Valgus osteotomy of the proximal femur can be performed for a number of different conditions including (among others) congenital or acquired coxa vara, fracture nonunion, avascular necrosis (AVN), or Legg-Calvé-Perthes disease (LCPD).
 Coxa vara is a deformity of the proximal femur associated with a neck–shaft angle of less than 110 degrees.11 It can be congenital or developmental in origin.
Coxa vara is a deformity of the proximal femur associated with a neck–shaft angle of less than 110 degrees.11 It can be congenital or developmental in origin.
 Femoral neck fracture nonunions can result (in part) from a vertically oriented fracture plane. A valgus osteotomy can improve mechanical loading at the fracture site and aid in fracture healing.
Femoral neck fracture nonunions can result (in part) from a vertically oriented fracture plane. A valgus osteotomy can improve mechanical loading at the fracture site and aid in fracture healing.
 AVN of the femoral head typically affects the anterosuperior region of the femoral head while sparing the medial and posterior regions. In certain cases, a valgus osteotomy (with flexion) can rotate better parts of the femoral head into the weight-bearing zone.
AVN of the femoral head typically affects the anterosuperior region of the femoral head while sparing the medial and posterior regions. In certain cases, a valgus osteotomy (with flexion) can rotate better parts of the femoral head into the weight-bearing zone.
 In LCPD, valgus osteotomy can help those hips in which the primary goal of containment is no longer possible owing to hinge abduction. Under these circumstances, a valgus osteotomy can relieve the hinging and improve congruency of the joint.
In LCPD, valgus osteotomy can help those hips in which the primary goal of containment is no longer possible owing to hinge abduction. Under these circumstances, a valgus osteotomy can relieve the hinging and improve congruency of the joint.
ANATOMY
 Valgus osteotomy creates an apex medial angulation in the proximal femur.
Valgus osteotomy creates an apex medial angulation in the proximal femur.
 In doing so, the medial aspects of the femoral head are rotated more centrally into the joint and, correspondingly, the lateral aspects of the head are rotated away from the joint.
In doing so, the medial aspects of the femoral head are rotated more centrally into the joint and, correspondingly, the lateral aspects of the head are rotated away from the joint.
 Adding flexion or extension at the osteotomy site similarly rotates the posterior (flexion) or anterior (extension) aspects of the femoral head into the joint.
Adding flexion or extension at the osteotomy site similarly rotates the posterior (flexion) or anterior (extension) aspects of the femoral head into the joint.
 Valgus osteotomy increases a patient’s effective hip abduction by the amount of correction and similarly reduces the patient’s adduction by an equivalent amount.
Valgus osteotomy increases a patient’s effective hip abduction by the amount of correction and similarly reduces the patient’s adduction by an equivalent amount.
 Length of the limb is increased by a valgus correction, which can be useful in cases of mild limb shortening (eg, LCPD).
Length of the limb is increased by a valgus correction, which can be useful in cases of mild limb shortening (eg, LCPD).
 Valgus correction moves the greater trochanter distally, which improves abductor mechanics.
Valgus correction moves the greater trochanter distally, which improves abductor mechanics.
PATHOGENESIS
 The pathogenesis of the deformity to be treated by valgus osteotomy is specific to the underlying condition.
The pathogenesis of the deformity to be treated by valgus osteotomy is specific to the underlying condition.
 The exact cause of developmental coxa vara is unknown, but one theory postulates that the varus deformity is due to a primary ossification defect in the medial femoral neck that results in a more vertical physis. The physiologic shearing stresses that occur during weight bearing fatigue the dystrophic bone in the medial femoral neck, resulting in progressive varus.7
The exact cause of developmental coxa vara is unknown, but one theory postulates that the varus deformity is due to a primary ossification defect in the medial femoral neck that results in a more vertical physis. The physiologic shearing stresses that occur during weight bearing fatigue the dystrophic bone in the medial femoral neck, resulting in progressive varus.7
 AVN of the femoral head is the final common pathway of a spectrum of disease processes that disrupt circulation to the femoral head including embolism from deep sea diving, alcohol use, corticosteroid use, hemoglobinopathies, chemotherapy, LCPD, and traumatic injuries to the hip.1
AVN of the femoral head is the final common pathway of a spectrum of disease processes that disrupt circulation to the femoral head including embolism from deep sea diving, alcohol use, corticosteroid use, hemoglobinopathies, chemotherapy, LCPD, and traumatic injuries to the hip.1
 Hinge abduction in LCPD can result from fragmentation and extrusion of the epiphysis, which can create coxa magna and/or a ridge of lateral bone. As a result, the lateral aspect of the deformed femoral head may impinge on the acetabulum with attempted abduction. Continued abduction creates a lateral hinge, which pulls the inferomedial portion of the head away from the acetabulum.8
Hinge abduction in LCPD can result from fragmentation and extrusion of the epiphysis, which can create coxa magna and/or a ridge of lateral bone. As a result, the lateral aspect of the deformed femoral head may impinge on the acetabulum with attempted abduction. Continued abduction creates a lateral hinge, which pulls the inferomedial portion of the head away from the acetabulum.8
NATURAL HISTORY
 As described by Weinstein et al,11 the most reliable factor for progression of coxa vara is the Hilgenreiner–epiphyseal angle (HEA), measured between the line of Hilgenreiner and a line parallel to the proximal femoral physis (FIG 1).
As described by Weinstein et al,11 the most reliable factor for progression of coxa vara is the Hilgenreiner–epiphyseal angle (HEA), measured between the line of Hilgenreiner and a line parallel to the proximal femoral physis (FIG 1).
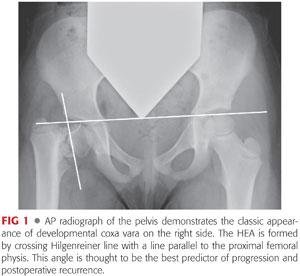
Patients with HEAs more than 60 degrees will invariably progress, whereas those between 45 and 60 degrees have a less defined prognosis and must be followed for progression of varus deformity or increased symptoms.
 AVN of femoral head typically results in progressive collapse, pain, and stiffness often necessitating total hip arthroplasty.1
AVN of femoral head typically results in progressive collapse, pain, and stiffness often necessitating total hip arthroplasty.1
 At maturity, patients with LCPD and unrelieved hinge abduction would generally be classified as Stulberg category IV (flattened femoral head with congruent acetabulum) or category V (flattened femoral head with a round acetabulum), both of which have been found to be associated with early-onset osteoarthritis.10
At maturity, patients with LCPD and unrelieved hinge abduction would generally be classified as Stulberg category IV (flattened femoral head with congruent acetabulum) or category V (flattened femoral head with a round acetabulum), both of which have been found to be associated with early-onset osteoarthritis.10
PATIENT HISTORY AND PHYSICAL FINDINGS
 History and physical findings are specific to the underlying condition.
History and physical findings are specific to the underlying condition.
 Typically, patients who would benefit from a valgus osteotomy have pain with ambulation that is relieved by rest.
Typically, patients who would benefit from a valgus osteotomy have pain with ambulation that is relieved by rest.
 There is generally 1 to 2 cm of limb shortening.
There is generally 1 to 2 cm of limb shortening.
 Abductor weakness often causes a Trendelenburg gait.
Abductor weakness often causes a Trendelenburg gait.
 Limited abduction is common, and occasionally, patients have frank adduction contractures.
Limited abduction is common, and occasionally, patients have frank adduction contractures.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Plain radiographs are generally diagnostic for the conditions that may require a valgus osteotomy.
Plain radiographs are generally diagnostic for the conditions that may require a valgus osteotomy.
