Melanin, hemoglobin, and water are the three chromophores targeted in skin-directed laser therapy. In normal skin, melanin is produced by melanocytes, transferred to keratinocytes, and confined to the epidermis. Hemoglobin is carried within red blood cells that circulate within blood vessels located in the dermis. Water is present in all layers of the skin but is increased in abundance in the dermis and subcutaneous tissue. Melanin, hemoglobin, and water each exhibit characteristic wavelengths of absorption. Exposure to these energy wavelengths induces excitation to a higher energy state. Lasers with corresponding wavelengths selectively target these chromophores to treat cutaneous pathology while limiting surrounding tissue damage.
18.3 Hyperpigmented Lesions
The pathogenesis of skin hyperpigmentation is complex. A single or combination of factor(s) such as photosensitivity, trauma, medication deposition, or neoplasia may result in the clinical observation of skin hyperpigmentation. The classification and treatment of pigmentary disorders is based upon the histological location of the pigment within the epidermis, dermis, or a combination of both the epidermis and dermis. Due to melanin’s wide spectrum of absorption, several lasers may be used to target this pigment. Ideal laser therapy targets the abnormal pigment without causing surrounding damage. In general, longer wavelength lasers penetrate deeper in the skin.3 Thus lasers with a shorter wavelength are commonly utilized for superficial pigmented lesions, whereas the longer wavelength lasers are employed for dermal pigmentation.
Continuous wave and quasicontinuous wave laser systems were the first laser systems implemented to treat pigmentary disorders. However, the pulse duration of these laser systems is longer than the thermal relaxation time of the melanosome, resulting in significant surrounding thermal damage with subsequent scarring and dyspigmentation.4 More recently, short-pulsed or quality-switched (QS) lasers were developed to selectively target pigment. These laser systems deliver high bursts of energy over a very short period of time and are thus more suitable for treating pigmentary disorders. The main laser systems in use today for pigmentary disorders are the 755-nm QS alexandrite laser, and the 532-nm or 1,064-nm QS neodymium:yttrium aluminum garnet (Nd:YAG) laser.5 Each laser targets melanin as the chromophore. The type of laser and laser settings are adjusted based upon the skin pathology and location of the pigmentation. Topical, local, and regional anesthesia may be applied for patient comfort. Pigmentary lesions should be treated in a nonoverlapping technique. A “snapping” sound indicates pigment destruction and an ash-gray or white discoloration is produced with treatment. Erythema, hyperpigmentation or hypopigmentation, purpura, and textural changes are possible side effects of each laser. Given the risk for pigmentary alteration, the ideal patient is one with a fair complexion (Fitzpatrick skin types I and II). However, if treatment for a patient with darker skin is indicated, the longer wavelength lasers are associated with less epidermal absorption and thus a decreased risk of posttreatment dyspigmentation.6 It is essential to perform a test spot on any darker skinned patients before treating large areas of the face. Judicious epidermal cooling and pretreatment and/or posttreatment with bleaching agents, topical steroids, and sun protection may also be of some benefit.
18.3.1 Epidermal Hyperpigmentation
Café-Au-Lait Macules
Café-au-lait macules (CALMs) are well-circumscribed, hyperpigmented macules that present in infancy and may be associated with several underlying genetic syndromes. The hyperpigmentation results from increased pigmentation within the basal layer of the epidermis. Although removal of CALMs with the 532-nm QS Nd:YAG laser has been reported, the results are variable with a high risk of recurrence.7,8,9,10 Ablative laser therapy using an erbium:yttrium aluminum garnet (Er:YAG) laser system has also been reported.11 Several treatments may be necessary for resolution.
18.3.2 Combined Epidermal and Dermal Hyperpigmentation
Becker Melanosis
Becker melanosis presents during the second or third decade of life as a unilateral hyperpigmented patch with increased hair growth commonly located on the anterior or posterior chest and shoulder (▶ Fig. 18.1). Although the exact pathogenesis is unclear, this lesion is regarded as a hamartoma of both ectoderm and mesoderm embryonic tissue with increased pigmentation in the basal layer of the epidermis, a variably increased quantity of melanocytes, hyperplasia of the pilosebaceous units, and often a smooth-muscle proliferation within the dermis.12 Ablative, nonablative, QS, and fractional laser systems have been reported to improve the appearance of Becker melanosis.10,13,14,15,16,17 Terminal hair density may be reduced with long-pulsed 694-nm ruby and 755-nm alexandrite lasers.14,17 In one comparative trial, a 2,940-nm Er:YAG laser proved to be more efficacious for pigment removal than a 1,064-nm QS Nd:YAG, with fewer treatment sessions required and prolonged results.16 The QS laser systems, however, are often the preferable method for removal due to their low-side effect profile. However, repeat procedures are often required for recurrent or residual pigmentation. Treatment modality should be based upon the patient’s skin type, comorbidities, and preference.
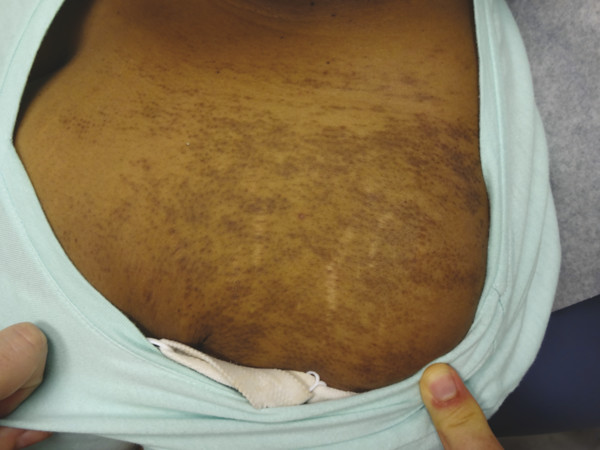
Fig. 18.1 Becker melanosis on the chest of a young woman.
Postinflammatory Hyperpigmentation
Postinflammatory hyperpigmentation results from melanin deposition in the epidermis or the dermis and presents as brown-to-gray macules and patches following trauma to the dermoepidermal junction (▶ Fig. 18.2). Therapy for these lesions has been attempted with 532-nm and 1,064-nm QS Nd:YAG lasers with inconsistent results.13,18 Since melanin is the chromophore for each laser system, postinflammatory hyperpigmentation is a common and often expected side effect of therapy. Laser therapy for this pigmentary phenomenon should be used with discretion.
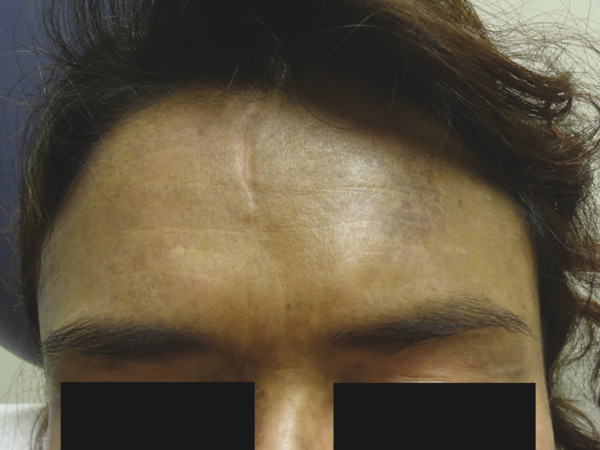
Fig. 18.2 Postinflammatory hyperpigmentation and hydroxychloroquine-induced pigmentation on the forehead of a woman with dermatomyositis.
18.3.3 Dermal Hyperpigmentation
Drug-Induced Pigmentation
Several systemic medications can cause skin hyperpigmentation. Minocycline and amiodarone are the two more common causative agents. Three clinical and histological categories of minocycline hyperpigmentation exist. A blue-black discoloration in previous sites of inflammation, commonly in acne scars, is characteristic of type I minocycline-associated hyperpigmentation, whereas type II produces blue-gray macules and patches on the surface of the shins, and type III presents with diffuse muddy-brown hyperpigmentation on sun-exposed skin. Histologically, type I and type II minocycline-associated hyperpigmentation display intracellular and extracellular pigmentation granules within the superficial dermis. To the contrary, type III reveals increased melanin production by melanocytes within the epidermis. Amiodarone-associated hyperpigmentation produces a slate-gray discoloration on photoexposed skin with intracellular yellow-brown pigment granules present histologically.12 Drug-induced hyperpigmentation may also be caused by chemotherapy agents, antimalarials, heavy metals, psychotropic medications, and other miscellaneous systemic medications (▶ Fig. 18.2). The treatment of choice for drug-induced pigmentation is discontinuation of the inciting agent. Although pigmentation fades over time after discontinuation of the implicated medication, laser therapy may hasten resolution. QS 755-nm alexandrite, QS 1,064-nm Nd:YAG , and fractional 1,550-nm diode laser systems have each been reported to improve minocycline-associated hyperpigmentation.19,20,21,22,23 Amiodarone-associated hyperpigmentation has been successfully treated with the QS 1,064-nm Nd:YAG.24 Several treatments are often necessary for complete resolution. Lasers with a higher wavelength may be better suited for individuals with darker skin types to avoid posttreatment dyspigmentation (Fitzpatrick skin types III–VI).
Nevus of Ota
Nevus of Ota is a unilateral bluish-brown patch on the periocular skin (▶ Fig. 18.3) predominantly affecting individuals with a darker skin type (Fitzpatrick skin types III–VI). A proliferation of spindled, pigmented melanocytes within the dermis causes the bluish hyperpigmentation. The term nevus of Ito is used when the hyperpigmentation is localized to the supraclavicular, scapular, or deltoid regions.12 The QS 755-nm alexandrite and QS 1,064-nm Nd:YAG laser systems, at varying fluences, have successfully lightened the hyperpigmentation.25,26,27 Postinflammatory hypopigmentation and hyperpigmentation are significant concerns in patients with nevus of Ota, given the propensity for these lesions to affect patients with a darker skin phenotype.27 Recently, decreased side effects were noted with low-fluence QS 1,064-nm Nd:YAG.26 Several treatments may be necessary to achieve significant clinical effect.
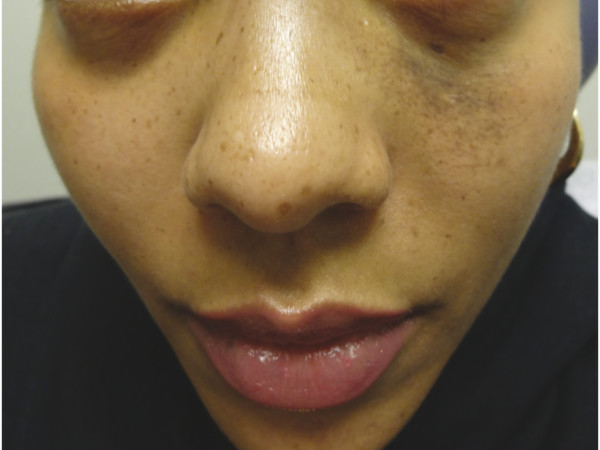
Fig. 18.3 Nevus of Ota on the periorbital skin of a young woman.
18.4 Benign Skin Lesions
18.4.1 Solar Lentigo and Ephelides
Solar lentigines and ephelides are benign hyperpigmented macules located on photoexposed skin. Solar lentigines result from ultraviolet light exposure. Histologically, an increased number of melanocytes and quantity of melanin is present within the epidermis. Although ephelides may also result from sun exposure, histological examination reveals an increased quantity of melanin within the epidermis without any increase in the number of melanocytes. Both solar lentigines and ephelides are amenable to laser therapy with the QS 755-nm alexandrite laser or the QS 532-nm Nd:YAG laser.4,10,28 Each lesion turns white after being treated. Typically, two to three treatments repeated every 6 to 8 weeks are necessary for complete resolution. Sun protection and sun avoidance should be emphasized to all patients to prevent recurrence of treated lesions or development of new lesions.
18.4.2 Seborrheic Keratoses
Seborrheic keratoses are benign, pink-to-brown epidermal proliferations with a classic stuck-on appearance commonly affecting adults and older individuals. Both ablative and nonablative laser systems may be utilized for removal.8,10,29,30,31 However, given the risks of scarring and dyspigmentation with the ablative lasers, QS 532-nm Nd:YAG or long-pulsed 755-nm alexandrite are used more commonly. Repeat treatment may be necessary for complete removal. The advantages of laser treatment versus more economical modalities such as cryotherapy or electrocautery are uncertain.
18.4.3 Melanocytic Nevi
Common acquired melanocytic nevi result from a proliferation of specialized melanocytes or nevoid cells and may be subclassified as junctional, compound, or dermal nevi depending upon the location of the nevus cells within the skin. Blue nevi are blue papules that result from heavily pigmented, spindled melanocytes localized in the dermis (▶ Fig. 18.4). Lastly, congenital melanocytic nevi are proliferations of melanocytes that are present at birth. These lesions may be epidermal, dermal, or both and are clinically classified based upon lesion size into small- , medium- , and giant-sized congenital nevi. Excision with histopathological evaluation is the standard of care for these lesions. The use of lasers for the removal of nevi remains controversial. Although normal-mode ruby, QS, and normal-mode 755-nm alexandrite; QS 532-nm, and 1,064-nm Nd:YAG; 585-nm pulsed dye laser (PDL); and the ablative laser systems may improve the clinical appearance of acquired, blue, or congenital melanocytic nevi, dermal melanocytic nevoid cells often remain after treatment and pose a risk for malignant transformation.32–44 In general, the longer wavelength lasers are better adapted to reach deeply situated melanocytes to treat blue, congenital, compound, or dermal nevi. The risk of malignant transformation, in addition to the side effects of each type of laser, should be discussed with the patient prior to treatment. Each patient should be monitored for recurrence or malignant transformation after therapy completion. In this author’s practice, laser surgery is not recommended to treat melanocytic nevi.
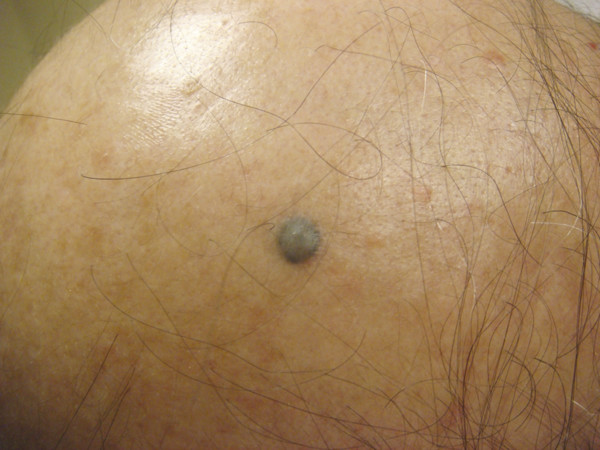
Fig. 18.4 Banal blue nevus on the scalp.









