Chapter 2 Tendon Nutrition and Healing
Outline
Traditionally, tendons have been thought of as being inert, hypovascular structures.1,2 However, subsequent research has shown that tendons are active metabolically, that tenocytes can replicate and heal experimental injuries, and that adhesions, while common, are not necessary for healing.3–6 This chapter will review current knowledge on tendon nutrition and healing.
Tendon Nutrition
Vascular Sources
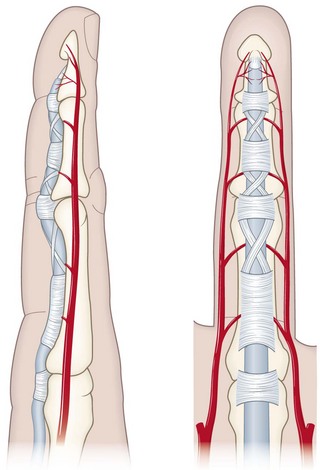
Tendons have two sources of vascular nutrition. Extrasynovial tendons are supplied by circumferential vessels in the paratenon, a multilayered system elegantly described by Giumberteau7 and illustrated in Chapter 31. Intrasynovial tendons, such as those in zone 2 in the hand, are supplied through the segmental vincular system.8–10 This segmental system results in predictable watershed zones within the flexor tendon (Figure 2-1), with intercalated regions on the palmar aspect of the tendon with little in the way of blood supply. The number and location of these zones also varies by digit, with the ring finger generally having the fewest vincula.10
Synovial Fluid
Intrasynovial tendons have a second source of nutrition, of course—synovial fluid. This mechanism is especially important in avascular and hypovascular regions of the tendon3,11–14 and is dependent on the pumping effect of digital motion. Tendons whose vincula are absent or damaged and that do not move are therefore deprived of nutrition, an important consideration for tendon repair and rehabilitation.
Tendon Healing
Biology of Tendon Healing
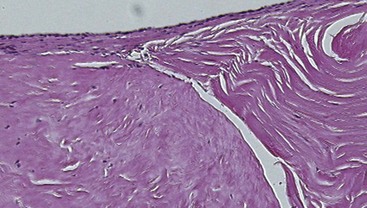
The extracellular matrix (ECM) is the principal component of tendon tissue and is responsible for its material properties.15–17 The major constituents of the ECM are type I collagen; proteoglycans, principally decorin, but also aggrecan in the gliding regions18–21; fibronectin; and elastin. This matrix is synthesized by tendon cells, or tenocytes. These cells are surrounded by the dense matrix; thus, although they are metabolically active, they do not participate much in the tendon healing process. Instead, undifferentiated cells in the epitenon do the heavy lifting for tendon healing, proliferating, migrating into the gap between the tendon ends, and finally uniting the cut tendon ends22,23 (Figure 2-2). Unfortunately, this is a two-edged sword; if these same cells migrate away from the tendon, toward the tendon sheath, they form adhesions, which restrict tendon motion. Often this is indeed the case, as the relatively ischemic tendon is surrounded by better vascularized tissue, which sends out vascular buds under the stimulation of vascular endothelial growth factor (VEGF).24–27
After tendon injury, the extracellular matrix undergoes significant changes, due to synthesis of new elements by the tenocytes, such as type III collagen,28–30 degradation of existing elements by various matrix metalloproteases, and remodeling of the resulting combination, under the influence of cytokines such as transforming growth factor (TGF)β as well as mechanical forces. Manipulation of these processes, to augment their action between the tendon ends while reducing them at the tendon’s gliding surface, is the goal of much research, as described later.
Healing of flexor tendons in zone 2 depends on the ability of the injured tendon to recruit fibroblasts and other cellular components to the site of injury.31 Normally these are circulating or locally derived undifferentiated (i.e., stem) cells that are recruited to the injury site by the expression of cytokines in the wound.24,27,32–34 Cytokine stimulation is also important in converting these undifferentiated cells into the tendon phenotype, characterized by the expression of markers such as tenomodulin and scleraxis.35,36 Bone marrow–derived stem cells (BMSCs) can also enter and participate in soft tissue healing.37–39
Effect of Physical Factors
Motion
Lacerated tendons that are immobilized, unless extremely well nourished, will heal with adhesions.40–42 While these adhesions can remodel, especially in an extrasynovial environment, in the region of the tendon sheath such adhesions are invariably associated with loss of motion. Thus, motion of flexor tendon repairs in critical. Duran and Houser43 suggested observing the repair intraoperatively and maintaining passive motion sufficient to cause 4 mm of tendon gliding, and separation of the profundus and superficialis repairs, if both tendons are injured. Evans has discussed similar concepts of limited active motion of repaired tendons.44
Loading
Clearly, some load must be applied to a flexor tendon if it is to move. The loads applied to a tendon by passive motion of a digit tend to be small, and tendons may buckle instead of glide, especially if the tendon repair has a high friction.45 A modification of the usual synergistic passive motion protocol in which the metacarpophalangeal joints are maintained in extension in both the flexion and extension phases of motion seems to provide better pull, on the order of 100 g, in both the flexion and extension directions, and may be useful in some cases.46,47 The use of higher loads, with active motion protocols, is somewhat controversial.48–50 My philosophy has been to tailor the loading to the stage of healing, as emphasized by Groth,51 as further described later.
Effect of Friction on the Results of Tendon Repair
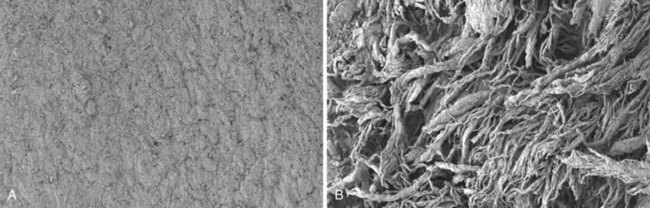
Animal studies over the past decade have shown convincingly that high friction repairs with knots and/or many loops on the surface, such as the modified Becker/MGH or Tsuge repairs, result in abrasion of the tendon sheath (Figure 2-3) and adhesion formation, even when factors such as rehabilitation method are optimized.52–54 Thus, the goal should be to use a high-strength, low-friction repair construct, such as the modified Kessler, Tajima, or Pennington repairs, and a low-friction suture material, of which there are many. Most recently, I have been using 3-0 polyester suture and a modified Pennington design.
Impact on Postoperative Management: Concept of the “Safe Zone”
Until the mid 1960s, most flexor tendon repairs were immobilized postoperatively for three weeks. This policy was based on the research of Mason and Allen,55 who had shown that canine flexor tendon repairs decreased in tensile strength for three weeks postoperatively. Subsequent clinical work by Verdan,56 Kleinert et al,57 and Duran et al58 showed that human flexor tendon repairs could be safely mobilized with a combination of active extension and passive flexion.
The use of early mobilization after tendon repair has resulted in improved outcomes for flexor tendon injuries.56,58–62 In animal models,45,53,63–67 earlier mobilization results in better final tendon gliding and tensile strength. More recently, the fine details of mobilization have been studied, specifically the effect of timing68–70 and the effect of differential motion of the wrist and finger joints on tendon loading and tendon gliding during the healing period.49,53,71 Active motion protocols have also been used, although, interestingly, the clinical results are not reliably better than passive protocols.72–75 Moreover, the addition of loading to motion in animal models has been shown to have little effect on the final result in terms of strength and motion.49,67,76 Thus, the available evidence suggests that motion, not load, is the critical factor.
Of course, there must be some load on the tendon if it is going to move; at the very least, the load must be sufficient to overcome the forces of friction. It is for this reason that low friction repairs are important—they minimize the load needed to initiate movement. Friction, though, is not the only concern. The force needed to overcome joint stiffness and to flex traumatized, edematous tissues must also be considered, as well as the weight of the distal digit itself; often these latter forces will far outweigh the frictional ones in magnitude, especially in an injured digits. So the minimum force needed to load the tendon will be a combination of the frictional force and the force needed to move the joints and soft tissues. The energy needed to flex the digit is often called the “work of flexion”.69,77,78
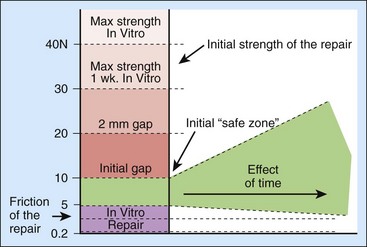
One might imagine that the maximum load that could be applied is the load that represents the breaking strength of the tendon, but that would be incorrect: long before the tendon breaks, it begins to gap, and gapping also increases friction, setting up a vicious circle that can lead to later rupture. So, really, the upper bound is not breaking strength but the force needed to create a gap, which is usually much less.50,62,79–87 The difference between the two forces—the force to initiate unloaded digital flexion and the gapping force—represents the “safe zone” in which rehabilitation can occur88 (Figure 2-4). Early on, this safe zone will be bounded by strictly mechanical parameters, related to the anatomy and biomechanics of the repair. Over time, though, the effects of tendon healing are added in; the general effect is usually to gradually widen the safe zone, enabling the rational use of a graded resistance program as outlined by Groth.51 The details of such programs are reviewed in Chapter 38.
Pharmacological Manipulation of Tendon Healing
Various pharmacological agents have been used in the past in an attempt to modify adhesion formation. Steroids, antihistamines, and beta-aminoproprionitrile have not been shown to clinically decrease scar formation.89,90 Ibuprofen and indomethacin have been found to have a small beneficial effect.91
The ideal pharmacological agent should have no systemic side effects, should be limited to a single application, and should be directed at growth factor expression and ECM production. 5-Fluorouracil (5-FU) may be such a drug. 5-FU is an antimetabolite that is used not only as a cancer chemotherapeutic agent but also to prevent adhesions in glaucoma filtration surgery. The exposure of a surgical field to 5-FU produces a focal inhibition of scarring. Blumenkranz et al92,93
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree