependent hair follicle miniaturization on the scalp.
- •
Several studies have suggested that follicular microinflammation is implicated in the pathogenesis of androgenetic alopecia, but to date, no immune modulatory therapies have been investigated for use in androgenetic alopecia alone.
- •
Androgenetic alopecia can arise in the setting of other forms of alopecia such as alopecia areata and cicatricial alopecias. As such, therapy for patients with androgenetic alopecia superimposed on other alopecias should be guided by the best-practice standards for all etiologies of hair loss.
- •
Topical immunotherapies and topical or intralesional corticosteroids are appropriate treatments for alopecia areata, while intralesional steroids are appropriate for frontal fibrosing alopecia.
- •
In this chapter, we discuss the use of topical and intralesional immunomodulatory agents for the treatment of alopecia areata or frontal fibrosing alopecia that may present in concert with androgenetic alopecia.
Background, definitions, and history
Androgenetic alopecia (AGA), otherwise known as male or female pattern hair loss, is the most common cause of hair loss, affecting 50% of men and 40% women by the age of 50 (level of evidence; 1a). , Despite its prevalence, only one medical treatment for women and two for men are currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of AGA: topical minoxidil for both and oral finasteride for men. Additionally, several low-level laser therapy (LLLT) devices have received 510(k) premarket medical device approval from the FDA for use in both men and women with AGA (level of evidence: 3b). Response to these treatments can vary and may achieve partial response in some, highlighting the need for therapies that target alternative mechanisms in the pathophysiology of alopecia and those that may work synergistically with the standard-of-care therapies already available.
AGA specifically arises in the setting of androgen-dependent hair follicle miniaturization on the scalp. Notably, histopathologic examination of the scalp skin of males and females with AGA demonstrates activated T cells, macrophages, and mast cells infiltrating the hair follicle region, suggesting that microinflammation could be implicated in the cause or occur as a consequence of miniaturization (level of evidence: 5). In one study, AGA patients with follicular microinflammation experienced hair regrowth after combination therapy with topical steroids, minocycline, and red light. Another study found that the histologic patterns of early AGA lesions were indistinguishable from those seen in lichen planopilaris (LPP), suggesting the lichenoid reaction leading to follicular destruction in these patients may be related to the underlying mechanisms driving AGA (level of evidence: 4). Although therapies targeting the immune system are widely used for alopecia areata (AA) or cicatricial alopecias such as LPP or central centrifugal cicatricial alopecia (CCCA), their utility in AGA requires further exploration. Given the potential immune mechanisms involved in AGA pathogenesis, immune modulation may represent a novel therapeutic avenue for some patients with AGA.
AGA often presents in combination with other forms of alopecia. For instance, AGA has been observed in both men and pre- and postmenopausal women diagnosed with frontal fibrosing alopecia (FFA) (level of evidence: 4). Oral 5α-reductase inhibitors are commonly used as part of a multitherapy approach to halt hair loss in FFA, suggesting that concurrent treatment of underlying AGA may be responsible for a portion of the response (level of evidence: 3a). , AA can also present concurrently with AGA or can present with hair loss in the androgen-dependent areas, effectively mimicking AGA (level of evidence: 4). As such, recognition and treatment of all underlying pathomechanisms of hair loss is paramount to achieving optimal hair regrowth and maximizing patient satisfaction. Herein we discuss the evidence behind and best practices for immunomodulatory therapies, with a specific focus on therapies that may be of use in patients with AGA and comorbid AA or cicatricial alopecia.
Topical irritants and sensitizers
Topical immunotherapy has proven to be an effective first-line treatment for autoimmune, noncicatricial alopecias such as AA. Current methods include application of an irritant (anthralin) or an allergic sensitizer (squaric acid dibutylester [SADBE] or diphenylcyclopropenone [DPCP]). The notable difference between irritant and allergic contact dermatitis is that once the topical agent is applied to the skin, the latter requires both a sensitization phase and an elicitation phase. Although the mechanisms of action of these therapies are not fully understood, they are believed to modulate proinflammatory cytokines (level of evidence: 5). Allergic sensitization is believed to also induce antigenic competition, reduce the CD4+/CD8+ T cell ratio, and lead to apoptosis of autoreactive T cells (level of evidence: 5).
Indications and patient selection
Use of topical immunotherapy should be limited to patients who have AA. Although distinction between AGA and AA is typically possible via history and clinical examination, diagnosis may be difficult if there is diffuse AA, or if AGA presents in the androgen-dependent areas (level of evidence: 5). For presentations that are clinically equivocal, biopsy may be necessary. The presence of highly positive CD3 infiltration or CD8 T-cell infiltration, especially in the intrafollicular region, is suggestive of AA ( Pearl 9.1 ). , For patients with AA, topical sensitizers (SADBE and DPCP) are first-line for moderate-to-severe hair loss (>10% of the scalp) or for those with chronic-relapsing AA (level of evidence: 5). Topical irritants (anthralin) remain a second-line option for moderate disease (level of evidence: 5). Generally, patients experiencing acute or rapidly progressive hair loss are not good candidates for topical irritants or sensitizers. Though the majority of higher-level evidence supports the use of topical immunotherapy for AA patients, at least one study showed that patients with patchy AA did not experience significant benefit (level of evidence: 3b). For these patients, intralesional steroids may be beneficial, as discussed later in this chapter. Notably, patients with serious concurrent medical illnesses and women who are pregnant or breastfeeding should not be treated with topical sensitizers (level of evidence: 4).
Distinguishing between alopecia areata (AA) and androgenetic alopecia (AGA) may be challenging if a patient presents with diffuse AA or with AGA in androgen-dependent areas. For presentations that are clinically equivocal, biopsy showing highly positive CD3 infiltration or CD8 T-cell infiltration, especially in the intrafollicular region, is suggestive of AA. ,
Expected outcomes
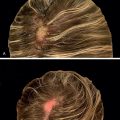
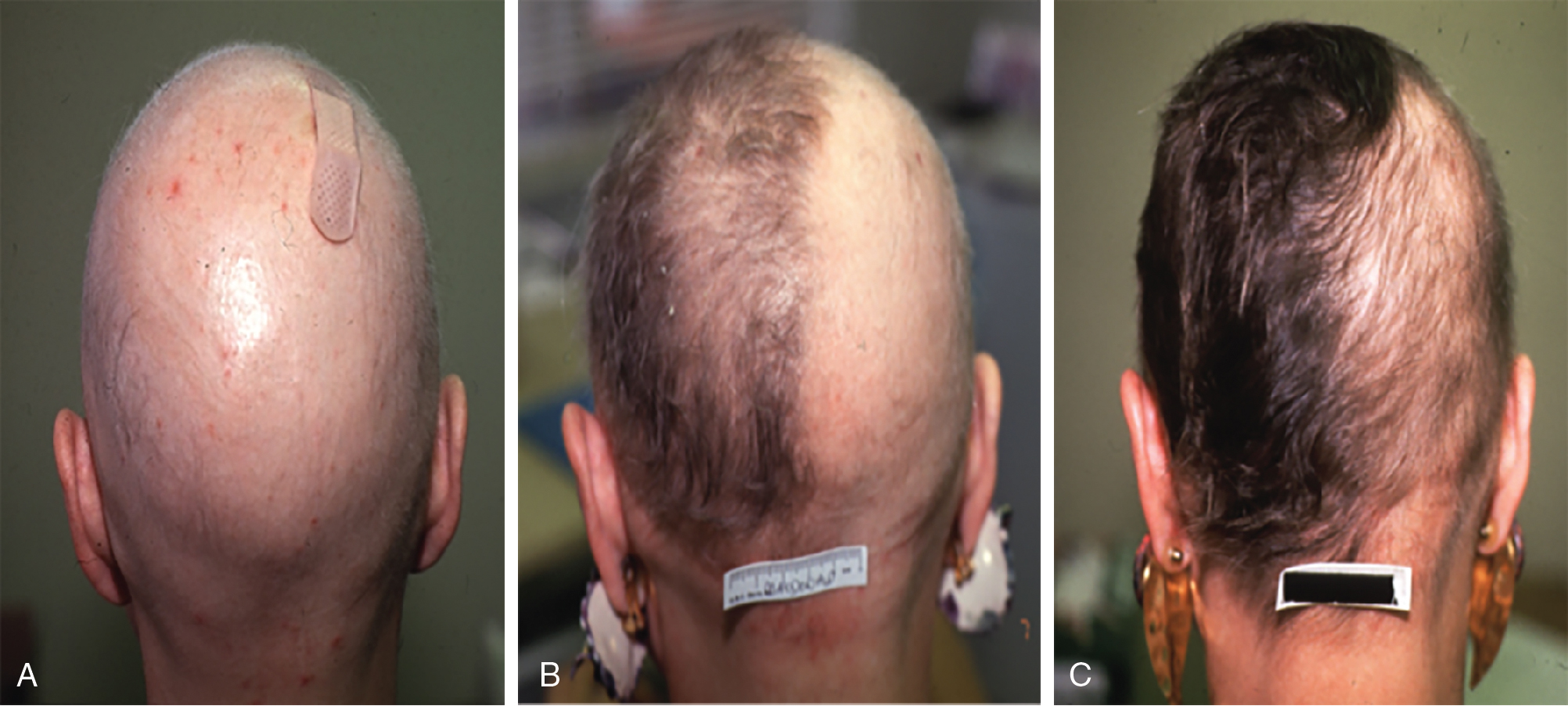
Although no randomized controlled trials evaluating the efficacy of topical sensitizers exist to date, the largest review of the use of topical sensitizers in AA estimated that 50% to 60% of patients with moderate-to-severe AA could be treated effectively with DPCP or SADBE (level of evidence: 4). However, the range of responses reported in the individual studies varied widely (9% to 87%). An observational study revealed that 77.9% of participants treated with DPCP experienced clinically significant hair growth at 32 months, defined as patient-reported cosmetically acceptable regrowth or regrowth resulting in more than 75% of scalp terminal hair coverage. These patients began to see hair growth at an average of 3 months after treatment initiation and achieved clinically significant regrowth at a median of 12.2 months ( Fig. 9.1 ). In general, patients who demonstrated terminal hair regrowth earlier in treatment or with fewer treatments had more favorable outcomes. Patients with earlier onset of hair loss or larger areas of hair loss at baseline were less likely to respond. Additionally, patients requiring higher peak concentration of therapy (>2.0% solution) had a decreased chance of significant regrowth. Unfortunately, response to treatment is often transient. One study found that after achieving clinically significant regrowth, 62.5% of patients experienced relapse, with a median time to relapse of 2.5 years. For patients with recalcitrant alopecic patches, concomitant intralesional steroids may be of use, as discussed later in this chapter.
Of note, one randomized controlled trial exists for topical anthralin, in which 0.5% anthralin was used as the “positive control” to study the efficacy of 20% azelaic acid in AA. This study found that cosmetically acceptable regrowth after anthralin treatment was achieved in 56.2% of patients who presented with patch-type alopecia with no evidence of relapse 8 weeks after completion of the treatment. This study was not placebo-controlled, however, making these results difficult to interpret (level of evidence: 1b). Similarly, there is observational evidence that 0.5% to 1.0% anthralin elicits a cosmetic response in 75% of patients with patch-type AA and 25% of patients with AA totalis (level of evidence: 4). , In one patient with long standing AA who failed several years of treatment with intralesional steroids and a trial of whole scalp anthralin 1% cream, treatment with anthralin 1% cream combined with calcipotriene 0.005% cream achieved visible hair regrowth that was maintained through follow-up at 8 months posttreatment (level of evidence: 5).
Treatment techniques and best practices
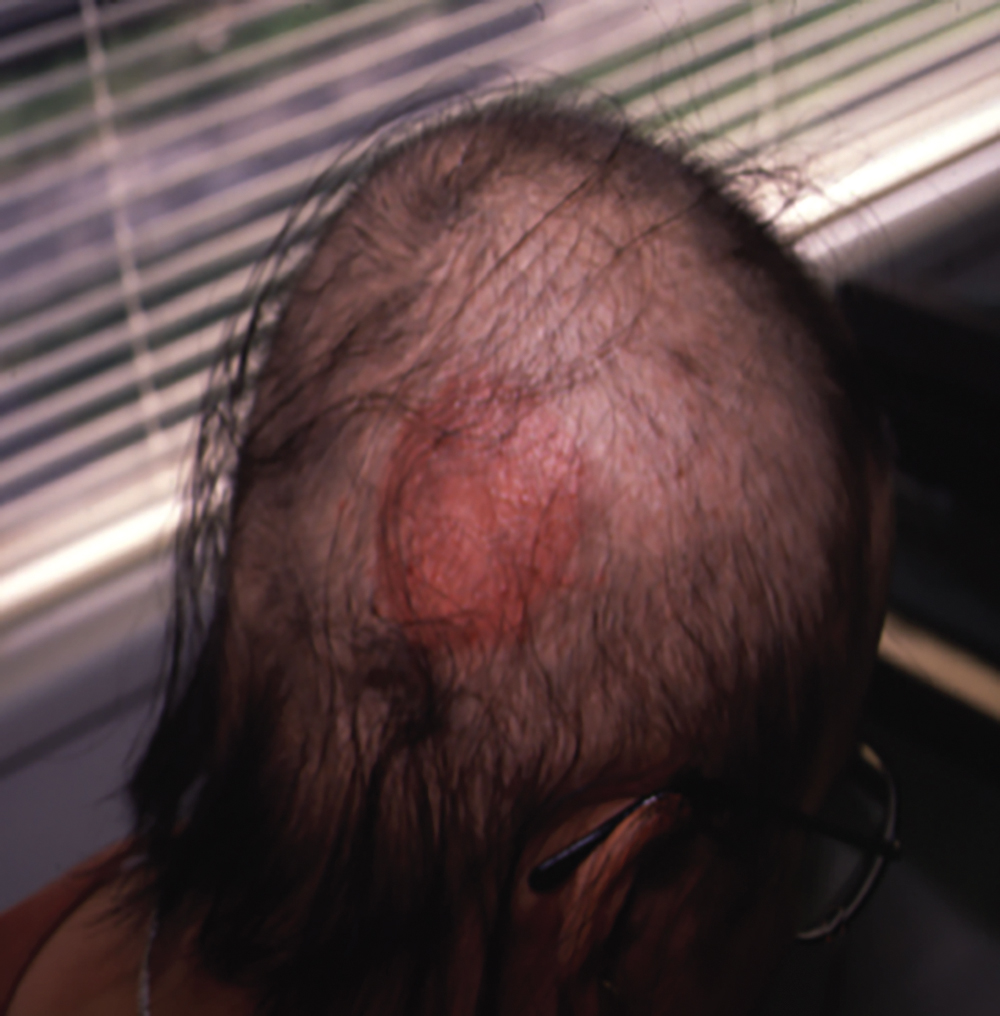
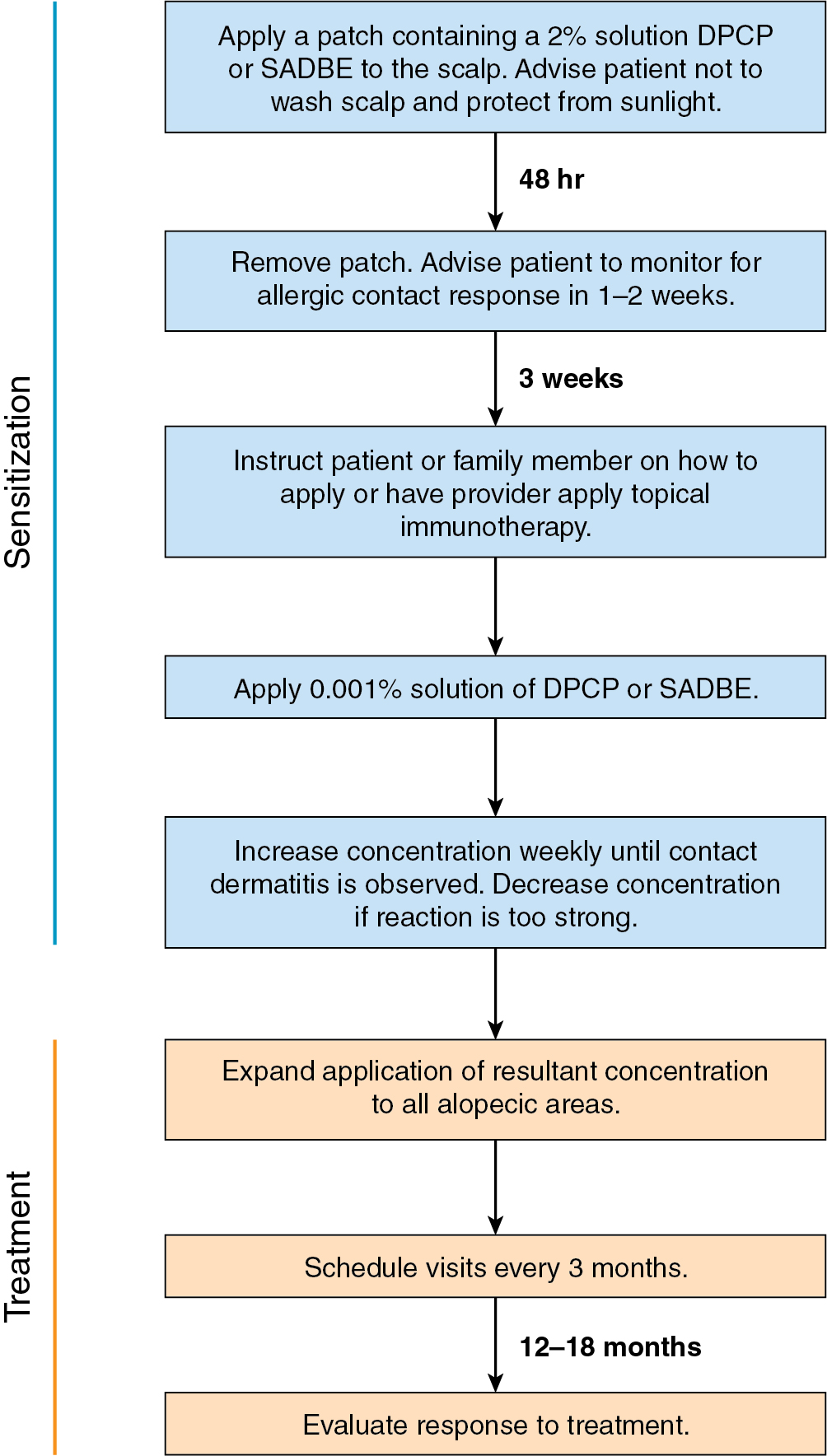
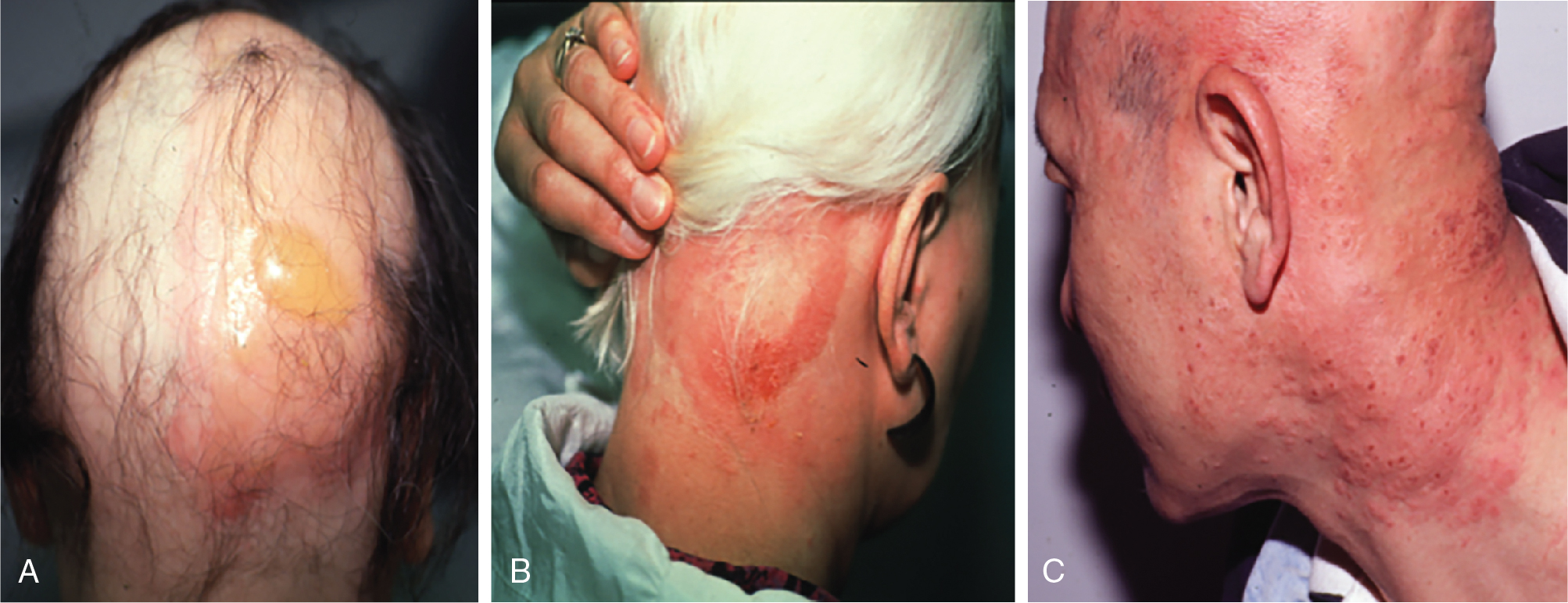
DPCP is the most commonly used topical sensitizer, though efficacy studies have shown that DPCP and SADBE are equally effective in treating AA (level of evidence: 4). , The patient can be sensitized with a patch containing a 2% solution of DPCP or SADBE diluted in acetone. The patch is applied to a small, 4-cm-diameter circular area on the scalp. The patient is instructed to avoid washing their head or removing the patch for 48 hours. After this time, the patch is removed. Approximately 2 weeks later, an allergic contact dermatitis should appear on the scalp ( Fig. 9.2 ). Three weeks after the sensitization, the patient should return to the office, either to have the clinician apply the topical immunotherapy or accompanied by someone who will apply the topical immunotherapy for them. Patients can also be instructed to apply the therapy themselves; however, they should be made aware of the severe allergic reactions that can occur in the case of a spill. The scalp is first treated with a weak solution of DPCP or SADBE using a cotton swab, starting as low as a 0.001% solution. Many clinicians initially choose to treat half of the scalp to distinguish treatment response versus spontaneous regrowth. A reinforced cotton applicator is saturated with the treatment chemical and applied to the scalp, first antero-posteriorly, then laterally ( Fig. 9.3 ). At weekly intervals, the concentration is increased until a mild dermatitis reaction is observed ( Pearl 9.2 ). The concentration that produces this dermatitis reaction is used as the maintenance concentration for subsequent treatments (level of evidence: 4). As such, the final concentration of solution varies by patient. In cases of excess inflammation, the concentration can be reduced. Similarly, in the case of insufficient response, the concentration can be increased. Communication with the patient about the degree of dermatitis experienced is extremely important. Once the correct concentration has been chosen, the application area can be gradually expanded until all alopecic areas are covered. Once all alopecic areas are treated with adequate response, visits can be scheduled at 3-month intervals. Treatment efficacy should be evaluated at 18 months. Afterward, maintenance therapy every 1 to 4 weeks may be beneficial for patients who exhibit clinically significant hair regrowth ( Fig. 9.4 ).
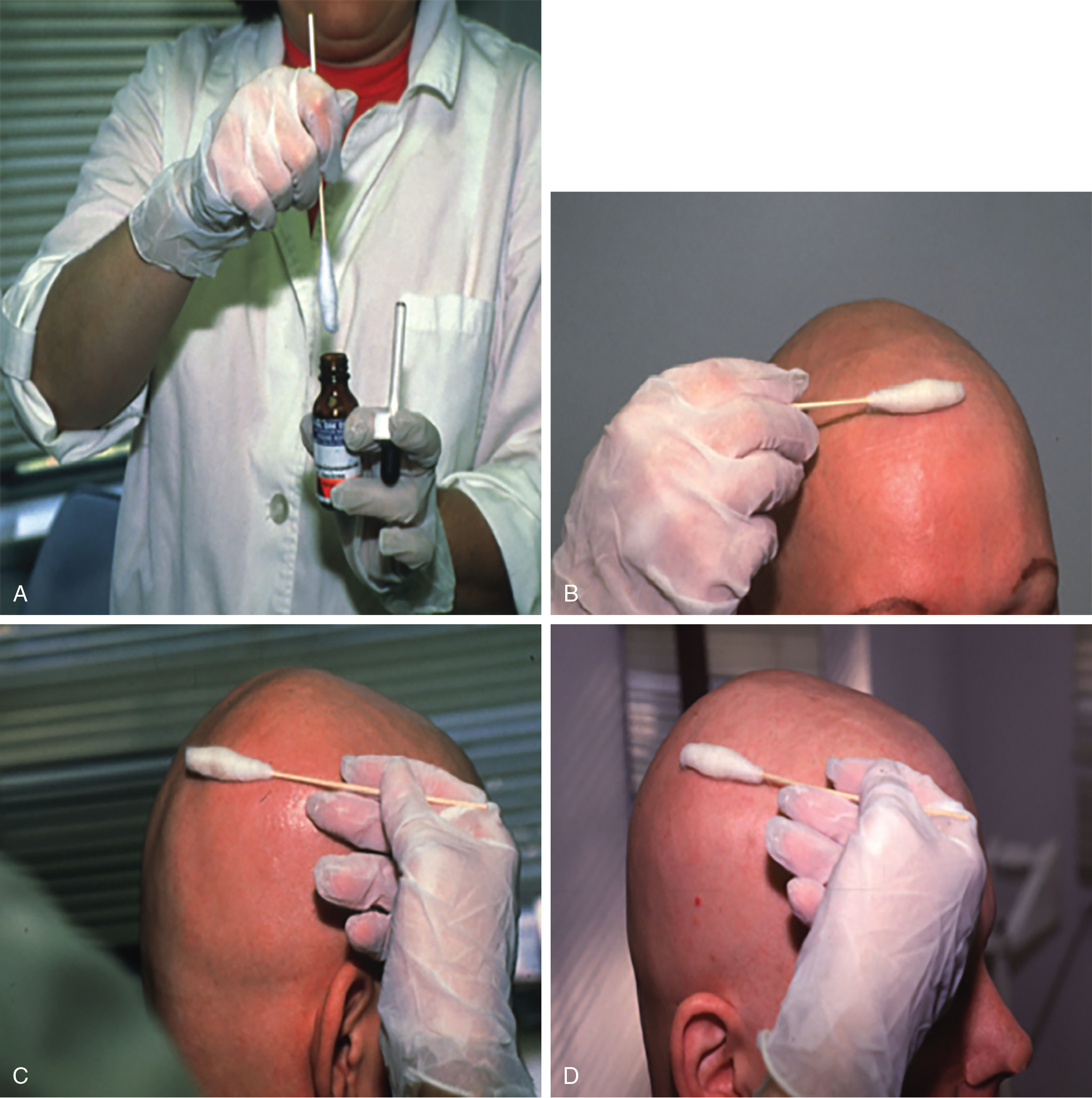
