Structure of the Skin
Kenneth R. Watson DO
The skin is the largest organ of the human body. It is composed of tissue that grows, differentiates, and renews itself constantly. Because the skin is a barrier between the internal organs and the external environment, it is uniquely subjected to noxious external agents and is also a sensitive reflection of internal disease. An understanding of the cause and effect of this complex interplay in the skin begins with knowledge of the basic structure of this organ.
Layers of the Skin
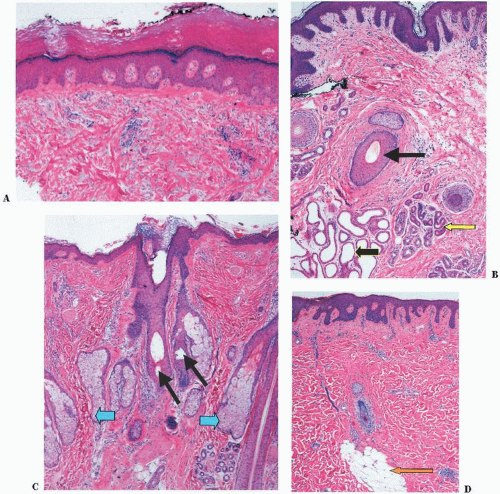
The skin is divided into three distinct layers. From the external surface inward, they are the epidermis, dermis, and subcutaneous tissue (Fig. 1-1). There are regional variations of these layers that probably represent adaptations to different functions, such as:
1. a thickened keratin layer of the epidermis on the palms and soles,
2. numerous nerve fibers within the fingertips for improved tactile function,
3. increased numbers of sebaceous glands on the face, and
4. thickened dermis on the back
Epidermis
The epidermis is the most superficial of the three layers of the skin and averages in thickness about the width of the mark of a sharp pencil (<1 mm). It contains several types of cells including keratinocytes, dendritic cells (melanocytes and Langerhans cells), and Merkel cells.
The keratinocytes, or keratin-forming cells, are by far the most common and develop into four identifiable layers of the epidermis (Fig. 1-2). From inside out, they are as follows:
Basal layer | } | Living epidermis |
Spinous layer | ||
Granular layer | ||
Keratin layer | Dead end-product |
The basal layer lies next to the dermis. This layer can be thought of as the stem cell layer of the epidermis, which is capable of progressive maturation into cell forms higher in the epidermis. It normally requires 3 or 4 weeks for the epidermis to replicate itself by the processes of division and differentiation. This cell turnover is greatly accelerated in diseases such as psoriasis in which the turnover rate may be as short as 2 to 3 days.
The spinous layer, or stratum malpighii, is made up of several layers of epidermal cells, which have a polyhedral shape. The cells of this layer are connected by intercellular bridges, which may be seen in routine sections.
The granular layer is composed of flatter cells containing protein granules called keratohyalin granules. In lichen planus, the granular cell layer is focally increased.
The outermost layer of the epidermis is the keratin (cornified) layer. It is made up of stratified layers of dead keratinized cells that are constantly shedding (Fig. 1-3). The protein in these cells is called keratin and is capable of absorbing vast amounts of water. This is readily seen during bathing, when the skin of the palms and the soles becomes white, swollen, and wrinkled. The keratin layer provides a major barrier of protection for the body. Mucous membranes, such as the oral and vaginal mucosa, do not have granular or keratin layers.
Immediately beneath the basal layer is the interface between the epidermis and the dermis known as the basement membrane zone or dermal-epidermal junction. It is difficult to visualize in routine hematoxylin and eosin stained sections but can clearly be seen as a thin band with periodic acid schiff (PAS) stains, due to the presence of mucopolysaccharides. Ultrastructurally, the basal cells are attached to the basement membrane by hemidesmosomes. Beneath the basal cells is an electron-clear layer known as the lamina lucida. Below this is a more electron-dense layer known as the lamina densa, which consists predominantly of type IV collagen. Anchoring filaments extend through the basement membrane zone to connect the surface membranes of the basal cells to the lamina densa. There are anchoring fibrils that attach the lamina densa to the papillary dermis.
Several blistering diseases occur due to defects in the basement membrane zone. Bullous pemphigoid antigens are present within the hemidesmosomes of the basement membrane zone. Circulating IgG antibodies bind to these antigens, resulting in the subepidermal blistering disease bullous pemphigoid. Epidermolysis bullosa represents a heterogeneous group of noninflammatory blistering disorders that can be divided into three subtypes based on the location of the blister. In epidermolysis bullosa simplex, the blister usually occurs through the basal cell layer. In the junctional
form, it occurs between the basal cells and the lamina lucida, probably due to a defect in the hemidesmosomes. In the dermolytic form, the blister occurs beneath the lamina densa in the area of the anchoring fibrils.
form, it occurs between the basal cells and the lamina lucida, probably due to a defect in the hemidesmosomes. In the dermolytic form, the blister occurs beneath the lamina densa in the area of the anchoring fibrils.
The melanin-forming cells, or melanocytes, are sandwiched between the more numerous keratin-forming cells in the basal layer. In routine hematoxylin and eosin stained sections, melanocytes have small, dark nuclei and clear cytoplasm, which is the result of shrinkage artifact. Approximately 10% of the cells in the basal layer are melanocytes. However, this varies depending on the body site and ethnic background of the individual. These melanocytes are dopa-positive because they stain darkly after contact with a solution of levorotatory 3,4-dihydroxyphenylalanine, or dopa. This laboratory reaction closely simulates physiologic melanin formation, in which the amino acid tyrosine is oxidized by the enzyme tyrosinase to form dopa. Dopa is then further changed, through a series of complex metabolic processes, to melanin. In
dermatopathology practices, melanocytes are most commonly recognized by showing positive immunoreactivity for S-100 protein, HMB-45, and Melan-A (MART-1), which may be useful in the diagnosis of melanocytic tumors such as malignant melanoma. Melanocytes may also be recognized using silver stains due to the fact that melanin is both argyrophilic and argentaffin. For example, the Fontana-Masson histochemical stain results in black cytoplasmic granules within melanocytes because of the ability of melanin to reduce ammoniated silver nitrate. Melanin may also be bleached, which is useful in identifying the neoplastic melanocytes that are obscured in heavily pigmented tumors.
dermatopathology practices, melanocytes are most commonly recognized by showing positive immunoreactivity for S-100 protein, HMB-45, and Melan-A (MART-1), which may be useful in the diagnosis of melanocytic tumors such as malignant melanoma. Melanocytes may also be recognized using silver stains due to the fact that melanin is both argyrophilic and argentaffin. For example, the Fontana-Masson histochemical stain results in black cytoplasmic granules within melanocytes because of the ability of melanin to reduce ammoniated silver nitrate. Melanin may also be bleached, which is useful in identifying the neoplastic melanocytes that are obscured in heavily pigmented tumors.
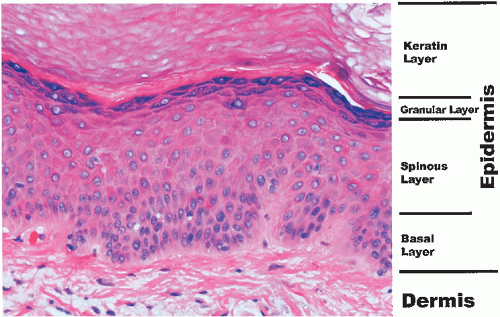 FIGURE 1-2 ▪ Histology of the epidermis. A photomicrograph from the palm. (Courtesy of Dr. K. Watson.) |
Melanin pigmentation of the skin, whether increased or decreased, is influenced by many local and systemic factors (see Chapter 30). Melanocyte-stimulating hormone from the pituitary is the most potent melanizing agent. Melanin is transferred from melanocytes to basal keratinocytes. Skin color is largely related to the amount of melanin present in basal cells. Exposure to ultraviolet light results in increased melanocyte concentration and function.
Langerhans cells are found scattered evenly throughout the epidermis. They are bone marrow-derived mononuclear cells. They are involved in cell-mediated hypersensitivity, antigen processing and recognition, stimulation of immunecompetent cells, and graft rejection. Sunlight suppresses their immune function. Their number is decreased in certain skin diseases, such as psoriasis. Staining with membrane adenosine triphosphatase and monoclonal antibodies such as S-100 protein and CD-1 a can be done for identification. Electron microscopy reveals that these cells contain characteristic racquet-shaped Birbeck granules. These cells proliferate in the disease Langerhans cell histiocytosis (formerly known as histiocytosis X), which may be isolated to the skin or may be part of a larger systemic process.
Merkel cells are located within the basal layer but may also be found within hair follicles and sweat ducts. They are assumed to function as touch receptors and are associated with fine unmyelinated nerve fibers. They are inconspicuous in routine sections. They may be recognized using immunostains for the neuroendocrine markers neuron-specific enolase, chromogranin, and synaptophysin. Ultrastructurally, they contain dense core neurosecretory granules. They give rise to primary neuroendocrine carcinoma of the skin (Merkel cell carcinoma).
Dermis
The dermis consists of connective tissue, cellular elements, and ground substance. It has a rich vascular and nerve supply and contains pilosebaceous, apocrine, and eccrine structures. Anatomically, it is divided into two compartments. The first contains thin collagen fibers, delicate elastic fibers, numerous capillaries, and abundant ground substance, which form a thin layer beneath the epidermis (papillary dermis) and surrounding adnexal structures (periadnexal dermis). Together, these are regarded as a single unit called the adventitial dermis. This is an important unit because it is altered together with the adjacent epithelium in many inflammatory diseases. The second compartment, known as the reticular or deep dermis, is composed of thick collagen bundles with intertwined elastic fibers. The reticular dermis is thick and comprises the bulk of the dermis. It contains less ground substance, vascular spaces, and cellular elements than the thin adventitial dermis.
The connective tissue component of the dermis consists of collagen fibers, including reticulin fibers, and elastic fibers. These fibers contribute to the support and elasticity of the skin.
Two different types of collagen are present within the dermis. Type I collagen is predominantly found within the thick fibers of the reticular dermis. Type III collagen, also known as reticulin,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree