Total hip arthroplasty (THA) is successfully performed around the world for the treatment of arthritis, osteonecrosis, and femoral neck fracture, with predictably excellent results. The criteria for successful THA are no different today than they were 50 years ago: The procedure should be safe, effective, and durable. There is no doubt about the effectiveness of THA relieving pain and improving function. Additionally, there have been dramatic advances in bioengineering that have improved the durability of implants so that most THA patients will never need to be concerned that their hip implant will need to be revised. With appropriate preoperative planning and precise surgical techniques, THA is a very safe procedure. Despite this, complications can and do occur, and it is imperative that we minimize the risks of surgery.
 Intraoperative complications during THA can range from minor, such as a subtle crack seen during broaching of the femur that is easily remedied with a cerclage cable, to major, such as a catastrophic vascular injury that could lead to loss of life or limb. A thorough understanding of the bony structure of the pelvis and the surrounding neurovascular anatomy is necessary to minimize risks of injury. This knowledge allows for optimal surgical exposure with safe placement of retractors. Complications can be due to direct injury such as vascular injury, peripheral nerve injury, or intraoperative fracture. Additional complications can be due to poor technical technique that can lead to infection or instability and dislocation. It is important to note that the incidence of all these complications is higher in revision surgery and surgeons and patients should be appropriately prepared and counseled on the possibility of their occurrence.
Intraoperative complications during THA can range from minor, such as a subtle crack seen during broaching of the femur that is easily remedied with a cerclage cable, to major, such as a catastrophic vascular injury that could lead to loss of life or limb. A thorough understanding of the bony structure of the pelvis and the surrounding neurovascular anatomy is necessary to minimize risks of injury. This knowledge allows for optimal surgical exposure with safe placement of retractors. Complications can be due to direct injury such as vascular injury, peripheral nerve injury, or intraoperative fracture. Additional complications can be due to poor technical technique that can lead to infection or instability and dislocation. It is important to note that the incidence of all these complications is higher in revision surgery and surgeons and patients should be appropriately prepared and counseled on the possibility of their occurrence.
 Although it is out of the scope of this chapter, perioperative complications after THA unrelated to surgical technique are often preventable. A thorough medical and surgical preoperative evaluation along with appropriate patient selection can dramatically impact the surgical outcomes of THA patients. Patients with significant comorbidities should be medically optimized prior to surgery. Those with diabetes or significant renal or liver disease are at increased risk for surgical site infection (SSI). Patients with morbid obesity need greater surgical complexity and have higher risks of wound complications, infections, and fractures. It is also imperative that the orthopaedic surgeon be actively involved in managing patients postoperatively to ensure the medical and physical well-being of their patients.
Although it is out of the scope of this chapter, perioperative complications after THA unrelated to surgical technique are often preventable. A thorough medical and surgical preoperative evaluation along with appropriate patient selection can dramatically impact the surgical outcomes of THA patients. Patients with significant comorbidities should be medically optimized prior to surgery. Those with diabetes or significant renal or liver disease are at increased risk for surgical site infection (SSI). Patients with morbid obesity need greater surgical complexity and have higher risks of wound complications, infections, and fractures. It is also imperative that the orthopaedic surgeon be actively involved in managing patients postoperatively to ensure the medical and physical well-being of their patients.
 Additionally, complications can develop from the implants and bearing surfaces themselves. Discussion regarding tribology and implant materials is found in other chapters. However, many of the complications we most commonly deal with as orthopaedic joint reconstruction surgeons are not typically due to technical mistakes but occur due to decisions we make. Using the latest breakthrough in THA is often a dangerous proposition and many intelligent surgeons have succumbed to this pressure only to find that they were faced with unexpected complications.
Additionally, complications can develop from the implants and bearing surfaces themselves. Discussion regarding tribology and implant materials is found in other chapters. However, many of the complications we most commonly deal with as orthopaedic joint reconstruction surgeons are not typically due to technical mistakes but occur due to decisions we make. Using the latest breakthrough in THA is often a dangerous proposition and many intelligent surgeons have succumbed to this pressure only to find that they were faced with unexpected complications.
 This chapter consists of a review of the anatomy surrounding the hip and then discusses the following complications: vascular injury, peripheral nerve injury, periprosthetic fracture, periprosthetic joint infection (PJI), and dislocation.
This chapter consists of a review of the anatomy surrounding the hip and then discusses the following complications: vascular injury, peripheral nerve injury, periprosthetic fracture, periprosthetic joint infection (PJI), and dislocation.
ANATOMY
 The hip is a diarthrodial synovial joint consisting of articulation of the femoral head with the acetabulum. The acetabulum is a concave socket formed within the innominate bone of the pelvis. At puberty, the ilium, ischium, and pubis unite together to form the acetabulum. The acetabulum is surrounded by vital neurovascular structures, and their anatomic locations are critical to understand.
The hip is a diarthrodial synovial joint consisting of articulation of the femoral head with the acetabulum. The acetabulum is a concave socket formed within the innominate bone of the pelvis. At puberty, the ilium, ischium, and pubis unite together to form the acetabulum. The acetabulum is surrounded by vital neurovascular structures, and their anatomic locations are critical to understand.
 Posteriorly, the sacrospinous ligament extends from lateral border sacrum to ischial spine. The sacrotuberous ligament is larger and extends from dorsum and lateral border sacrum and posterior surface ilium to the ischial tuberosity. The attachments of the sacrospinous and sacrotuberous ligaments enclose the lesser and greater sciatic notches, respectively, forming the greater and lesser foramina. Structures passing through the greater sciatic foramina are the piriformis muscle; sciatic nerve; superior and inferior gluteal nerve and artery; internal pudendal nerve, artery, and vein; nerve to the obturator internus muscle; nerve to the quadratus femoris; and the posterior cutaneous nerve of the thigh. Structures passing through the lesser sciatic foramina are the tendon of the obturator internus, nerve to the obturator internus, pudendal nerve, and the internal pudendal artery. The superior gluteal nerve and artery exit above the piriformis. The inferior gluteal nerve and artery as well as the sciatic nerve exit posterior to the hip joint, inferior to the piriformis, and superior to the superior gemellus muscle (FIG 1).
Posteriorly, the sacrospinous ligament extends from lateral border sacrum to ischial spine. The sacrotuberous ligament is larger and extends from dorsum and lateral border sacrum and posterior surface ilium to the ischial tuberosity. The attachments of the sacrospinous and sacrotuberous ligaments enclose the lesser and greater sciatic notches, respectively, forming the greater and lesser foramina. Structures passing through the greater sciatic foramina are the piriformis muscle; sciatic nerve; superior and inferior gluteal nerve and artery; internal pudendal nerve, artery, and vein; nerve to the obturator internus muscle; nerve to the quadratus femoris; and the posterior cutaneous nerve of the thigh. Structures passing through the lesser sciatic foramina are the tendon of the obturator internus, nerve to the obturator internus, pudendal nerve, and the internal pudendal artery. The superior gluteal nerve and artery exit above the piriformis. The inferior gluteal nerve and artery as well as the sciatic nerve exit posterior to the hip joint, inferior to the piriformis, and superior to the superior gemellus muscle (FIG 1).
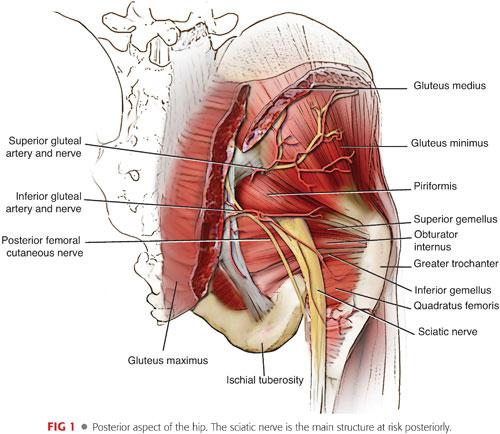
 The sciatic nerve is the largest nerve in the body. It arises from the sacral plexus (L4–S3) and exits through the greater sciatic foramen anterior and medial to the piriformis muscle. The sciatic nerve will split into the common peroneal and tibial nerves at the popliteal fossa. Proximally, the common peroneal portion of the sciatic nerve sits more laterally and has thinner connective tissue coverage. When the sciatic nerve is injured, it is typically the common peroneal branch that is affected.44 There are anatomic variations of the sciatic nerve as it emerges from the greater sciatic notch. Eighty-four percent of the time, it exits as one nerve distal to the piriformis. Sixteen percent of the time, there can be a branch passing proximally, posteriorly, or through the muscle.
The sciatic nerve is the largest nerve in the body. It arises from the sacral plexus (L4–S3) and exits through the greater sciatic foramen anterior and medial to the piriformis muscle. The sciatic nerve will split into the common peroneal and tibial nerves at the popliteal fossa. Proximally, the common peroneal portion of the sciatic nerve sits more laterally and has thinner connective tissue coverage. When the sciatic nerve is injured, it is typically the common peroneal branch that is affected.44 There are anatomic variations of the sciatic nerve as it emerges from the greater sciatic notch. Eighty-four percent of the time, it exits as one nerve distal to the piriformis. Sixteen percent of the time, there can be a branch passing proximally, posteriorly, or through the muscle.
 Anteriorly, the neurovascular structures enter the thigh under the inguinal ligament within the femoral triangle. The borders of the triangle are the sartorius laterally, pectineus medially, and the inguinal ligament superiorly. Within the triangle, from lateral to medial, are the femoral nerve, artery, and vein and the lymphatic vessels. The muscles that make up the floor of the femoral triangle, from lateral to medial, are the iliacus, psoas, pectineus, and adductor longus muscles. The lateral femoral cutaneous nerve lies on the surface of the iliacus muscle and exits the pelvis under the lateral attachment of the inguinal ligament. It can be found medially and travels superficially to the sartorius muscle about 6 to 8 cm below the anterior superior iliac spine. The femoral artery lies on the surface of the iliopsoas and delivers the profunda femoris, which supplies the anteromedial portion of the thigh, perforators, and vastus lateralis. The lateral and medial femoral circumflex arteries arise from the profunda femoris. The lateral femoral circumflex artery gives off an ascending branch toward the greater trochanter that is at risk during anterolateral approaches. The medial femoral circumflex artery, which provides the majority of blood flow to the femoral head, proceeds posteriorly between the iliopsoas and pectineus and lies anteriorly to the quadratus femoris (FIG 2).
Anteriorly, the neurovascular structures enter the thigh under the inguinal ligament within the femoral triangle. The borders of the triangle are the sartorius laterally, pectineus medially, and the inguinal ligament superiorly. Within the triangle, from lateral to medial, are the femoral nerve, artery, and vein and the lymphatic vessels. The muscles that make up the floor of the femoral triangle, from lateral to medial, are the iliacus, psoas, pectineus, and adductor longus muscles. The lateral femoral cutaneous nerve lies on the surface of the iliacus muscle and exits the pelvis under the lateral attachment of the inguinal ligament. It can be found medially and travels superficially to the sartorius muscle about 6 to 8 cm below the anterior superior iliac spine. The femoral artery lies on the surface of the iliopsoas and delivers the profunda femoris, which supplies the anteromedial portion of the thigh, perforators, and vastus lateralis. The lateral and medial femoral circumflex arteries arise from the profunda femoris. The lateral femoral circumflex artery gives off an ascending branch toward the greater trochanter that is at risk during anterolateral approaches. The medial femoral circumflex artery, which provides the majority of blood flow to the femoral head, proceeds posteriorly between the iliopsoas and pectineus and lies anteriorly to the quadratus femoris (FIG 2).
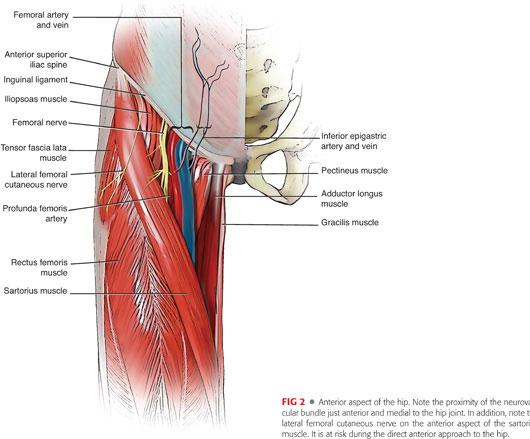
 The femoral nerve (L2–L4) lies on the psoas muscle belly and passes through the femoral triangle as the most lateral structure. It is at risk to injury during the approach to the hip with retractors rather than with reaming or drilling.
The femoral nerve (L2–L4) lies on the psoas muscle belly and passes through the femoral triangle as the most lateral structure. It is at risk to injury during the approach to the hip with retractors rather than with reaming or drilling.
 Inferiorly, the obturator nerve and artery exit the pelvis via the obturator canal. The posterior branches run inferiorly to the transverse acetabular ligament.
Inferiorly, the obturator nerve and artery exit the pelvis via the obturator canal. The posterior branches run inferiorly to the transverse acetabular ligament.
 Superiorly, the superior gluteal nerve and artery run between the gluteus minimus and medius to the tensor fascia lata. Branches of the superior gluteal nerve can pass within 5 cm to the greater trochanter.
Superiorly, the superior gluteal nerve and artery run between the gluteus minimus and medius to the tensor fascia lata. Branches of the superior gluteal nerve can pass within 5 cm to the greater trochanter.
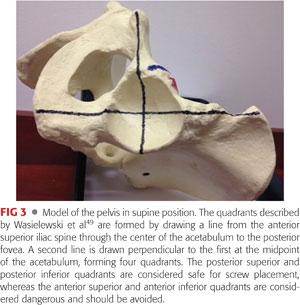
 Because it is common to place screws for cementless acetabular component fixation during THA, it is necessary to understand the anatomy deep to the acetabulum as well. Running along the inner cortical surface of the acetabulum are the external iliac, femoral and obturator vessels, and nerves. Wasielewski et al49 described a four-quadrant system for safe placement of acetabular screws (FIG 3). The quadrants are formed by drawing a line from the anterior superior iliac spine through the center of the acetabulum to the posterior fovea, forming acetabular halves. A second line is drawn perpendicular to the first at the midpoint of the acetabulum, forming four quadrants. The posterior superior and posterior inferior quadrants contain the best bone stock and are considered safe for screw placement. The anterior superior and anterior inferior quadrants are considered dangerous and should be avoided due to risks to the external iliac and obturator vessels and nerves, respectively.
Because it is common to place screws for cementless acetabular component fixation during THA, it is necessary to understand the anatomy deep to the acetabulum as well. Running along the inner cortical surface of the acetabulum are the external iliac, femoral and obturator vessels, and nerves. Wasielewski et al49 described a four-quadrant system for safe placement of acetabular screws (FIG 3). The quadrants are formed by drawing a line from the anterior superior iliac spine through the center of the acetabulum to the posterior fovea, forming acetabular halves. A second line is drawn perpendicular to the first at the midpoint of the acetabulum, forming four quadrants. The posterior superior and posterior inferior quadrants contain the best bone stock and are considered safe for screw placement. The anterior superior and anterior inferior quadrants are considered dangerous and should be avoided due to risks to the external iliac and obturator vessels and nerves, respectively.
Vascular Injury
 Vascular injury is one of the most catastrophic complications in THA and can lead to loss of life or limb. The reported incidence of injury is between 0.08% and 0.25%.8,16,33,35 Sharma et al46 reported an overall mortality rate of 7% and a 15% incidence of limb loss in patients with vascular injury after THA. The most important treatment for vascular injury is prevention. Surgeons must be keenly aware of the surrounding anatomy and have a high index of suspicion if excessive bleeding is encountered or unexplained hypotension exists during the surgery or postoperatively. Besides the obvious monitoring for acute hypotension performed by the anesthesiologist, careful monitoring of patients for changes in vital signs in the recovery room postoperatively is paramount to prompt recognition of a vascular injury. Although direct injury to vessels can occur that would lead to hemorrhage, indirect injury can occur during routine manipulation of the limb as vessels can be stretched, torqued, and compressed. Strain on the vessels can cause intimal tears and flaps that can fully clot off arteries.
Vascular injury is one of the most catastrophic complications in THA and can lead to loss of life or limb. The reported incidence of injury is between 0.08% and 0.25%.8,16,33,35 Sharma et al46 reported an overall mortality rate of 7% and a 15% incidence of limb loss in patients with vascular injury after THA. The most important treatment for vascular injury is prevention. Surgeons must be keenly aware of the surrounding anatomy and have a high index of suspicion if excessive bleeding is encountered or unexplained hypotension exists during the surgery or postoperatively. Besides the obvious monitoring for acute hypotension performed by the anesthesiologist, careful monitoring of patients for changes in vital signs in the recovery room postoperatively is paramount to prompt recognition of a vascular injury. Although direct injury to vessels can occur that would lead to hemorrhage, indirect injury can occur during routine manipulation of the limb as vessels can be stretched, torqued, and compressed. Strain on the vessels can cause intimal tears and flaps that can fully clot off arteries.
 Generally, the most common vessels at risk during THA are the external iliac artery and vein, femoral artery and vein, profunda femoris artery and vein, and obturator artery and vein. All of these vessels can be at risk regardless of the approach to the hip joint as they come into play mainly during exposure of the acetabulum. The risk of vascular injury increases substantially during conversion and revision procedures. The need to remove implants and/or cement along with the presence of scar tissue, heterotopic ossification, and bony abnormalities all contribute to the higher risk. Additionally, patients with a history of trauma, previous radiation, congenital malformation, and existing peripheral vascular disease are at increased risk for vascular injury.
Generally, the most common vessels at risk during THA are the external iliac artery and vein, femoral artery and vein, profunda femoris artery and vein, and obturator artery and vein. All of these vessels can be at risk regardless of the approach to the hip joint as they come into play mainly during exposure of the acetabulum. The risk of vascular injury increases substantially during conversion and revision procedures. The need to remove implants and/or cement along with the presence of scar tissue, heterotopic ossification, and bony abnormalities all contribute to the higher risk. Additionally, patients with a history of trauma, previous radiation, congenital malformation, and existing peripheral vascular disease are at increased risk for vascular injury.
 Precise understanding of the anatomy surrounding the hip is critically important for a safe exposure. Protecting the vessels with appropriate retractors and avoidance of straying into the soft tissue can help avoid injury. Direct injury can occur from a variety of sources: scalpels, osteotomes, retractors, bone fragments, reamers, drills, screws, cement, and the implants themselves. Careful dissection and placement of retractors during exposure through a variety of approaches leads to a safe outcome. It is also the surgeon’s responsibility to ensure retractors held by their assistants do not drift or migrate into unsafe positions.
Precise understanding of the anatomy surrounding the hip is critically important for a safe exposure. Protecting the vessels with appropriate retractors and avoidance of straying into the soft tissue can help avoid injury. Direct injury can occur from a variety of sources: scalpels, osteotomes, retractors, bone fragments, reamers, drills, screws, cement, and the implants themselves. Careful dissection and placement of retractors during exposure through a variety of approaches leads to a safe outcome. It is also the surgeon’s responsibility to ensure retractors held by their assistants do not drift or migrate into unsafe positions.
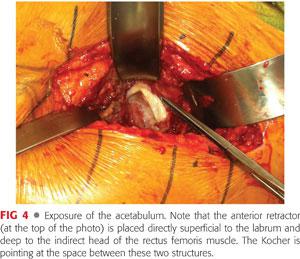
 Anteriorly, the external iliac and femoral vessels are at risk. They pass anteriorly to the hip joint on the surface of the iliopsoas muscle. Cadaveric dissection has shown that the femoral vessels lie within an inch of the anterior capsule of the hip.11 Therefore, it is critical that the anterior retractor be placed directly onto the anterior acetabular wall, superficial to any labral tissue but deep to the indirect head of the rectus femoris and the iliopsoas muscles (FIG 4). If it is noted that the retractor has pierced into the muscle, it should be carefully removed and visualized for blood extravasation. Inferiorly, the obturator vessels are at risk during dissection, retractor placement, or implant/cement removal. The profunda femoris vessels are at risk during instrumentation of the femur, especially when placing cerclage wires.1 However, the major risk of vascular injury during THA comes from the use of acetabular screws. As indicated in the Anatomy section, the quadrant system described by Wasielewski et al49 is crucial to avoiding injury to the external iliac and obturator vessels when drilling and inserting acetabular screws. Screws should only be placed in the posterior superior and posterior inferior quadrants (see FIG 3).
Anteriorly, the external iliac and femoral vessels are at risk. They pass anteriorly to the hip joint on the surface of the iliopsoas muscle. Cadaveric dissection has shown that the femoral vessels lie within an inch of the anterior capsule of the hip.11 Therefore, it is critical that the anterior retractor be placed directly onto the anterior acetabular wall, superficial to any labral tissue but deep to the indirect head of the rectus femoris and the iliopsoas muscles (FIG 4). If it is noted that the retractor has pierced into the muscle, it should be carefully removed and visualized for blood extravasation. Inferiorly, the obturator vessels are at risk during dissection, retractor placement, or implant/cement removal. The profunda femoris vessels are at risk during instrumentation of the femur, especially when placing cerclage wires.1 However, the major risk of vascular injury during THA comes from the use of acetabular screws. As indicated in the Anatomy section, the quadrant system described by Wasielewski et al49 is crucial to avoiding injury to the external iliac and obturator vessels when drilling and inserting acetabular screws. Screws should only be placed in the posterior superior and posterior inferior quadrants (see FIG 3).
 During revision surgery, safe component removal is also paramount. If implants or cement have migrated into the pelvis, vascular surgical support should be on hand during the procedure. Lewallen24 has suggested that intrapelvic mobilization of vessels be performed prior to hip exposure. Another option is to place an intravascular balloon sheath in the common femoral artery prior to hip exposure. It could be inflated to tamponade the vessel if major bleeding is encountered on intrapelvic implant removal. Emergency vascular surgery intervention for repair could be performed in a somewhat managed fashion while the bleeding is controlled.
During revision surgery, safe component removal is also paramount. If implants or cement have migrated into the pelvis, vascular surgical support should be on hand during the procedure. Lewallen24 has suggested that intrapelvic mobilization of vessels be performed prior to hip exposure. Another option is to place an intravascular balloon sheath in the common femoral artery prior to hip exposure. It could be inflated to tamponade the vessel if major bleeding is encountered on intrapelvic implant removal. Emergency vascular surgery intervention for repair could be performed in a somewhat managed fashion while the bleeding is controlled.
 The hallmark signs of vascular injury are sudden hemorrhage (pulsatile if arterial), hypotension, tachycardia, decreasing hemoglobin, low urine output, and/or thigh or abdominal distention. If massive bleeding occurs, apply direct pressure to the area and obtain immediate vascular assistance. The anesthesiologist should administer blood products immediately. If the patient is hemodynamically stable, angiography and transcatheter embolization can be performed. If the patient is unstable, immediate exploration must be performed.3
The hallmark signs of vascular injury are sudden hemorrhage (pulsatile if arterial), hypotension, tachycardia, decreasing hemoglobin, low urine output, and/or thigh or abdominal distention. If massive bleeding occurs, apply direct pressure to the area and obtain immediate vascular assistance. The anesthesiologist should administer blood products immediately. If the patient is hemodynamically stable, angiography and transcatheter embolization can be performed. If the patient is unstable, immediate exploration must be performed.3
 Early recognition and prompt action is necessary (whether intraoperatively or in the postoperative period) to save the patient’s life and/or limb in the case of vascular complications. These unfortunate outcomes are real and a high index of suspicion is critical in avoiding their occurrence.
Early recognition and prompt action is necessary (whether intraoperatively or in the postoperative period) to save the patient’s life and/or limb in the case of vascular complications. These unfortunate outcomes are real and a high index of suspicion is critical in avoiding their occurrence.
Peripheral Nerve Injury
 The overall incidence of major nerve injury after primary THA is 0.6% to 2.9% regardless of approach.12,20,38,44 The incidence has been reported from 3.2% to7.6% after revision THA, and nerve injury was found in 5.2% of patients undergoing primary THA for congenital dislocation or dysplasia of the hip.22,41,44 Additionally, it is generally accepted that lengthening the lower extremity greater than 4 cm is a risk factor for neurapraxia13; however, other studies have shown that there is no relation between the amount of lengthening and nerve injury.38
The overall incidence of major nerve injury after primary THA is 0.6% to 2.9% regardless of approach.12,20,38,44 The incidence has been reported from 3.2% to7.6% after revision THA, and nerve injury was found in 5.2% of patients undergoing primary THA for congenital dislocation or dysplasia of the hip.22,41,44 Additionally, it is generally accepted that lengthening the lower extremity greater than 4 cm is a risk factor for neurapraxia13; however, other studies have shown that there is no relation between the amount of lengthening and nerve injury.38
 The two largest and most critical structures are the femoral and sciatic nerves (see FIGS 1 and 2). The superior gluteal and obturator nerves are also at risk, and, with the increasing use of the direct anterior approach, lateral femoral cutaneous neurapraxia is commonly seen. The most common motor nerve injury is to the sciatic nerve followed by the superior gluteal, obturator, and femoral nerves, respectively. The common peroneal portion of the sciatic nerve is more often injured due to its anatomic characteristics. As noted, the peroneal portion is more lateral and has thinner connective tissue coverage, which makes it more susceptible to injury.44
The two largest and most critical structures are the femoral and sciatic nerves (see FIGS 1 and 2). The superior gluteal and obturator nerves are also at risk, and, with the increasing use of the direct anterior approach, lateral femoral cutaneous neurapraxia is commonly seen. The most common motor nerve injury is to the sciatic nerve followed by the superior gluteal, obturator, and femoral nerves, respectively. The common peroneal portion of the sciatic nerve is more often injured due to its anatomic characteristics. As noted, the peroneal portion is more lateral and has thinner connective tissue coverage, which makes it more susceptible to injury.44
 Although nerve injuries can occur with any approach to the hip, certain nerves can be at higher risk depending on the dissection method. During acetabular exposure via the posterior approach, the sciatic nerve should be palpated and protected with a posterior Hohmann retractor. The position of the leg is important during exposure of the proximal femur. Increased tension on the sciatic nerve is seen with flexion, internal rotation, and adduction. Therefore, placing a soft roll under the thigh to avoid adduction during femoral preparation can decrease tension on the nerve. During the lateral, anterolateral, and direct anterior approaches, the femoral nerve is at risk with the anterior acetabular retractor. As mentioned earlier, precise placement along the bone and deep to the indirect head of the rectus is important to protect the nerve. Specifically with the direct anterior approach, the lateral femoral cutaneous nerve is affected frequently. In one report, 81% of patients reported neurapraxia; however, this did not lead to any functional limitations.17 The superior gluteal nerve is at risk during the direct lateral (Hardinge) approach. The nerve passes within 5 cm of the tip of the greater trochanter; therefore, avoidance of extending the split in the gluteus medius more than 5 cm should protect the nerve.
Although nerve injuries can occur with any approach to the hip, certain nerves can be at higher risk depending on the dissection method. During acetabular exposure via the posterior approach, the sciatic nerve should be palpated and protected with a posterior Hohmann retractor. The position of the leg is important during exposure of the proximal femur. Increased tension on the sciatic nerve is seen with flexion, internal rotation, and adduction. Therefore, placing a soft roll under the thigh to avoid adduction during femoral preparation can decrease tension on the nerve. During the lateral, anterolateral, and direct anterior approaches, the femoral nerve is at risk with the anterior acetabular retractor. As mentioned earlier, precise placement along the bone and deep to the indirect head of the rectus is important to protect the nerve. Specifically with the direct anterior approach, the lateral femoral cutaneous nerve is affected frequently. In one report, 81% of patients reported neurapraxia; however, this did not lead to any functional limitations.17 The superior gluteal nerve is at risk during the direct lateral (Hardinge) approach. The nerve passes within 5 cm of the tip of the greater trochanter; therefore, avoidance of extending the split in the gluteus medius more than 5 cm should protect the nerve.
 Similar to vascular injuries, damage to peripheral nerves can occur from direct mechanical compression, distraction, or thermal injury. If deformity exists, or if excessive scar, heterotopic ossification, prior radiation, or previous hardware is present, the risk of injury is increased. Understanding of the surrounding anatomy is again critical to protecting these structures. During revision THA, entrapment of nerves with cerclage wires has been reported.28 The femoral, sciatic, and obturator nerves can be adherent or encased in acetabular bone cement.27 If there is posterior wall deficiency, the sciatic nerve can adhere to a porous-coated acetabular component and be at risk with removal of the implant. However, when a nerve injury is diagnosed postoperatively, it is frequently impossible to determine its origin. The cause of nerve injury after THA is still poorly understood and is likely multifactorial.
Similar to vascular injuries, damage to peripheral nerves can occur from direct mechanical compression, distraction, or thermal injury. If deformity exists, or if excessive scar, heterotopic ossification, prior radiation, or previous hardware is present, the risk of injury is increased. Understanding of the surrounding anatomy is again critical to protecting these structures. During revision THA, entrapment of nerves with cerclage wires has been reported.28 The femoral, sciatic, and obturator nerves can be adherent or encased in acetabular bone cement.27 If there is posterior wall deficiency, the sciatic nerve can adhere to a porous-coated acetabular component and be at risk with removal of the implant. However, when a nerve injury is diagnosed postoperatively, it is frequently impossible to determine its origin. The cause of nerve injury after THA is still poorly understood and is likely multifactorial.
 Patients who have had damage to a peripheral nerve will present with motor and sensory deficits in the anatomic distribution and dermatome of that particular nerve. If a direct injury to the nerve has occurred, the nerve deficit will typically be evident immediately after surgery. However, nerve palsies can occur for up to 36 hours or more postoperatively. Neurovascular monitoring of all postoperative THA patients is vitally important for the first 2 days after surgery. Immediate evaluation in the recovery room is paramount. Early nerve palsy can be the first sign of a vascular injury and this should be ruled out immediately.
Patients who have had damage to a peripheral nerve will present with motor and sensory deficits in the anatomic distribution and dermatome of that particular nerve. If a direct injury to the nerve has occurred, the nerve deficit will typically be evident immediately after surgery. However, nerve palsies can occur for up to 36 hours or more postoperatively. Neurovascular monitoring of all postoperative THA patients is vitally important for the first 2 days after surgery. Immediate evaluation in the recovery room is paramount. Early nerve palsy can be the first sign of a vascular injury and this should be ruled out immediately.
 Accurate serial physical examination results regarding neurovascular status as well as wound assessment for drainage or swelling should be recorded. Immediate postoperative radiographs should be evaluated to assess the location of implants, screws, cerclage wires, cement, or bone fragments. Computed tomography (CT) scans or magnetic resonance imaging (MRI) should be obtained for further clarification of hardware position or the presence of an expanding hematoma if there is any uncertainty. An MRI of the lumbar spine (if spinal anesthesia was performed) should be obtained if there is concern for an epidural hematoma. Urgent evacuation of the hematoma has been shown to improve outcomes.7 If hardware is causing damage to a nerve, it should be removed.24 To prevent nerve injuries, intraoperative monitoring with somatosensory evoked potentials has been recommended by some; however, it is time consuming and can give a significant number of false-positive results.43 Additionally, in a study comparing THA cases using monitoring versus those that did not, the use of monitoring did not decrease the number of nerve injuries.41
Accurate serial physical examination results regarding neurovascular status as well as wound assessment for drainage or swelling should be recorded. Immediate postoperative radiographs should be evaluated to assess the location of implants, screws, cerclage wires, cement, or bone fragments. Computed tomography (CT) scans or magnetic resonance imaging (MRI) should be obtained for further clarification of hardware position or the presence of an expanding hematoma if there is any uncertainty. An MRI of the lumbar spine (if spinal anesthesia was performed) should be obtained if there is concern for an epidural hematoma. Urgent evacuation of the hematoma has been shown to improve outcomes.7 If hardware is causing damage to a nerve, it should be removed.24 To prevent nerve injuries, intraoperative monitoring with somatosensory evoked potentials has been recommended by some; however, it is time consuming and can give a significant number of false-positive results.43 Additionally, in a study comparing THA cases using monitoring versus those that did not, the use of monitoring did not decrease the number of nerve injuries.41
Prognosis
 Neurologic recovery after injury is variable. Improvement may occur for 2 to 3 years and is unpredictable. Where some authors suggest that recovery is associated with the extent of injury, others found no correlation.24,38 Direct injury to nerves tends to have a better outcome than stretch injuries. Femoral nerve palsy improves better than sciatic nerve palsies and isolated peroneal palsies tend to do better than complete sciatic nerve palsies.13,50 If the nerve palsy involves only sensory and not motor, or if the motor function begins to return in the early postoperative period, then prognosis is good for recovery.44
Neurologic recovery after injury is variable. Improvement may occur for 2 to 3 years and is unpredictable. Where some authors suggest that recovery is associated with the extent of injury, others found no correlation.24,38 Direct injury to nerves tends to have a better outcome than stretch injuries. Femoral nerve palsy improves better than sciatic nerve palsies and isolated peroneal palsies tend to do better than complete sciatic nerve palsies.13,50 If the nerve palsy involves only sensory and not motor, or if the motor function begins to return in the early postoperative period, then prognosis is good for recovery.44
Intraoperative Periprosthetic Fracture
 Periprosthetic fractures can be challenging for both inexperienced and seasoned orthopaedic surgeons. Intraoperative femur fractures occur in approximately 0.1% to 3.2% of primary cemented THA and 3% to 5.4% of primary uncemented THA. The rate of fracture during revision surgery is significantly higher with some reporting an incidence of 30%.5,31 Intraoperative acetabular fractures are less common. Fractures can occur during bone preparation; when dislocating or relocating the hip, preparing the bone, inserting a new implant; or, if revision surgery, removal of preexisting implants.
Periprosthetic fractures can be challenging for both inexperienced and seasoned orthopaedic surgeons. Intraoperative femur fractures occur in approximately 0.1% to 3.2% of primary cemented THA and 3% to 5.4% of primary uncemented THA. The rate of fracture during revision surgery is significantly higher with some reporting an incidence of 30%.5,31 Intraoperative acetabular fractures are less common. Fractures can occur during bone preparation; when dislocating or relocating the hip, preparing the bone, inserting a new implant; or, if revision surgery, removal of preexisting implants.
 Patients with osteoporosis, deformity, osteolysis, bone defects, and small size are at increased risk for fracture. Sheth et al47 reviewed over 5000 THA patients and found female gender, increasing age, and hip dysplasia to have an increased risk for fracture. Additional risk factors include a diagnosis of rheumatoid arthritis, Paget disease, and chronic steroid usage.26 Surgical techniques and the design of implants can also increase the risk of intraoperative fracture. Minimally invasive surgical (MIS) approaches are associated with a higher risk of intraoperative fracture.18,19 As with many complications, understanding the risk factors for fracture can help to prevent them from occurring.