After the sensitization period, treatment is initiated with a weak solution of diphenylcyclopropenone (DPCP) or squaric acid dibutylester (SADBE), starting as low as a 0.001% solution and increasing in concentration at weekly intervals until contact dermatitis is elicited.
Treatment with anthralin similarly involves inducing a dermatitis reaction on the scalp. Anthralin cream 0.5% to 1.0% is commonly used, although these concentrations are not currently commercially available and may need to be prepared by a compounding pharmacy, which may not be cost effective for the patient. The compound can be applied daily to affected areas for 15 to 20 minutes, then washed off. On a weekly basis, the amount of time the cream is applied is increased by 5 minutes up to a maximum of 1 hour, or until a low-grade dermatitis develops. Once the optimal contact time is determined, the patient should continue to apply anthralin daily to the affected area for that fixed contact time. Clinical response should be evaluated at 3 months. For patients with recalcitrant AA who have failed anthralin alone, anthralin plus calcipotriene may be tried. For these patients, anthralin 1% cream and calcipotriene 0.005% cream should initially be applied for 5 minutes, increasing in duration to 90 minutes as tolerated for 5 days a week.
Prevention and management of adverse events
For both topical sensitizers and irritants, avoidance of sun exposure to the area is highly recommended for the entire treatment period. Though topical sensitizers are generally well tolerated, clinically significant adverse events have occurred in 56.8% of patients undergoing DPCP for AA, with the most common adverse effects including blistering (45.3%), hyperpigmentation (12.2%), autoeczematization (10.1%), hypopigmentation (2.0%), and symptomatic lymphadenopathy (2.0%). Anecdotally, the authors have rarely observed facial edema. Blistering and autoeczematous reactions are often caused by incorrect selection of concentration, and management includes washing off the treatment and applying topical corticosteroids ( Fig. 9.5 ). In severe cases, oral corticosteroids may be used. Cervical and occipital lymphadenopathy are common and resolve with decreasing concentration; however, they should be reviewed with the patient to avoid any potentially unnecessary anxiety ( Fig. 9.6 ). Hyper- or hypopigmentation may occur, especially in darker skin, and, rarely, vitiligo affecting the treatment area can occur (level of evidence: 4) , ( Fig. 9.7 ). In the case of therapy-induced vitiligo, treatment should be immediately discontinued and topical steroids applied. Phototherapy (PUVA or nb-UVB) may be used with variable response. Complete repigmentation in these cases is rare.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree