Abstract
The specific dermatoses of pregnancy represent a heterogeneous group of inflammatory skin diseases and include pemphigoid gestationis (PG), polymorphic eruption of pregnancy (PEP), intrahepatic cholestasis of pregnancy (ICP), and atopic eruption of pregnancy (AEP). Pruritus is the primary symptom and should be taken seriously in a pregnant woman. Clinical characteristics, in particular the morphology and distribution of lesions as well as the timing of their appearance relative to the stage of gestation, are crucial for diagnosis. The diagnoses of PG and ICP are confirmed via specific immunofluorescence and laboratory findings, respectively. PEP and AEP are distressing only to the mother because of associated pruritus, whereas PG may be associated with prematurity and small-for-gestational age neonates and ICP poses an increased risk for fetal distress, prematurity, and stillbirth. Corticosteroids and antihistamines control PG, PEP and AEP, whereas ICP is best treated with ursodeoxycholic acid.
Keywords
pregnancy dermatosis, pruritic inflammatory dermatoses of pregnancy, pregnancy, postpartum flare, pemphigoid gestationis, polymorphic eruption of pregnancy, intrahepatic cholestasis of pregnancy, atopic eruption of pregnancy, corticosteroids in pregnancy, antihistamines in pregnancy, ursodeoxycholic acid.
For decades, the specific dermatoses of pregnancy represented a confusing group of overlapping entities, understood largely through anecdotes and case reports. However, more recent reviews have simplified and condensed the list of pregnancy-specific dermatoses ( Table 27.1 ) . For example, impetigo herpetiformis is now generally recognized as pustular psoriasis, perhaps induced by the relative hypocalcemia of pregnancy (see Ch. 8 ). Also, prurigo annularis, first reported in 1941 (without histology or laboratory information), and linear IgM disease of pregnancy (described in 1988) have not been subsequently reported, yet are frequently perpetuated in reviews.
| CLASSIFICATION OF THE DERMATOSES OF PREGNANCY | |
|---|---|
| Classification | Synonym(s) |
| Pemphigoid gestationis * | Herpes gestationis † Gestational pemphigoid |
| Polymorphic eruption of pregnancy (PEP) | Pruritic urticarial papules and plaques of pregnancy (PUPPP) † Toxic erythema of pregnancy Late-onset prurigo of pregnancy Toxemic rash of pregnancy |
| Intrahepatic cholestasis of pregnancy (ICP) | Cholestasis of pregnancy † Obstetric cholestasis Cholestatic jaundice of pregnancy Pruritus/prurigo gravidarum |
| Atopic eruption of pregnancy (AEP) | Prurigo of pregnancy * , † Prurigo gestationis (Besnier) Early-onset prurigo of pregnancy (Nurse) Papular dermatitis of pregnancy (Spangler) Pruritic folliculitis of pregnancy * Linear IgM disease of pregnancy Eczema in pregnancy |
* Former classification by Holmes & Black (1983) .
Much of the confusion can be dispelled by reviewing the original articles: Besnier (1904) first used the term “prurigo gestationis” to include all patients with pregnancy-related dermatoses, other than those with pemphigoid gestationis. Costello (1941) subsequently referred to these patients as “prurigo gestationis of Besnier” and estimated an incidence of 2% of all pregnancies. Bourne (1962) characterized a subset of patients with intensely pruritic papules or urticarial plaques that tended to appear during the later part of the third trimester, coining the term “toxaemic rash of pregnancy”. Initial lesions typically developed within the abdominal striae of short women who experienced excessive weight gain during pregnancy. However, he offered no histopathologic or laboratory details.
Spangler et al. (also 1962) reported on a series of women with intensely pruritic, widely scattered, excoriated papules. Initial onset was during the second or third trimester and all patients suffered recurrences during subsequent gestations. The hallmarks of Spangler’s cases were biochemical: elevated urinary human chorionic gonadotropin (HCG), decreased plasma hydrocortisone, and decreased serum half-life of hydrocortisone. Liver function tests and histopathology were not reported and immunofluorescence (IF) was not yet available. Most notably, Spangler’s papular dermatitis was associated with high fetal wastage, a finding now thoroughly discredited.
Nurse (1968) cited all of the above literature but ignored it, dividing patients with (non-pemphigoid gestationis) pregnancy eruptions into “early” and “late” forms. The late papular/urticarial form clearly overlapped with Bourne’s “toxaemic rash of pregnancy”, and has since been described as “pruritic urticarial papules and plaques of pregnancy” (PUPPP), “toxic erythema of pregnancy”, and, most recently, “polymorphic eruption of pregnancy” (PEP). Nurse’s “early-onset” patients and Spangler’s “papular dermatitis” patients were undoubtedly drawn from the same clinical spectrum, and subsequently these entities were reclassified as forms of “prurigo of pregnancy” (see Table 27.1 ).
Pruritic folliculitis of pregnancy was added to the list of pregnancy-specific diseases in 1981, yet all six of the original patients were thought to have papular dermatitis clinically. They were classified as having a different entity based solely upon shared histologic features, that is, sterile folliculitis. In spite of their clinical similarity to the patients reported by Spangler, none had appropriate biochemical investigations. In 1998, an adaptation of the Holmes and Black classification scheme (1983) was published and listed pruritic folliculitis as a variant of prurigo of pregnancy (see Table 27.1 ).
Introduction of the term “atopic eruption of pregnancy (AEP)” represents the most recent revision to the classification of dermatoses of pregnancy . Based upon an analysis of over 500 pregnant women with pruritus , significant overlap, both clinical and histologic, was noted amongst those patients with the diagnoses of prurigo of pregnancy, pruritic folliculitis of pregnancy, and eczema in pregnancy (~50% of the patients in this series) . AEP was thought to be a useful diagnostic term that encompassed these three disorders and reminded the clinician that the eczema was more likely to be of new onset as opposed to a flare of previously diagnosed atopic dermatitis.
Because of the lack of primary lesions, intrahepatic cholestasis of pregnancy (ICP) had long been omitted from the list of pregnancy dermatoses. However, failure to appreciate ICP in a pregnant woman with widespread excoriations or even prurigo nodularis surely, in retrospect, accounts for some of the confusion in terminology. Furthermore, ICP is the most important diagnosis to exclude in a pregnant patient with pruritus, as it is associated with significant fetal risk (see below).
Pemphigoid Gestationis
▪ Gestational pemphigoid ▪ Herpes gestationis
- ▪
Rare, pruritic, vesiculobullous eruption that develops during late pregnancy or the immediate postpartum period
- ▪
Linear C3 deposition along the basement membrane zone (BMZ) by direct IF
- ▪
IgG1 autoantibodies are directed against a transmembrane hemidesmosomal protein (BP180; BPAG2; collagen XVII)
- ▪
Increased risk of prematurity and small-for-gestational age neonates; the risk correlates with disease severity
- ▪
Commonly recurs in subsequent pregnancies
Introduction
Pemphigoid gestationis is a rare, self-limited, autoimmune bullous disease. It is the most clearly characterized dermatosis of pregnancy and the only one that may also affect the skin of the newborn.
History
Milton first coined the term “herpes gestationis” in 1872 and Bulkley (1874) canonized the term “as embodying the clinical characters of the eruption and signifying at the same time the sex and state of the body in which it appears”. Little additional insight was gained until IF techniques revealed complement deposition along the BMZ in 1973, a feature now accepted as essential for the diagnosis of pemphigoid gestationis.
Epidemiology
The incidence of pemphigoid gestationis has been estimated at 1 : 1700–1 : 50 000 pregnancies , correlating with the prevalence of HLA-DR3 and -DR4 in different populations. While occurring primarily during pregnancy and the immediate postpartum period, pemphigoid gestationis has rarely developed in association with trophoblastic tumors (hydatidiform mole, choriocarcinoma). Interestingly, no case of a pemphigoid-like disease has been reported in men with choriocarcinoma, who have a biochemically similar yet entirely syngenic tumor; of note, the nuclear genome in placental tissue (and hence choriocarcinoma in women) is primarily paternal in origin. Patients with a history of pemphigoid gestationis appear to be at increased risk for the development of Graves disease .
Pathogenesis
Historically, pemphigoid gestationis was thought to be caused by an anti-BMZ “serum factor” (the “herpes gestationis [HG] factor”) that induces C3 deposition along the dermal–epidermal junction. This factor is now known to be complement-fixing autoantibodies of the IgG1 subclass directed against a 180 kDa transmembrane hemidesmosomal protein (BP180; BPAG2; collagen XVII). As in patients with bullous pemphigoid (BP), it is the non-collagenous (NC) segment closest to the plasma membrane of the basal keratinocyte, NC16A, that constitutes the immunodominant region of BP180 (see Fig. 31.9 ). Circulating antibodies are almost exclusively directed against this domain, as demonstrated by ELISA and immunoblot studies of maternal or neonatal sera.
What initiates the production of autoantibody remains enigmatic. Because the antibodies also bind to amniotic basement membrane (a structure derived from fetal ectoderm and antigenically similar to skin), attention has focused on immunogenetics and potential cross-reactivity between placental tissue and skin. Immunogenetic studies have revealed a marked increase in HLA antigens DR3 or DR4, and, curiously, nearly 50% of patients have the simultaneous presence of both. There is essentially a 100% incidence of anti-HLA antibodies in patients with a history of pemphigoid gestationis . Since the only source of disparate HLA antigens is typically the placenta (which is primarily of paternal origin), the universal finding of anti-HLA antibodies implies a high frequency of immunologic insult during gestation. Women with pemphigoid gestationis also have increased expression of MHC class II antigens (DR, DP, DQ) within the villous stroma of their chorionic villi. It has therefore been proposed that pemphigoid gestationis is a disease, initiated by the aberrant expression of MHC class II antigens (of paternal haplotype), that serves to initiate an allogeneic response to placental BMZ, which then cross-reacts with skin .
Clinical Features
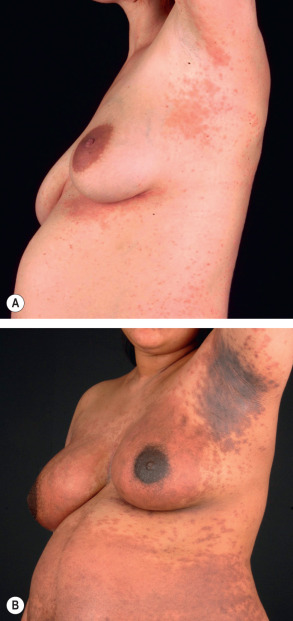
Pemphigoid gestationis may develop during any trimester as well as immediately postpartum, but classically it presents during late pregnancy. There is an abrupt onset of cutaneous lesions on the trunk, in particular the abdomen and often within or immediately adjacent to the umbilicus ( Fig. 27.1 ). Rapid progression to a generalized pemphigoid-like eruption then occurs, with pruritic urticarial papules and plaques, followed by clustered (herpetiform) vesicles or tense bullae on an erythematous base. The eruption may involve the entire body, sparing only the mucous membranes. While the clinical presentation and course may vary considerably, spontaneous improvement during late gestation is common. This is followed, however, by a flare at the time of delivery in 75% of patients. Such flares may be dramatic, with an explosive onset of blistering within hours.
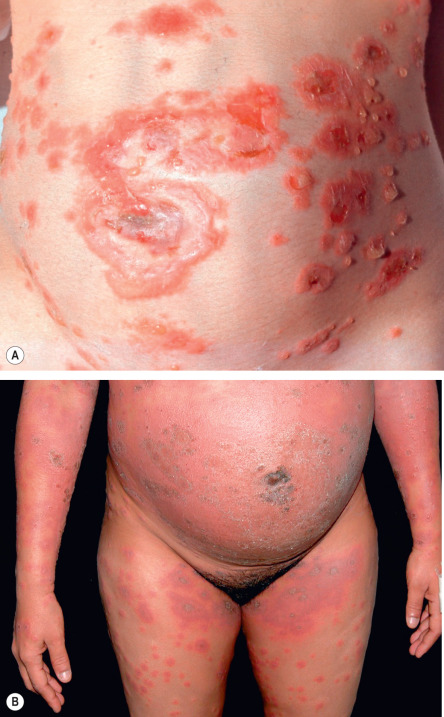
Most disease activity spontaneously remits during the weeks to months following delivery, but there are isolated reports of a protracted course postpartum. Flares and/or recurrences in association with menstruation are common, and in 25–50% of patients, they may also be induced by oral contraceptives ( Table 27.2 ). Pemphigoid gestationis may not develop during the patient’s first pregnancy, but, once established, it is quite likely to recur in subsequent pregnancies, usually with an earlier onset and more severe course. “Skipped” pregnancies have been observed in 5–8% of women.