 Anteroposterior (AP) x-rays of the hip in coxa vara will demonstrate a reduced neck–shaft angle and a wider more vertically oriented proximal femoral physis. Classically, a triangular metaphyseal fragment will be seen in the inferior neck surrounded by physis, giving an inverted Y pattern11 (see FIG 1).
Anteroposterior (AP) x-rays of the hip in coxa vara will demonstrate a reduced neck–shaft angle and a wider more vertically oriented proximal femoral physis. Classically, a triangular metaphyseal fragment will be seen in the inferior neck surrounded by physis, giving an inverted Y pattern11 (see FIG 1).
 Femoral neck nonunions typically result in medial collapse and varus deformity. Subtle nonunions can be confirmed by computed tomography (CT) scan.
Femoral neck nonunions typically result in medial collapse and varus deformity. Subtle nonunions can be confirmed by computed tomography (CT) scan.
 On the AP view, AVN typically results in sclerosis and/or collapse of the lateral and superior regions of the femoral head. On the frog view, the anterior head is more commonly affected.
On the AP view, AVN typically results in sclerosis and/or collapse of the lateral and superior regions of the femoral head. On the frog view, the anterior head is more commonly affected.
 Hinge abduction in LCPD is best diagnosed using dynamic arthrography. Although pooling of dye medially is often considered diagnostic for hinge abduction, this can simply reflect an area of flattening of the epiphysis. It is most accurate to determine whether the lateral edge of the femoral head is able to rotate underneath the lip of the acetabulum and labrum with abduction (FIG 2). Congruency is generally improved when the hip is adducted.
Hinge abduction in LCPD is best diagnosed using dynamic arthrography. Although pooling of dye medially is often considered diagnostic for hinge abduction, this can simply reflect an area of flattening of the epiphysis. It is most accurate to determine whether the lateral edge of the femoral head is able to rotate underneath the lip of the acetabulum and labrum with abduction (FIG 2). Congruency is generally improved when the hip is adducted.
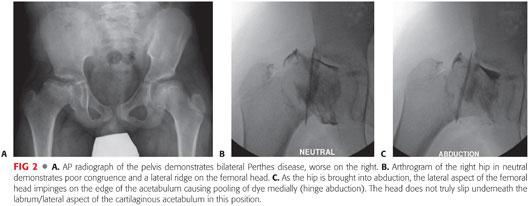
The arthrogram should also be studied in AP and lateral projections with abduction, adduction, and internal and external rotation to determine the position that maximizes congruency and relieves impingement.
DIFFERENTIAL DIAGNOSIS
 Congenital or acquired coxa vara
Congenital or acquired coxa vara
 Femoral neck nonunion
Femoral neck nonunion
 AVN
AVN
 LCPD with hinge abduction or poor congruency
LCPD with hinge abduction or poor congruency
 Pathologic bone condition causing progressive varus deformity (eg, fibrous dysplasia, osteogenesis imperfecta, renal osteodystrophy)
Pathologic bone condition causing progressive varus deformity (eg, fibrous dysplasia, osteogenesis imperfecta, renal osteodystrophy)
NONOPERATIVE MANAGEMENT
 Mild forms of coxa vara, precollapse AVN, and early-stage LCPD may be managed conservatively.
Mild forms of coxa vara, precollapse AVN, and early-stage LCPD may be managed conservatively.
 Depending on the circumstance, short courses of anti-inflammatory medications, protected weight bearing, and/or activity modification can be helpful.
Depending on the circumstance, short courses of anti-inflammatory medications, protected weight bearing, and/or activity modification can be helpful.
 In LCPD, if hinge abduction is seen on radiographs but the patient is symptom-free, an osteotomy can still improve the prognosis. In that scenario, it would be reasonable to wait until the patient is symptomatic before proceeding with surgery.
In LCPD, if hinge abduction is seen on radiographs but the patient is symptom-free, an osteotomy can still improve the prognosis. In that scenario, it would be reasonable to wait until the patient is symptomatic before proceeding with surgery.
SURGICAL MANAGEMENT
 The valgus osteotomy is indicated for unacceptable varus deformity, fracture nonunion, and certain cases of AVN among others.
The valgus osteotomy is indicated for unacceptable varus deformity, fracture nonunion, and certain cases of AVN among others.
 In LCPD, valgus osteotomy is considered a salvage operation for late cases in which the femoral head has developed a lateral ridge that can no longer be brought under the acetabulum (hinge abduction) or to improve congruency of an aspherical hip (FIG 3).
In LCPD, valgus osteotomy is considered a salvage operation for late cases in which the femoral head has developed a lateral ridge that can no longer be brought under the acetabulum (hinge abduction) or to improve congruency of an aspherical hip (FIG 3).
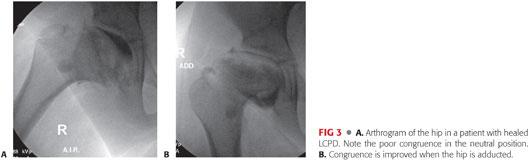
Preoperative Planning
 Clinically or radiographically assess limb lengths to determine if concomitant shortening is necessary.
Clinically or radiographically assess limb lengths to determine if concomitant shortening is necessary.
 Carefully evaluate preoperative range of motion including flexion, extension, and rotation.
Carefully evaluate preoperative range of motion including flexion, extension, and rotation.
 Specifically evaluate a patient’s adduction and abduction prior to surgery to guide the amount of correction. Keep in mind that adduction will be decreased and abduction will be increased by the amount of osteotomy correction. Generally, it is preferable to achieve at least 20 degrees of abduction after surgery.
Specifically evaluate a patient’s adduction and abduction prior to surgery to guide the amount of correction. Keep in mind that adduction will be decreased and abduction will be increased by the amount of osteotomy correction. Generally, it is preferable to achieve at least 20 degrees of abduction after surgery.
 If an arthrogram was performed, it should be reviewed carefully. In cases of hinge abduction, extension, flexion, or rotation may be required in addition to valgus to fully relieve impingement and maximize congruency.
If an arthrogram was performed, it should be reviewed carefully. In cases of hinge abduction, extension, flexion, or rotation may be required in addition to valgus to fully relieve impingement and maximize congruency.
 Review preoperative AP and frog lateral radiographs to determine the native neck–shaft angle and size of bone.
Review preoperative AP and frog lateral radiographs to determine the native neck–shaft angle and size of bone.
 Based on range-of-motion measurements and preoperative radiographs, one should determine the desired amount of valgus correction.
Based on range-of-motion measurements and preoperative radiographs, one should determine the desired amount of valgus correction.
 Implant choice is critical as the placement and shape of the implant, rather than the saw cut, will determine the final position of the osteotomy.
Implant choice is critical as the placement and shape of the implant, rather than the saw cut, will determine the final position of the osteotomy.
 The author prefers a 130-degree nonoffset blade plate, which lateralizes the femoral shaft and preserves head–shaft offset (FIG 4A).
The author prefers a 130-degree nonoffset blade plate, which lateralizes the femoral shaft and preserves head–shaft offset (FIG 4A).
Using a standard blade plate or proximal femoral locking plate can medialize the femoral shaft excessively such that the limb resembles a “post” without any physiologic offset (FIG 4B).
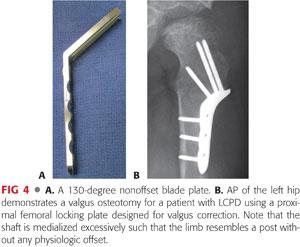
 Depending on the size and weight of the patient, both 3.5- and 4.5-mm plate options exist for one particular manufacturer (Orthopediatrics, Warsaw, IN). The width of the 3.5-mm blade plate system is 11 mm. The width of the 4.5-mm blade plate system is 14 mm.
Depending on the size and weight of the patient, both 3.5- and 4.5-mm plate options exist for one particular manufacturer (Orthopediatrics, Warsaw, IN). The width of the 3.5-mm blade plate system is 11 mm. The width of the 4.5-mm blade plate system is 14 mm.
 The blade plate should occupy 50% to 75% of the width of the femoral neck on the lateral projection for optimum strength.
The blade plate should occupy 50% to 75% of the width of the femoral neck on the lateral projection for optimum strength.
 To calculate the angle for insertion of the blade plate relative to the femoral shaft, the angle of the planned correction is subtracted from 130 degrees.
To calculate the angle for insertion of the blade plate relative to the femoral shaft, the angle of the planned correction is subtracted from 130 degrees.
Example: For a desired 20 degrees of valgus correction, the blade is inserted at 110 degrees from the shaft. With the blade at 110 degrees, the shaft must come into 20 degrees of valgus to accommodate a 130-degree fixed-angle blade plate.
Positioning
 The patient is placed supine on a radiolucent surgical table with a small bump under the ipsilateral pelvis to facilitate access to the lateral femur. Too large of a bump can distort orthogonal imaging.
The patient is placed supine on a radiolucent surgical table with a small bump under the ipsilateral pelvis to facilitate access to the lateral femur. Too large of a bump can distort orthogonal imaging.
 The surgeon should check that sufficient AP and frog lateral radiographs can be obtained.
The surgeon should check that sufficient AP and frog lateral radiographs can be obtained.
 The entire limb should be draped free to allow manipulation of the limb during surgery.
The entire limb should be draped free to allow manipulation of the limb during surgery.
Approach
 The standard lateral approach to the proximal femur is used.
The standard lateral approach to the proximal femur is used.
TECHNIQUES
 Exposure
Exposure
Skin incision is made in line with the proximal femur starting a few centimeter proximal to the vastus ridge and extending distally approximately 10 to 12 cm depending on the size of the patient and the intended implant.
The fascia lata is split in line with the fibers over the palpated lateral border of the femur.
The vastus lateralis fascia is incised longitudinally about 5 to 10 mm anterior to the intermuscular septum and is elevated atraumatically from the femur. Perforating vessels are identified and cauterized.
Proximally, the fascia of the vastus lateralis is opened anteriorly with the electrocautery along the vastus ridge, creating an L shape (TECH FIG 1A).
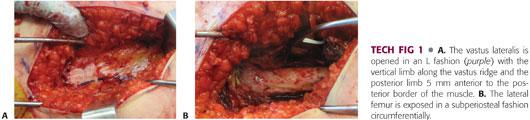
The periosteum is incised along the anterolateral femur, and subperiosteal dissection is performed circumferentially just proximal to the level of the lesser trochanter. The exposure should be extended sufficiently distal to allow shortening of the bone (if necessary) and application of the plate (TECH FIG 1B).
 Valgus Osteotomy of the Femur Using a Cannulated Blade Plate
Valgus Osteotomy of the Femur Using a Cannulated Blade Plate
Guidewire Placement
In a cannulated blade plate system, the guidewire establishes the position of the chisel which in turn dictates the position and trajectory of the blade. Therefore, precise placement is critical.
To provide a reference, a standard Kirschner (K) wire is inserted perpendicular to the femoral shaft at the intended osteotomy site (usually at the proximal aspect of the lesser trochanter).
The entry site for the cannulated blade plate guidewire is proximal to the osteotomy site at a distance specified by the implant design. This typically ends up being just distal to the vastus ridge and trochanteric apophysis. The entry site should be centered on the lateral aspect of the femur in the sagittal plane (TECH FIG 2A).
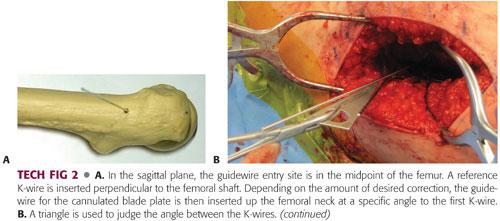
Using a triangle template to guide the trajectory (chosen based on the amount of intended correction), the guidewire is inserted into the femoral neck.
To use the previous example, if a 20-degree correction is desired and a 130-degree implant is being used, the guidewire should be placed 110 degrees from the shaft. A 110-degree angle with the shaft corresponds to a 20-degree angle compared to the perpendicular K-wire (90 + 20). Therefore, a 20-degree triangle is used to confirm the angle between the wires (TECH FIG 2B,C).
In the lateral plane, the wire should parallel the proposed track of the blade plate (ie, centered in the femoral neck) (TECH FIG 2D).
The guidewire is advanced short of the physis, and length is measured.
The guidewire should be inserted bit further than the intended blade plate depth to prevent dislodgement during chisel placement.
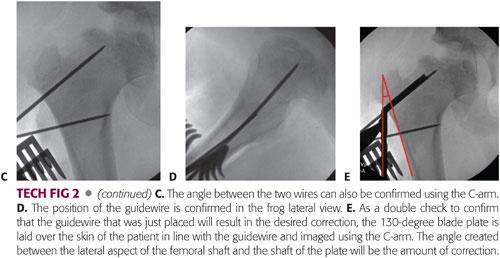
As a double check to confirm that the guidewire that was just placed will result in the desired correction, the 130-degree blade plate is laid over the skin of the patient in line with the guidewire and imaged using the C-arm. The angle created between the lateral aspect of the femoral shaft and the shaft of the plate will be the amount of correction. This can be estimated visually or measured using a goniometer (TECH FIG 2E).
Chisel Insertion
The cannulated chisel is now inserted over the guidewire (TECH FIG 3A).
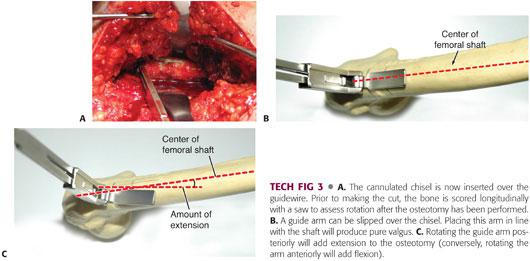
For pure valgus, the chisel is rotated so that it is perpendicular to the lateral shaft of the femur.
To add extension or flexion, the chisel is rotated posteriorly or anteriorly from perpendicular, respectively. The desired amount of flexion or extension should be marked on the bone (TECH FIG 3B,C).
A guide arm that can be slid over the chisel is helpful to visualize the amount of flexion or extension.
The chisel should be frequently backed out a bit during insertion (using a slap hammer) to prevent incarceration.
The path of the chisel is checked periodically with fluoroscopy.
The chisel is backed up to loosen it before making the osteotomy. The surgeon should verify it has actually backed up by checking the depth measurement or by taking a C-arm image.
Making the Osteotomy
Prior to making the cut, the bone is scored longitudinally with electrocautery or a saw across the osteotomy site, or K-wires can be placed proximal and distal to the osteotomy site to assess rotation after the osteotomy has been performed (see TECH FIG 3A).
A transverse osteotomy is made using an oscillating saw at the site of the original, perpendicularly directed K-wire (generally at the proximal aspect of the lesser trochanter). The K-wire can be left in place to help guide the saw cut so that it is perpendicular to the femoral shaft or the trajectory can be confirmed using the C-arm.
A single transverse cut is the simplest means of performing the osteotomy and generally heals quite well, but it does not provide the maximum surface area for osteotomy healing.
If better apposition is desired, a step cut can be made in the lateral cortex or a wedge can be removed (TECH FIG 4).
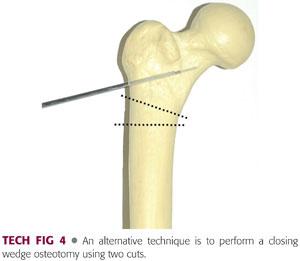
Hohmann or other retractors can be placed around the femur subperiosteally to protect soft tissue structures during the osteotomy.
If the femur needs to be shortened, the distal fragment can be delivered out of the wound. This allows the surgeon to measure, mark, and cut the bone as needed.
Blade Plate Placement
A bone tenaculum clamp is now placed around the greater trochanter to provide control of the proximal fragment.
The chisel is removed, and the cannulated blade plate is inserted over the guidewire. The exchange should be done quickly to minimize the chance that the proper rotation is lost.
The blade plate should be inserted by hand initially to prevent deviation from the desired path. It can then be impacted into place using a mallet.
A frog lateral view should be obtained during blade plate insertion to be sure that it is following the guidewire.
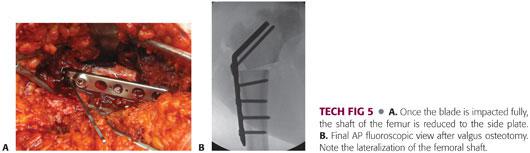
Once impacted fully, the shaft of the femur is reduced to the side plate and secured with a bone reduction clamp (TECH FIG 5A).
Rotation is confirmed using the previously placed orientation line or K-wires.
The side plate is secured to the femur using standard techniques with the initial screws inserted in compression (TECH FIG 5B).
In certain implant systems (Orthopediatrics), an additional locking screw can be placed through the shoulder of the plate into the proximal fragment.
Closure
The vastus lateralis is repaired using interrupted absorbable sutures at the vastus ridge followed by a running suture along its posterior border.
The fascia lata is closed in a watertight fashion using interrupted heavy absorbable suture (eg, no. 1 Vicryl), followed by layered closure of the dermis (2-0 Vicryl) and the subcuticular layer (4-0 Monocryl).
PEARLS AND PITFALLS | |
Chisel incarceration |
|
| |
Loss of guidewire |
|
| |
Inaccurate correction |
|
| |
Avoiding implant cutout |
|
| |
| |
POSTOPERATIVE CARE
 Patients are made toe-touch weight bearing with crutches. Younger patients and those who are expected to be noncompliant with crutches and activity limitations can be placed in a single-leg spica cast. Weight bearing is generally advanced at 4 to 6 weeks based on radiographic healing of the osteotomy.
Patients are made toe-touch weight bearing with crutches. Younger patients and those who are expected to be noncompliant with crutches and activity limitations can be placed in a single-leg spica cast. Weight bearing is generally advanced at 4 to 6 weeks based on radiographic healing of the osteotomy.
 Elective hardware removal is controversial. I prefer hardware removal 1 year postoperatively or after bony union is obtained in patients in whom the likelihood of future surgery, including joint arthroplasty, is high. This is particularly true of younger patients who can develop bone growth over their implants.
Elective hardware removal is controversial. I prefer hardware removal 1 year postoperatively or after bony union is obtained in patients in whom the likelihood of future surgery, including joint arthroplasty, is high. This is particularly true of younger patients who can develop bone growth over their implants.
OUTCOMES
 Coxa vara
Coxa vara
If adequate valgus is achieved, the triangular defect will spontaneously close by 3 to 6 months after surgery in nearly all patients.
Between 50% and 89% of operated hips sustain a premature closure of the proximal femoral physis, which occurs 1 to 2 years postoperatively and has not been found to correlate with patient age, surgical trauma, or degree of valgus.6,9
Recurrence has been reported in 30% to 70% of patients, although correction of the HEA to less than 38 degrees has been shown to have a 95% success rate.4
These patients must be monitored for recurrent varus deformity or significant leg length discrepancy that may require further surgical intervention.
