Hip reimplantation refers to the insertion of another prosthesis after removal of the original, infected prosthesis. This may be performed as a single- or two-staged procedure, using either cemented or uncemented components.
ANATOMY
 Hip reimplantation surgery is most commonly performed via the posterolateral or direct lateral (transgluteal) approaches. Either approach may be combined with a trochanteric osteotomy to provide further exposure.
Hip reimplantation surgery is most commonly performed via the posterolateral or direct lateral (transgluteal) approaches. Either approach may be combined with a trochanteric osteotomy to provide further exposure.
 The sciatic nerve is at risk during the posterolateral approach to the hip. In patients with severe scarring, it may be necessary to formally expose the nerve before proceeding with the implantation itself. The sciatic nerve typically emerges deep and inferior to the piriformis muscle and then passes superficial to the obturator internus muscle. However, the sciatic nerve is prone to significant variation in its course and, in some patients, may emerge proximal to, or even emerging through, the piriformis muscle belly.
The sciatic nerve is at risk during the posterolateral approach to the hip. In patients with severe scarring, it may be necessary to formally expose the nerve before proceeding with the implantation itself. The sciatic nerve typically emerges deep and inferior to the piriformis muscle and then passes superficial to the obturator internus muscle. However, the sciatic nerve is prone to significant variation in its course and, in some patients, may emerge proximal to, or even emerging through, the piriformis muscle belly.
 In a direct lateral approach, the function of the abductors may be compromised if sufficient care is not taken to avoid injury to the superior gluteal nerve, located on average 5 cm proximal to the greater trochanter.
In a direct lateral approach, the function of the abductors may be compromised if sufficient care is not taken to avoid injury to the superior gluteal nerve, located on average 5 cm proximal to the greater trochanter.
 For acetabular reconstruction, screw fixation is often necessary. The safest zone for the insertion of acetabular screws, so as to avoid neurovascular structures, is the posterior superior quadrant.18
For acetabular reconstruction, screw fixation is often necessary. The safest zone for the insertion of acetabular screws, so as to avoid neurovascular structures, is the posterior superior quadrant.18
PATHOGENESIS
 The prevalence of infection is 0.7% to 2% following primary hip arthroplasty and 3% to 4% for revisions.1,10
The prevalence of infection is 0.7% to 2% following primary hip arthroplasty and 3% to 4% for revisions.1,10
 The organisms most commonly isolated in infected total hip replacements are Staphylococcus aureus, S. epidermidis, and gram-negative bacteria, with an increasing prevalence of antibiotic-resistant bacteria.2,19
The organisms most commonly isolated in infected total hip replacements are Staphylococcus aureus, S. epidermidis, and gram-negative bacteria, with an increasing prevalence of antibiotic-resistant bacteria.2,19
 Infected prostheses may be classified as one of the following subtypes:17
Infected prostheses may be classified as one of the following subtypes:17
Type I—positive intraoperative cultures in an otherwise grossly normal joint
Type II—early postoperative infection (occurring within 4 weeks of the primary surgery)
Type III—acute hematogenous (occurring in a patient with otherwise well-functioning joint with <4 weeks of symptoms)
Type IV—late chronic infections (>4 weeks of symptoms)
 Patients with unexpected positive intraoperative cultures may initially be treated with antibiotics alone. Types II and III infections can frequently be successfully treated with débridement of the joint, liner exchange, and retention of the implant. Type IV (ie, chronic) infections generally indicate removal of all implants and complete revision.
Patients with unexpected positive intraoperative cultures may initially be treated with antibiotics alone. Types II and III infections can frequently be successfully treated with débridement of the joint, liner exchange, and retention of the implant. Type IV (ie, chronic) infections generally indicate removal of all implants and complete revision.
 This difference in management, based on the chronicity of the infection, is due to the predictable establishment of a bacterial biofilm on the implants, which may prohibit effective clearance of the infection without prosthesis removal. The development of biofilms occurs to a degree that varies depending on the particular bacterial species involved. The surgical management described in the following text is concerned with cases in which prosthesis revision is required.
This difference in management, based on the chronicity of the infection, is due to the predictable establishment of a bacterial biofilm on the implants, which may prohibit effective clearance of the infection without prosthesis removal. The development of biofilms occurs to a degree that varies depending on the particular bacterial species involved. The surgical management described in the following text is concerned with cases in which prosthesis revision is required.
PATIENT HISTORY AND PHYSICAL FINDINGS
 The main presenting symptom of patients with a periprosthetic infection is pain, which is often present at rest.
The main presenting symptom of patients with a periprosthetic infection is pain, which is often present at rest.
 Delayed wound healing, persistent wound drainage, and a history of superficial wound infection after the primary procedure are highly suggestive of infection.
Delayed wound healing, persistent wound drainage, and a history of superficial wound infection after the primary procedure are highly suggestive of infection.
 Risk factors for infection include a history of diabetes mellitus, chronic skin lesions, the use of corticosteroids, any type of immunocompromisation, and the duration of the primary surgery.1
Risk factors for infection include a history of diabetes mellitus, chronic skin lesions, the use of corticosteroids, any type of immunocompromisation, and the duration of the primary surgery.1
 Initial assessment should begin with a general hip examination.
Initial assessment should begin with a general hip examination.
 The hip wound is examined for warmth, erythema, fluctuance, discharging sinuses, and the presence of any hematoma.
The hip wound is examined for warmth, erythema, fluctuance, discharging sinuses, and the presence of any hematoma.
 The abductors are palpated and their function assessed.
The abductors are palpated and their function assessed.
 Pulses are palpated and a full neurologic examination is performed with particular attention to the function of the sciatic nerve.
Pulses are palpated and a full neurologic examination is performed with particular attention to the function of the sciatic nerve.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Infection is monitored by serial assessment of the erythrocyte sedimentation rate (ESR) (normal <30 mm per hour) and C-reactive protein (CRP) (normal <10 mg/L). A normal CRP has a negative predictive value for infection of over 95%.16
Infection is monitored by serial assessment of the erythrocyte sedimentation rate (ESR) (normal <30 mm per hour) and C-reactive protein (CRP) (normal <10 mg/L). A normal CRP has a negative predictive value for infection of over 95%.16
 ESR and CRP may be elevated if the patient has other inflammatory systemic diseases (eg, rheumatic disease) and these tests therefore may not be completely reliable in such cases.
ESR and CRP may be elevated if the patient has other inflammatory systemic diseases (eg, rheumatic disease) and these tests therefore may not be completely reliable in such cases.
 A hip aspiration with a white cell count of greater than 3000 cells/μL and more than 80% neutrophils is highly predictive for infection. Gram stain has a very low sensitivity for infection.5
A hip aspiration with a white cell count of greater than 3000 cells/μL and more than 80% neutrophils is highly predictive for infection. Gram stain has a very low sensitivity for infection.5
 Three samples are obtained at the time of hip aspiration. A positive culture result is considered to be one in which growth is obtained in two separate specimens.
Three samples are obtained at the time of hip aspiration. A positive culture result is considered to be one in which growth is obtained in two separate specimens.
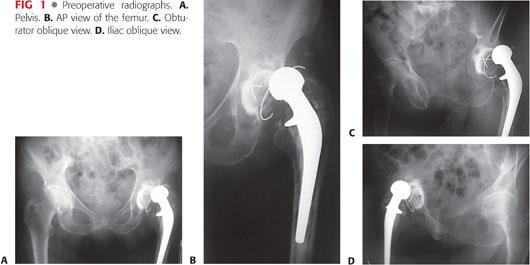
 Radiographs are obtained, including an anteroposterior (AP) view of the pelvis, lateral view of the hip, and Judet views, if necessary, to assess the integrity of the acetabular columns (FIG 1). In some cases, AP and lateral views of the full length of the femur may be necessary. Bone defects should be estimated on plain radiographs and appropriate reconstructive prostheses made available.
Radiographs are obtained, including an anteroposterior (AP) view of the pelvis, lateral view of the hip, and Judet views, if necessary, to assess the integrity of the acetabular columns (FIG 1). In some cases, AP and lateral views of the full length of the femur may be necessary. Bone defects should be estimated on plain radiographs and appropriate reconstructive prostheses made available.
 Computed tomography scans are helpful to ascertain the magnitude of acetabular bone defects.
Computed tomography scans are helpful to ascertain the magnitude of acetabular bone defects.
DIFFERENTIAL DIAGNOSIS
 Adverse reactions to metal ions can manifest with symptoms and signs similar to those of an infected prosthesis, although these cases can typically be differentiated on the basis of serologic testing, imaging studies, and joint aspiration.
Adverse reactions to metal ions can manifest with symptoms and signs similar to those of an infected prosthesis, although these cases can typically be differentiated on the basis of serologic testing, imaging studies, and joint aspiration.
NONOPERATIVE MANAGEMENT
 For some patients with significant comorbidities or previous multiple failed reimplantations, further surgery may not be a viable option. In such cases, the decision may be made to leave the infected prosthesis in situ and to use long-term suppressive antibiotic therapy.
For some patients with significant comorbidities or previous multiple failed reimplantations, further surgery may not be a viable option. In such cases, the decision may be made to leave the infected prosthesis in situ and to use long-term suppressive antibiotic therapy.
SURGICAL MANAGEMENT
 The aims of surgical treatment are to eradicate infection, minimize morbidity, and restore function.
The aims of surgical treatment are to eradicate infection, minimize morbidity, and restore function.
 Prosthetic reimplantation may be performed as a single operation, immediately following the removal of an infected implant, or as a two-stage procedure, weeks or months after removal of the initial infected components. In either case, it is essential to ensure a sterile surgical field prior to hip reimplantation.
Prosthetic reimplantation may be performed as a single operation, immediately following the removal of an infected implant, or as a two-stage procedure, weeks or months after removal of the initial infected components. In either case, it is essential to ensure a sterile surgical field prior to hip reimplantation.
 In a one-stage procedure, at least one of the implants, typically the femoral component, needs to be inserted with antibiotic-loaded bone cement.
In a one-stage procedure, at least one of the implants, typically the femoral component, needs to be inserted with antibiotic-loaded bone cement.
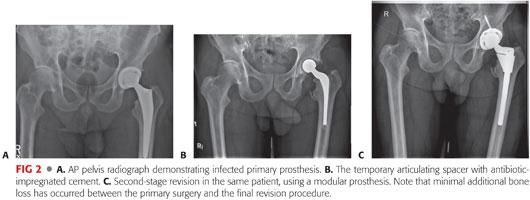
 Many surgeons favor a two-stage approach to hip reimplantation surgery, typically with an antibiotic-loaded spacer in situ, between the two procedures. An articulating spacer can be used both as a vehicle to deliver high-dose local antibiotics and as a means of preserving the articular space while preserving overall limb function ahead of the definitive reimplantation (FIG 2).
Many surgeons favor a two-stage approach to hip reimplantation surgery, typically with an antibiotic-loaded spacer in situ, between the two procedures. An articulating spacer can be used both as a vehicle to deliver high-dose local antibiotics and as a means of preserving the articular space while preserving overall limb function ahead of the definitive reimplantation (FIG 2).
 In two-stage procedures, the definitive hip reimplantation surgery can be scheduled once the patient has completed their antibiotic course and demonstrated a trend toward normalization of their inflammatory markers. A routine preoperative hip aspiration is not required in most cases.
In two-stage procedures, the definitive hip reimplantation surgery can be scheduled once the patient has completed their antibiotic course and demonstrated a trend toward normalization of their inflammatory markers. A routine preoperative hip aspiration is not required in most cases.
 The selection of prostheses for both femoral and acetabular reconstruction is determined by a number of factors, including the quality and quantity of remaining host bone for osseointegration or cementation, the status of the surrounding soft tissues, and the surgeon’s preference.
The selection of prostheses for both femoral and acetabular reconstruction is determined by a number of factors, including the quality and quantity of remaining host bone for osseointegration or cementation, the status of the surrounding soft tissues, and the surgeon’s preference.
 In some low-demand patients and those with a history of previous failed reimplantation surgery, a resection arthroplasty may be considered.
In some low-demand patients and those with a history of previous failed reimplantation surgery, a resection arthroplasty may be considered.
Preoperative Planning
 It is important to anticipate the need for specialized implants and instruments before surgery, taking note of factors such as offset, leg length inequality, bone stock, and joint stability.
It is important to anticipate the need for specialized implants and instruments before surgery, taking note of factors such as offset, leg length inequality, bone stock, and joint stability.
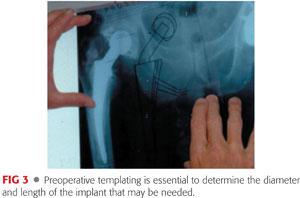
 Careful preoperative templating is essential to anticipate implant size, length, and offset (FIG 3).
Careful preoperative templating is essential to anticipate implant size, length, and offset (FIG 3).
 Insufficiency of hip abductors may require a constrained acetabular implant, larger diameter femoral head components, or a dual mobility cup.
Insufficiency of hip abductors may require a constrained acetabular implant, larger diameter femoral head components, or a dual mobility cup.
 The pathology laboratory should be informed of the possibility that intraoperative frozen section analysis may be required in equivocal cases of persistent infection. These tissue samples are taken at the soft tissue interface adjacent to the removed spacer implant. In addition, if intraoperative cultures are obtained, the microbiology laboratory should be alerted for the need for a prolonged incubation of 14 days.
The pathology laboratory should be informed of the possibility that intraoperative frozen section analysis may be required in equivocal cases of persistent infection. These tissue samples are taken at the soft tissue interface adjacent to the removed spacer implant. In addition, if intraoperative cultures are obtained, the microbiology laboratory should be alerted for the need for a prolonged incubation of 14 days.
 Alternative surgical plans are useful to have at hand in case of unexpected intraoperative findings or complications, which may include the need to reinsert an antibiotic spacer prosthesis if there is evidence of ongoing infection.
Alternative surgical plans are useful to have at hand in case of unexpected intraoperative findings or complications, which may include the need to reinsert an antibiotic spacer prosthesis if there is evidence of ongoing infection.
Positioning
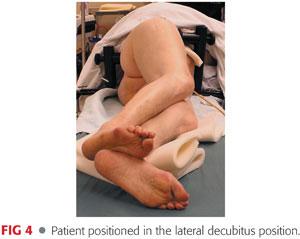
 The patient is positioned in the lateral decubitus position with anterior and posterior supports (FIG 4).
The patient is positioned in the lateral decubitus position with anterior and posterior supports (FIG 4).
 The pelvis must be vertical, and it must be confirmed that the supports are stable. The patient’s back is kept straight with their shoulders vertical.
The pelvis must be vertical, and it must be confirmed that the supports are stable. The patient’s back is kept straight with their shoulders vertical.
 A clinical note is made of any leg length discrepancy prior to prepping the limb. This allows for intraoperative monitoring of leg length adjustments.
A clinical note is made of any leg length discrepancy prior to prepping the limb. This allows for intraoperative monitoring of leg length adjustments.
 Patient positioning must be performed under surgeon supervision because errors in positioning may result in acetabular component malalignment.
Patient positioning must be performed under surgeon supervision because errors in positioning may result in acetabular component malalignment.
Approach
 The surgical approach is chosen after careful preoperative consideration of important factors, including the following:
The surgical approach is chosen after careful preoperative consideration of important factors, including the following:
Previous approach
Anatomic location and extent of bone loss
Anticipated instability
Function of the abductors
Surgeon preference and training
 The main options are as follows:
The main options are as follows:
Posterolateral approach
Direct lateral (transgluteal) approach
Trochanteric osteotomy
Trochanteric slide osteotomy
Extended trochanteric osteotomy (ETO)
TECHNIQUES
 Hip Exposure and Removal of Antibiotic Spacers
Hip Exposure and Removal of Antibiotic Spacers
The posterior approach is often used for surgical exposure.
The sciatic nerve is identified and protected throughout the procedure. This is facilitated by placement of the foot on a padded stand with the knee in flexion and the hip in slight abduction during the exposure.
Initial visualization of the nerve is often obscured by scar tissue from previous operations and care should be taken to verify the nerve’s precise location.
The short external rotators and posterior capsule are identified and incised as a composite flap. These are tagged with sutures for later repair. The gluteus maximus tendon insertion is released in many cases in order to improve mobilization of the femur.
Tissue samples are obtained from within the hip joint and sent for microbiology. We do not recommend obtaining frozen sections routinely.
The hip is dislocated, with flexion and internal rotation of the femur.
Anterior capsular scar tissue may need to be debulked to improve exposure. Exposure is further aided by releasing the anterior femoral capsule, with a femoral retractor placed to displace the femur anteriorly within the wound (TECH FIG 1A).
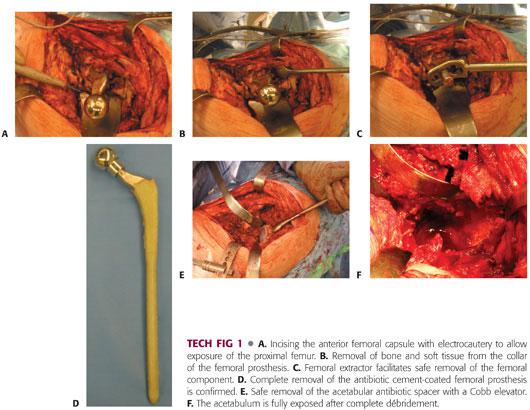
Removal of cement, soft tissue, and bone from the shoulder of the prosthesis and greater trochanter facilitates removal of the preexisting antibiotic implant spacer and reduces the risk of greater trochanter fracture (TECH FIG 1B).
A femoral extractor should be used to remove the femoral antibiotic spacer (TECH FIG 1C), ensuring complete removal of all cement (TECH FIG 1D).
The acetabular antibiotic spacer is carefully removed, with an osteotome to break the cement and a gouge to remove the liner if present. Care is taken to ensure no further bone loss (TECH FIG 1E).
Femoral débridement is performed with reverse hooks, curettes and brushes, and pulsed lavage.
Acetabular débridement is performed using a combination of curettes, rongeurs, and Cobb elevators to remove any residual soft tissue, ensuring complete exposure of the acetabulum (TECH FIG 1F).
The bone stock of the posterior acetabular walls is verified by palpation.
 Acetabular Reimplantation
Acetabular Reimplantation
The acetabulum is reamed incrementally to obtain a concentric, hemispheric surface, taking care to preserve the rim of the acetabulum (TECH FIG 2A).
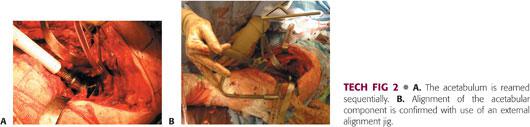
An implant 1 to 2 mm larger than the diameter of the last reamer is used in order to obtain a press-fit.
The implant is inserted in 40 degrees of lateral inclination and 15 to 20 degrees of anteversion (TECH FIG 2B).
Ascertain that the component is uniformly in contact with the underlying host bone.
Supplementary screw fixation is required in most cases, with placement in the posterior superior quadrant of the cup.
The appropriate trial liner is placed into the acetabulum for later trial reduction after femoral canal preparation.
 Two-Stage Reimplantation with an Uncemented, Extensively Porous-Coated Femoral Stem
Two-Stage Reimplantation with an Uncemented, Extensively Porous-Coated Femoral Stem
The diameter, offset, and length of the femoral implant are anticipated by careful preoperative templating.
The femoral canal is sequentially reamed until cortical resistance is encountered over a length of at least 5 to 6 cm (TECH FIG 3A).
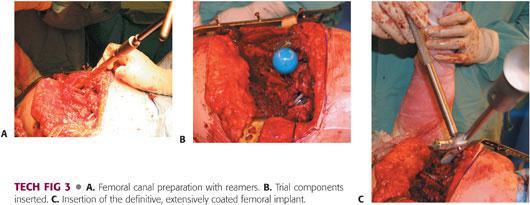
Torsional stability of the implant may be ensured by gently torquing the femoral trial with the broach handle.
Trial reduction is performed, ensuring satisfactory leg lengths, soft tissue tension, range of motion, and a stable hip (TECH FIG 3B).
Under-reaming the femoral canal by 0.5 mm compared with the diameter of the actual femoral implant is confirmed by checking with a “hole gauge.”
In an extensively porous-coated femoral component, 5 to 6 cm of diaphyseal fit (so-called scratch fit) is required to provide axial and rotational stability.
The final implant is inserted into the femoral canal. It should be inserted by hand as close as possible to its final position; otherwise, it should be reamed line to line to avoid inadvertent femoral fracture (TECH FIG 3C).
The final implant seating is achieved by gentle impaction with a mallet.
 Two-Stage Reimplantation with Uncemented, Tapered, Fluted Femoral Stem
Two-Stage Reimplantation with Uncemented, Tapered, Fluted Femoral Stem
Curettes and reverse hooks are used to débride fibrous tissue from the femoral canal (TECH FIG 4A).
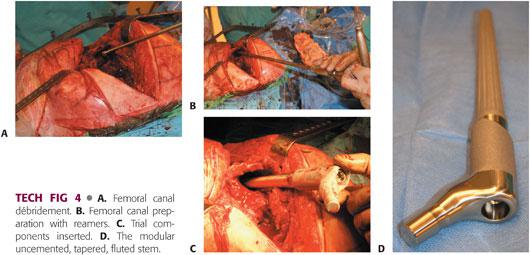
Femoral canal reaming is performed with tapered reamers, with the depth and diameter guided by preoperative templating, until endosteal contact is made (TECH FIG 4B).
The aim of diaphyseal reaming is to ensure implant stability, which will resist stem subsidence.
The length of the stem, as determined by preoperative templating, should be at least two cortical diameters distal to any potential stress risers, for example, the tip of an ETO.
Unlike fully porous-coated cylindrical stems, under-reaming the femoral canal by 0.5 mm compared with the diameter of the actual femoral implant is not recommended. Line-to-line reaming is preferred.
Proximal femoral preparation is then performed using conical reamers.
Torsional stability of the implant may be ensured by gently torquing the femoral trial with the broach handle.
Trial reduction is performed for assessment of correct femoral stem anteversion, limb length, soft tissue tension, range of motion, and hip stability (TECH FIG 4C).
The modularity of the uncemented tapered fluted femoral stem allows independent adjustment of femoral anteversion (TECH FIG 4D).
PEARLS AND PITFALLS | |
Implant removal and acetabular reconstruction |
|
| |
Femoral reimplantation with extensively porous-coated femoral stem |
|
| |
Femoral reimplantation with uncemented, tapered, fluted stem |
|
| |
Cemented reimplantation surgery |
|
| |
POSTOPERATIVE CARE
 Postoperative care is individualized, depending on the precise nature of the reimplantation procedure.
Postoperative care is individualized, depending on the precise nature of the reimplantation procedure.
 The quality of implant fixation, severity of preoperative bone loss, intraoperative stability, and patient compliance influence the amount of weight bearing permitted and the specific restrictions on hip range of movement.
The quality of implant fixation, severity of preoperative bone loss, intraoperative stability, and patient compliance influence the amount of weight bearing permitted and the specific restrictions on hip range of movement.
 If a transgluteal (direct lateral) approach was used, restriction of active abduction may be necessary.
If a transgluteal (direct lateral) approach was used, restriction of active abduction may be necessary.
 Clear postoperative instructions and frequent communication with the multidisciplinary team are essential. Instructions include postoperative blood work, deep venous thrombosis prophylaxis, and perioperative antibiotic requirements.
Clear postoperative instructions and frequent communication with the multidisciplinary team are essential. Instructions include postoperative blood work, deep venous thrombosis prophylaxis, and perioperative antibiotic requirements.
 All intraoperative specimens sent for microbiology at the time of reimplantation must be carefully monitored for any positive culture growths.
All intraoperative specimens sent for microbiology at the time of reimplantation must be carefully monitored for any positive culture growths.
OUTCOMES
 An uncemented two-stage procedure may successfully eradicate infection in 89% to 93% of cases.2,7,8,11
An uncemented two-stage procedure may successfully eradicate infection in 89% to 93% of cases.2,7,8,11
 Single-stage reimplantation with the use of antibiotic-loaded cement has a success rate of 77% to 91%.3,4,6,15
Single-stage reimplantation with the use of antibiotic-loaded cement has a success rate of 77% to 91%.3,4,6,15
 A two-stage procedure with the use of antibiotic-containing bone cement in the reimplantation procedure attains a success rate of 90% to 95%.9,13
A two-stage procedure with the use of antibiotic-containing bone cement in the reimplantation procedure attains a success rate of 90% to 95%.9,13
COMPLICATIONS
 Recurrent infection after reimplantation is a devastating complication and is associated with a poor outcome.14
Recurrent infection after reimplantation is a devastating complication and is associated with a poor outcome.14
 Infection with a methicillin-resistant organism is associated with a higher rate of treatment failure.12
Infection with a methicillin-resistant organism is associated with a higher rate of treatment failure.12
 Recurrent infection may be either recurrence of the initial infection or a new infection by a different organism, which is often due to multiple patient risk factors and indicates host failure.11
Recurrent infection may be either recurrence of the initial infection or a new infection by a different organism, which is often due to multiple patient risk factors and indicates host failure.11
 Hip dislocation, leg length discrepancy, venous thromboembolism, nerve and vessel injury, fracture, and a small mortality risk are potential complications, as they are for any revision arthroplasty.
Hip dislocation, leg length discrepancy, venous thromboembolism, nerve and vessel injury, fracture, and a small mortality risk are potential complications, as they are for any revision arthroplasty.
REFERENCES
1. Aggarwal VK, Tischler EH, Lautenbach C, et al. Mitigation and education. J Arthroplasty 2014;29(2 suppl):19–25.
2. Biring GS, Kostamo T, Garbusz DS, et al. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer. J Bone Joint Surg Br 2009;91B:1431–1437.
3. Buchholz HW, Elson RA, Engelbrecht E, et al. Management of deep infection of total hip replacement. J Bone Joint Surg Br 1981;63B:342–353.
4. Callaghan JJ, Katz PR, Johnston RC. One-stage revision surgery of the infected hip: a minimum 10-year follow-up study. Clin Orthop Relat Res 1999;369:139–143.
5. Dinnenn A, Guyot A, Clements J, et al. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J 2013;95-B:554–557.
6. Elson RA. One-stage exchange in the treatment of the infected total hip arthroplasty. Semin Arthroplasty 1994;5:137–141.
7. Faddad FS, Muirhead-Allwood SK, Manktelow AR, et al. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br 2000;82B:689–694.
8. Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty 1999;14:175–181.
9. Garvin KL, Evans BG, Salvati EA, et al. Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop Relat Res 1994;298:97–105.
10. Garvin KL, Hanssen AD. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am 1995;77:1576–1588.
11. Kraay MJ, Goldberg V, Fitzgerald SJ, et al. Cementless two-staged total hip replacement for deep periprosthetic infection. Clin Orthop Relat Res 2005;441;243–249.
12. Leung F, Richards CJ, Garbuz DS, et al. Two-stage total hip arthroplasty: how often does it control methicillin-resistant infection? Clin Orthop Relat Res 2011;469:1009–1015.
13. Lieberman JR, Callaway GH, Salvati EA, et al. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res 1994;301:205–212.
14. Pagnano MW, Trousdale RT, Hanssen AD. Outcome after reinfection following reimplantation hip arthroplasty. Clin Orthop Relat Res 1997;338:192–204.
15. Raut VV, Siney PD, Wroblewski BM. One-stage revision of total hip arthroplasty for deep infection: long-term follow-up. Clin Orthop Relat Res 1995;321:202–207.
16. Spangehl MJ, Masri BA, O’Connell JX, et al. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am 1999;81-A:672–683.
17. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996;78(4):512–523.
18. Wasielewski RC, Cooperstien LA, Kruger MP, et al. Acetabular anatomy and the transacetabular fixation of screws in total hip arthroplasty. J Bone Joint Surg Am 1990;72A:501–508.
19. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 2012;65:158–168.
< div class='tao-gold-member'>