| DERMATOSES OF PREGNANCY – FETAL RISK, INVOLVEMENT OF NEWBORN SKIN, AND RISK OF RECURRENCE | |||
|---|---|---|---|
| Dermatosis | Fetal risk | Newborn skin involvement | Risk of recurrence |
| Pemphigoid gestationis | Increased risk of prematurity and small-for-gestational age neonates; risk correlates with disease severity | Mild and transient lesions of pemphigoid gestationis in up to 10% | Commonly recurs (“skipped” pregnancies in only 5–8% of women); recurrences induced by oral contraceptives in 25–50% |
| Polymorphic eruption of pregnancy | None | None | Usually does not recur |
| Intrahepatic cholestasis of pregnancy | Increased risk of premature labor (20–60%), intrapartal fetal distress (20–30%), and stillbirths (1–2%) | None | Recurrence in 45–70% of subsequent pregnancies; may be triggered by oral contraceptives |
| Atopic eruption of pregnancy | None | None | Commonly recurs due to atopic diathesis |
Approximately 10% of newborns develop mild skin involvement due to passive transfer of maternal antibodies and this resolves spontaneously within days to weeks (see Ch. 34 ). There seems to be an increased risk of prematurity and small-for-gestational age neonates, presumably due to chronic placental insufficiency. Recently, it was shown that this risk correlates with disease severity, i.e. occurrence of blistering and early onset, and not with the use of systemic corticosteroids .
Pathology
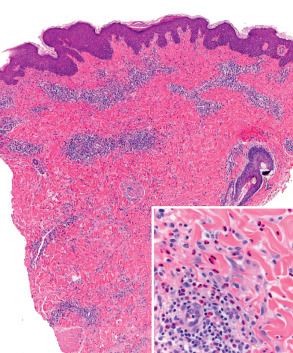
The classic histologic finding of a subepidermal vesicle is seen in the minority of patients. Instead, a nonspecific mixed cellular infiltrate containing a variable number of eosinophils is more common. The presence of eosinophils is the most constant histologic feature of pemphigoid gestationis ( Fig. 27.2 ).
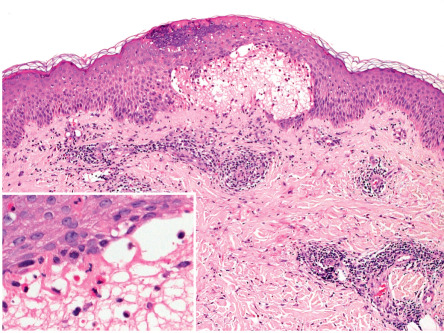
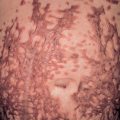
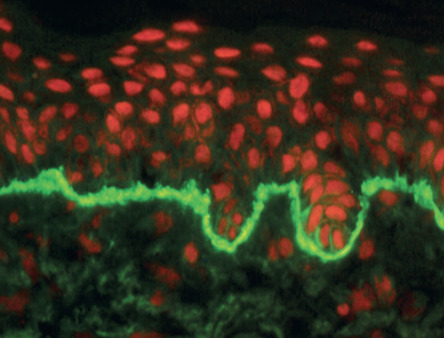
The essential component for the diagnosis of pemphigoid gestationis is a linear deposition of C3 along the BMZ of perilesional skin by direct IF microscopy ( Fig. 27.3 ). This is observed in 100% of patients, and linear IgG deposition is seen in 30% of patients. When salt-split skin specimens are employed for conventional indirect IF, deposition of IgG along the bottom of the epidermal fragment is observed in 30% of patients. However, complement-added indirect IF reveals the circulating anti-BMZ IgG1 autoantibodies in essentially all patients. Determination of antibody titers via BP180-NC16A ELISA may be helpful in following disease activity and monitoring therapy.
An increased incidence of anti-thyroid antibodies has been documented, but clinically apparent thyroid dysfunction is uncommon . Routine laboratory investigations are normal.
Differential Diagnosis
The most frequent considerations in the differential diagnosis are PEP and drug eruptions. PEP is a particularly challenging exclusion, given the difficulty of distinguishing PEP and urticarial lesions of pemphigoid gestationis. IF and, more recently, the BP180-NC16A ELISA are key to the differentiation and are especially relevant in helping the patient plan for future pregnancies.
Treatment
The primary goal in treating this self-limited disease is to relieve pruritus and suppress blister formation. In mild cases, the use of potent topical corticosteroids combined with emollients and systemic antihistamines may be adequate. However, systemic corticosteroids remain the cornerstone of therapy ( Table 27.3 ). Most patients respond to 0.5 mg/kg of prednisolone daily; the dose is tapered as soon as blister formation is suppressed. The common flare associated with delivery usually requires a temporary increase in dosage. Those rare patients with refractory disease may benefit from plasmapheresis during pregnancy. Persistent disease after delivery is uncommon and is treated like BP.
| SPECIAL CONSIDERATIONS FOR CORTICOSTEROID AND ANTIHISTAMINE USE DURING PREGNANCY | |
|---|---|
| Corticosteroids | |
| Topical |
|
| Systemic |
|
| Antihistamines | |
| Systemic |
|
Anecdotal alternatives to corticosteroids (dapsone, doxycycline or minocycline ± nicotinamide, pyridoxine, cyclosporine) or adjuvants (methotrexate, cyclophosphamide, gold, IVIg) have been tried. None of these medications, with the possible exception of cyclosporine, are safe prior to term and thus should be avoided.
Polymorphic Eruption of Pregnancy
▪ Pruritic urticarial papules and plaques of pregnancy (PUPPP) ▪ Bourne’s “toxaemic rash of pregnancy” ▪ Nurse’s “late-onset prurigo” of pregnancy ▪ Toxic erythema of pregnancy
- ▪
Urticarial papules and plaques that usually first appear within striae distensae during the latter portion of the third trimester or immediately postpartum
- ▪
Development of polymorphous features (vesicles, erythema, target, and eczematous lesions) with disease progression
- ▪
Most frequent in primiparous women
- ▪
Nonspecific histologic features, negative IF, and normal routine laboratory evaluation
- ▪
No maternal or fetal risks; usually does not recur
Introduction
Polymorphic eruption of pregnancy (PEP), formerly known as PUPPP, is a common gestational dermatosis. It is characterized by a typical clinical presentation, normal laboratory tests, and negative IF or ELISA.
History
The term PUPPP, introduced by Lawley et al. in 1979, focuses on the initial clinical findings in this disorder but overlooks its later polymorphous features. In order to encompass the entire clinical spectrum, the term “polymorphic eruption of pregnancy” has been introduced and is now generally accepted.
Epidemiology
The incidence is ~1 in 160 deliveries . It is seen predominantly in primiparous women and tends not to recur in subsequent pregnancies. There is neither an autoimmune diathesis nor an association with a specific HLA type.
Pathogenesis
The cause of PEP is unknown. Reference has been made to increased maternal weight gain and an increased frequency of multiple-gestation pregnancies . It has therefore been suggested that rapid, late stretching of abdominal skin may lead to damage of connective tissue and elicitation of an allergic-type reaction, resulting in the initial appearance of the eruption within striae . The lesions then become generalized as the inflammatory response develops cross-reactivity to collagen in otherwise normal-appearing skin. Immune tolerance during subsequent pregnancies might prevent recurrence. Additional theories include increased levels of progesterone in association with multiple gestations and peripheral chimerism (deposition of fetal DNA) that favors skin with increased vascularity and damaged collagen.
Clinical Features
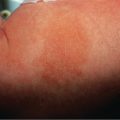
Pruritic erythematous and edematous papules and plaques usually first appear within the abdominal striae, typically with periumbilical sparing ( Fig. 27.4 ). Onset is most often during the latter part of the third trimester (85%) or in the immediate postpartum period (15%) . The eruption typically spreads over a matter of days, but in general spares the face, palms and soles. While pruritic urticarial papules are the initial lesions in almost all patients, approximately half will develop more polymorphic features as the disease evolves, including widespread erythema, target lesions, tiny vesicles, and eczematous plaques ( Fig. 27.5 ). Irrespective of whether the eruption starts during pregnancy or postpartum, lesions resolve over an average of 4 weeks.
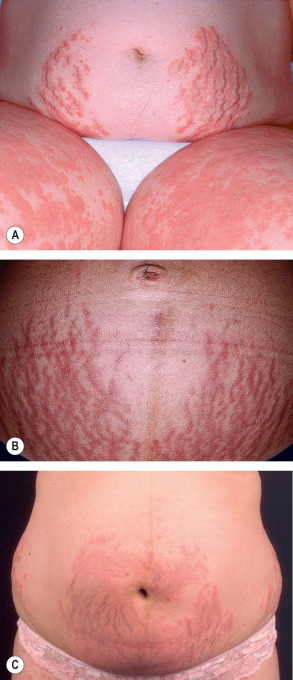
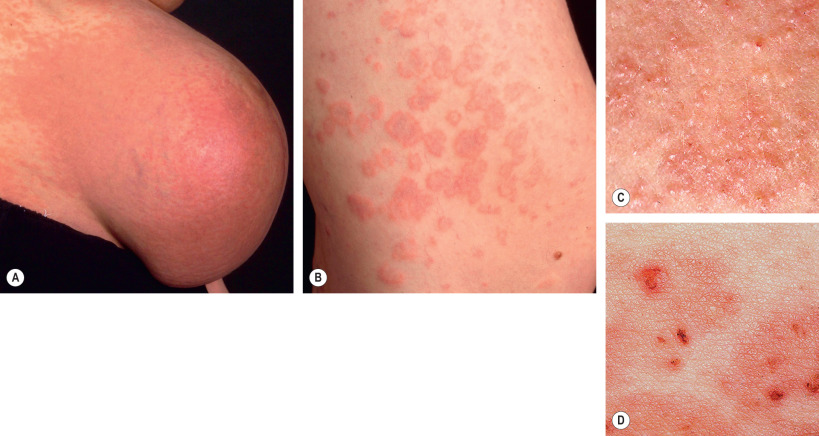
There are no maternal or fetal morbidities, and recurrences are unusual except for subsequent multiple-gestation pregnancies. To date, only a single possible case of newborn skin involvement has been described; however, the possibility of pemphigoid gestationis was not excluded by IF studies . Thus, it is generally agreed that neonatal skin is not affected by PEP.
Pathology
Skin biopsy specimens reveal nonspecific findings. Epidermal changes vary from modest spongiosis to acanthosis with hyperkeratosis and parakeratosis, depending upon the stage of the disease. The dermis shows a nonspecific perivascular lymphocytic infiltrate with a variable degree of dermal edema and a variable number of neutrophils or eosinophils. Early lesions may resemble arthropod bite reactions, with a deeper dermal infiltrate and an absence of epidermal changes ( Fig. 27.6 ). The histologic correlate of microvesiculation is severe epidermal spongiosis and/or dermal edema. Direct IF reveals no relevant abnormalities and indirect IF is negative. Routine laboratory evaluation is normal.
Differential Diagnosis
Since lesions of PEP may show microvesiculation, contact dermatitis must be considered. Drug eruptions, urticaria, or viral exanthems may also be in the clinical differential diagnosis. The most important entity to exclude is urticarial pemphigoid gestationis, whose lesions tend to appear earlier during gestation, have no association with abdominal striae and often involve the umbilicus, along with positive IF of perilesional skin.
Treatment
The majority of patients benefit from topical corticosteroids and oral antihistamines. More severe disease with a distressing degree of pruritus can be safely treated with a short course of systemic corticosteroids (see Table 27.3 ). Since the disease is self-limited and without serious sequelae, a conservative approach is justified.
Intrahepatic Cholestasis of Pregnancy
▪ Cholestasis of pregnancy ▪ Obstetric cholestasis ▪ Cholestatic jaundice of pregnancy ▪ Pruritus/prurigo gravidarum
- ▪
Pruritus without primary skin lesions with an onset during the third trimester
- ▪
Secondary changes correlate with disease duration and vary from subtle excoriations to severe prurigo nodularis
- ▪
Elevated total serum bile acid levels are diagnostic; histology is nonspecific and IF is negative
- ▪
Increased risk of prematurity, intrapartum fetal distress, and stillbirths
- ▪
Recurs in 45–70% of subsequent pregnancies
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a rare form of genetically linked, hormone-dependent, reversible cholestasis. It typically presents late during pregnancy with dramatic pruritus. Although maternal prognosis is usually good (a small minority may develop steatorrhea and vitamin K deficiency), fetal risk is significant. As a result, ICP is the most important pruritic gestational condition to consider and promptly diagnose and treat in order to prevent fetal impairment.
History
ICP was first described in 1907 by Kehrer. In the past, much of the confusion arose from applying descriptive names such as “pruritus gravidarum” for ICP without skin changes or “prurigo gravidarum” if skin lesions associated with scratching were present. While the first term was often combined with the mild unexplained pruritus that occurs in many women during pregnancy (most likely related to an atopic diathesis), the latter was confused with “prurigo of pregnancy” (see below). Because laboratory evaluation, including measurement of serum bile acid levels, was performed in only a minority of women, ICP was often missed.
Epidemiology
There are striking geographic and ethnic differences in the incidence of ICP. For example, it is most commonly observed in South America, with the highest incidence rates in Bolivia and Chile (9–16%), particularly among Araucanian Indian women (~30%). In contrast, rates of 0.1% to 1.5% have been described in Europe and North America, with relative “hot spots” in Scandinavia and the Baltic states (1–2%) . Some of the variance is probably explained by different reporting criteria; however, endemic clustering and a positive family history in up to 50% of those affected point towards a genetic predisposition. A higher incidence of ICP is also seen in multiple-gestation pregnancies, which may be related to higher hormonal levels (e.g. estrogen) in these patients.
Pathogenesis
The key element is reduced excretion of bile acids, which leads to increased serum levels. This not only provokes severe pruritus in the mother, but also may have deleterious effects on the fetus. Toxic bile acids crossing the placenta can lead to acute fetal anoxia due to abnormal uterine contractility and vasoconstriction of chorionic veins as well as impaired fetal cardiomyocyte function . One predisposing factor is mutations in genes (e.g. ABCB4 ) that encode bile transporter proteins . While mild dysfunction of these canalicular transporters may not lead to clinical symptoms in non-pregnant individuals, when the transporters’ capacity to secrete substrates is exceeded (as occurs in the setting of high levels of sex hormones during pregnancy), signs and symptoms of cholestasis can develop. Other contributing factors are the cholestatic effect of estrogen and progesterone metabolites, which peak late during pregnancy, and hepatitis C viral infection (see below). Furthermore, dietary factors such as selenium deficiency and increased intestinal permeability (“leaky gut“) have been suggested as possible triggers.
Clinical Features
Patients typically present during their last trimester with a sudden onset of intense, generalized pruritus that often starts on the palms and soles. No primary skin lesions are seen, and secondary changes due to scratching vary from subtle excoriations early on to pronounced prurigo nodularis in those with pruritus of longer duration ( Fig. 27.7 ). The extensor surfaces of the extremities, buttocks, and abdomen are usually most severely affected.
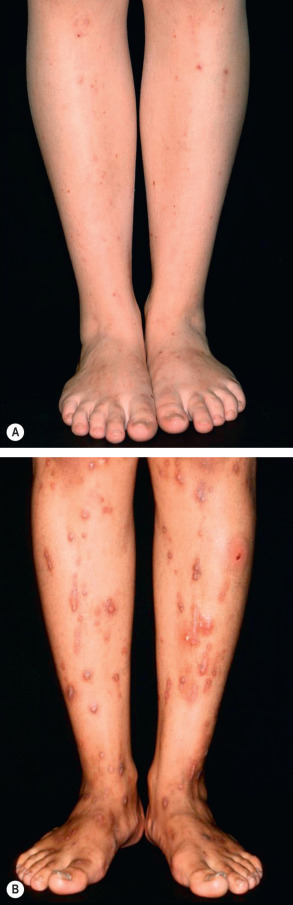
Although jaundice is often mentioned as a common finding in ICP, it actually occurs in only 10% of patients. Jaundice is usually a complication in those with the most severe and prolonged episodes of ICP. In such patients, concomitant extrahepatic cholestasis may be associated with steatorrhea and subsequent vitamin K deficiency, leading to an increased risk of intra- and postpartum hemorrhage.
Pruritus typically persists until delivery and then it resolves spontaneously within days. A protracted course is very unusual and should prompt one to exclude other liver diseases, especially primary biliary cirrhosis. Recurrence during subsequent pregnancies occurs in 45–70% of patients, and recurrence with oral contraceptives is routine. No detectable abnormalities are generally present between gestations.
ICP is associated with significant fetal risk, in particular an increase in premature births (20–60%), intrapartum fetal distress (20–30%; e.g. meconium staining of amniotic fluid, abnormal fetal heart rate), and fetal loss (1–2%) . Fetal risk correlates with the elevation in serum bile acid levels, especially when levels exceed 40 µmol/l . Thus, prompt diagnosis and treatment is essential, as is close obstetric surveillance.
Pathology
Histologic findings in the skin and the liver are nonspecific and direct IF of perilesional skin is negative. The diagnosis is confirmed by an increase in total serum bile acid levels (>11 µmol/l in a pregnant woman; normal range in non-pregnant women, 0–6 µmol/l). Levels may range from 3 to 100 times normal. During pregnancy, alkaline phosphatase levels typically increase (placental origin) even in the absence of ICP, and γ-glutamyl transferase levels are usually lower than in the non-pregnant state. Serum levels of transaminases are usually elevated in those with ICP, but may be normal in 30% of patients . In women with jaundice, conjugated (direct) bilirubin levels are increased and the prothrombin time may be prolonged. Hepatic ultrasonography generally is normal but may reveal gallstones in jaundiced patients, who are at increased risk for their development.
Differential Diagnosis
In the absence of primary lesions, the clinical differential diagnosis includes other causes of primary pruritus (see Ch. 6 ), including those that lead to cholestatic pruritus. Viral hepatitis is a common disorder and should be excluded by appropriate serologies. Of note, a history of hepatitis C viral infection is considered a risk factor for the development of ICP, and in one study, 20% of the women who were HCV RNA-positive developed ICP .