 Femoral neck nonunion
Femoral neck nonunion
One study reported an 86% union rate at a mean of 20 weeks following valgus osteotomy for femoral neck nonunion in young patients.5
 AVN
AVN
One series reported improved hip function (based on Harris Hip Score) in six patients treated with valgus osteotomy for posttraumatic AVN.3
Comparison of magnetic resonance imaging (MRI) scans before and after valgus correction demonstrated resorption of the necrotic segment of the femoral head and its remodeling in all six patients with segmental osteonecrosis.3
 LCPD/hinge abduction
LCPD/hinge abduction
Three recent publications2,8,12 reported subjectively satisfactory results (by varying standards) in 66% to 94% of patients at 5 to 10 years of follow-up. Average Iowa hip scores ranged from 86 to 93. Further hip surgery had been performed on 10% to 20% of patients.
Two of the three recent articles noted no significant changes in the Sharp angle and percent coverage.2,8 One study noted a significant difference in the percent coverage and superior joint space.12
Bankes et al2 reported two factors associated with favorable remodeling, both concerning the timing of surgery: when the osteotomies were carried out during the healing phase of Perthes disease and when they were carried out in patients with open triradiate cartilages.
COMPLICATIONS
 Nonunion is rare in otherwise healthy patients.
Nonunion is rare in otherwise healthy patients.
 Hardware failure most commonly occurs via the blade plate breaking out of the proximal fragment when too small of a bone bridge was preserved.
Hardware failure most commonly occurs via the blade plate breaking out of the proximal fragment when too small of a bone bridge was preserved.
 Infection
Infection
ACKNOWLEDGMENTS
 Thank you to Ellen M. Raney, Michael B. Millis, and Joshua A. Strassberg for their work on previous related chapters from the first edition.
Thank you to Ellen M. Raney, Michael B. Millis, and Joshua A. Strassberg for their work on previous related chapters from the first edition.
REFERENCES
1. Assouline-Dayan Y, Chang C, Greenspan A, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002;32(2):94–124.
2. Bankes MJ, Catterall A, Hashemi-Nejad A. Valgus extension osteotomy for ‘hinge abduction’ in Perthes’ disease: results at maturity and factors influencing radiographic outcome. J Bone Joint Surg Br 2000;82(4):548–554.
3. Bartonicek J, Vavra J, Bartoska R, et al. Operative treatment of avascular necrosis of the femoral head after proximal femur fractures in adolescents. Int Orthop 2012;36(1):149–157.
4. Carroll K, Coleman S, Stevens P. Coxa vara: surgical outcomes of valgus osteotomy. J Pediatr Orthop 1997;17:220–224.
5. Ghosh B, Bhattacharjya B, Banerjee K, et al. Management of the non-united neck femur fracture by valgus osteotomy—a viable alternative. J Indian Med Assoc 2012;110(11):819–820.
6. Kehl D, LaGrone M, Lovell W. Developmental coxa vara. Orthop Trans 1983;7:475.
7. Pylkkanen P. Coxa vara infantum. Acta Orthop Scand 1960;48(suppl 48):1–120.
8. Raney EM, Grogan DP, Hurley ME, et al. The role of proximal femoral valgus osteotomy in Legg-Calve-Perthes disease. Orthopedics 2002;25:513–517.
9. Schmidt TL, Kalamchi A. The fate of the capital femoral physis and acetabular development in developmental coxa vara. J Pediatr Orthop 1982;2(5):534–538.
10. Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calve-Perthes disease. J Bone Joint Surg Am 1981;63:1095–1108.
11. Weinstein JN, Kuo KN, Millar EA. Congenital coxa vara. A retrospective review. J Pediatr Orthop 1984;4:70–77.
12. Yoo WJ, Choi IH, Chung CY, et al. Valgus femoral osteotomy for hinge abduction in Perthes’ disease: decision-making and outcomes. J Bone Joint Surg Br 2004;86:726–730.
< div class='tao-gold-member'>