Treatment
Since fetal prognosis correlates with disease severity, the therapeutic goal is reduction of serum bile acid levels. This allows prolongation of the pregnancy and lessens both fetal risk and maternal symptoms. To date, the only successful agent has been oral ursodeoxycholic acid (UDCA) . It is a naturally occurring, hydrophilic, non-toxic bile acid that has been used for a variety of cholestatic liver diseases. Although the exact mechanism of action in ICP is still not fully understood, there is evidence that UDCA corrects the maternal serum bile acid profile, decreases the passage of maternal bile acids to the fetoplacental unit, and improves the function of the bile acid transport system across the trophoblast. UDCA is safe for mother and fetus, with its only side effect being mild diarrhea. Use of UDCA for ICP is off-label as it is only approved for primary biliary cirrhosis. The recommended oral dose is 15 mg/kg daily or, independent of body weight, 1 g daily. It should be started as early as possible and administered until delivery.
The use of S-adenosylmethionine, dexamethasone, epomediol, silymarin, phenobarbital or activated charcoal is not recommended as none have been shown to decrease fetal risk. Cholestyramine is contraindicated as it can further reduce vitamin K absorption and increase the risk of bleeding . In jaundiced patients, the prothrombin time should be monitored, and intramuscular vitamin K administered as necessary. Close interdisciplinary collaboration with the obstetrician is essential and close monitoring of the fetus is recommended.
Atopic Eruption of Pregnancy
▪ Prurigo of pregnancy ▪ Besnier’s “prurigo gestationis” ▪ Nurse’s “early-onset prurigo” of pregnancy ▪ Spangler’s “papular dermatitis of pregnancy” ▪ Pruritic folliculitis of pregnancy ▪ Eczema in pregnancy
- ▪
Eczematous and/or papular skin lesions in a patient with an atopic diathesis in whom other specific dermatoses have been excluded
- ▪
Most common pruritic disorder during pregnancy
- ▪
Generally appears earlier than other pregnancy-related dermatoses (75% before the third trimester)
- ▪
Nonspecific histology; negative direct IF; elevated serum IgE levels in up to 70% of patients
- ▪
No maternal or fetal risks; commonly recurs in subsequent pregnancies
Introduction
Atopic eruption of pregnancy (AEP) is defined as either an exacerbation or the first occurrence of eczematous and/or papular skin changes during pregnancy in atopic individuals. As the majority of patients belong to the second group, the atopic link is often overlooked, leading to a number of different diagnoses, as evidenced by the many synonyms.
History
In retrospect, an association with atopy dates back to the first reports. When Besnier described the disorder “prurigo gestationis” in 1904, “prurigo” was the term dermatologists used for atopic dermatitis (Besnier was the first to note the association between atopic dermatitis, allergic rhinitis, and asthma). Nurse, in 1968, described accompanying eczematous features in most of the 31 patients in his “early-onset” prurigo group. In 1983, Holmes and Black were the first to suggest that “prurigo of pregnancy” could simply result from pregnancy-related pruritus in women with an atopic diathesis rather than being a distinct entity .
Epidemiology
AEP is by far the most common pruritic disorder in pregnant women and it tends to appear earlier than the other pregnancy-related dermatoses . Its incidence is not known but may be as high as 1 in 5 to 1 in 20.
Pathogenesis
To prevent fetal rejection, a normal pregnancy is characterized by a lack of strong maternal cell-mediated immune function and reduced Th1 cytokine production (e.g. IL-12, interferon-γ) as well as a dominant humoral immune response with increased Th2 cytokine production (e.g. IL-4, IL-10). This natural switch towards a dominant Th2 response, which worsens the imbalance already present in most atopic patients, is thought to favor the development of AEP .
Clinical Features
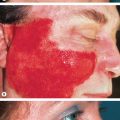
In contrast to the other specific dermatoses of pregnancy, AEP appears earlier, often during the first trimester, with 75% of patients presenting before the third trimester. Approximately 20% of women experience an exacerbation of pre-existing atopic dermatitis, while the remaining 80% develop atopic skin changes for the first time during pregnancy. Two-thirds of patients present with eczematous lesions ( Fig. 27.8 ), often involving “atopic sites” such as the face, neck, and flexural aspects of the extremities. One-third develop a papular eruption on the trunk and extremities, composed of either classic prurigo lesions or small erythematous papules ( Fig. 27.9 ). Findings typically include xerosis (often marked) and other signs of the underlying atopic diathesis (see Ch. 12 ). Fetal and maternal prognoses are excellent and recurrences in subsequent pregnancies are common.