Patients with osteoporosis, deformity, osteolysis, bone defects, and small size are at increased risk for fracture. Sheth et al47 reviewed over 5000 THA patients and found female gender, increasing age, and hip dysplasia to have an increased risk for fracture. Additional risk factors include a diagnosis of rheumatoid arthritis, Paget disease, and chronic steroid usage.26 Surgical techniques and the design of implants can also increase the risk of intraoperative fracture. Minimally invasive surgical (MIS) approaches are associated with a higher risk of intraoperative fracture.18,19 As with many complications, understanding the risk factors for fracture can help to prevent them from occurring.
Fractures of the Femur
 The most commonly used system that describes periprosthetic femur fracture patterns and can guide treatment is the Vancouver classification described by Masri et al.29 This system is based on the location of the fracture and the fixation of the stem. Type A fractures are stable fractures involving only the greater trochanter (AG) or the lesser trochanter (AL). Type B fractures occur around the stem or just below its tip. Type B1 indicates a fracture involves the stem where the stem remains well fixed. Type B2 also involves the stem; however, the stem is loose. Type B3 fractures involve a loose stem with poor available bone stock. Type C fractures occur distally beyond the stem tip and therefore do not affect the fixation of the implant. The Vancouver system for intraoperative fractures keeps the same anatomic definitions (types A, B, C); however, additional subtypes were added to indicate the quality of the fracture. Subtype 1 refers to a simple cortical perforation; subtype 2 represents a nondisplaced, linear fracture; and subtype 3 indicates a displaced or unstable fracture.10
The most commonly used system that describes periprosthetic femur fracture patterns and can guide treatment is the Vancouver classification described by Masri et al.29 This system is based on the location of the fracture and the fixation of the stem. Type A fractures are stable fractures involving only the greater trochanter (AG) or the lesser trochanter (AL). Type B fractures occur around the stem or just below its tip. Type B1 indicates a fracture involves the stem where the stem remains well fixed. Type B2 also involves the stem; however, the stem is loose. Type B3 fractures involve a loose stem with poor available bone stock. Type C fractures occur distally beyond the stem tip and therefore do not affect the fixation of the implant. The Vancouver system for intraoperative fractures keeps the same anatomic definitions (types A, B, C); however, additional subtypes were added to indicate the quality of the fracture. Subtype 1 refers to a simple cortical perforation; subtype 2 represents a nondisplaced, linear fracture; and subtype 3 indicates a displaced or unstable fracture.10
Surgical risks
 Surgical exposure is critical in performing a safe, well-functioning hip replacement. If exposure is compromised due to MIS, morbid obesity, or muscular body habitus, the risk of periprosthetic fracture increases.18 Straining to access the femoral canal can cause perforation or fracture during reaming, broaching, or implantation. The use of cementless implants inherently increases the risk of fracture. In an effort to gain press-fit stability, the strength of the underlying bone can be overcome. Stem design has been shown to affect fracture rates. Revision of femoral components carries the highest risk of fracture. The weakened bone is at risk due to osteolysis or stress shielding. Fracture can occur due to implant or cement removal. The presence of an endosteal pedestal can lead to eccentric reaming or broaching that can cause perforation. Long straight reamers and implants inserted into the curved bow of the femur also increase the risk of perforation and fracture.
Surgical exposure is critical in performing a safe, well-functioning hip replacement. If exposure is compromised due to MIS, morbid obesity, or muscular body habitus, the risk of periprosthetic fracture increases.18 Straining to access the femoral canal can cause perforation or fracture during reaming, broaching, or implantation. The use of cementless implants inherently increases the risk of fracture. In an effort to gain press-fit stability, the strength of the underlying bone can be overcome. Stem design has been shown to affect fracture rates. Revision of femoral components carries the highest risk of fracture. The weakened bone is at risk due to osteolysis or stress shielding. Fracture can occur due to implant or cement removal. The presence of an endosteal pedestal can lead to eccentric reaming or broaching that can cause perforation. Long straight reamers and implants inserted into the curved bow of the femur also increase the risk of perforation and fracture.
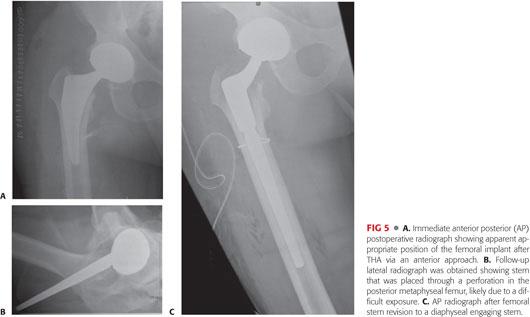
 During surgery, a high index of suspicion and thorough exposure and inspection of the surrounding bone are fundamental to immediate diagnosis and treatment of intraoperative fractures. Fractures can occur during impaction or removal of implants or during reaming, broaching, or dislocation of the hip. During impaction of the implants, any of the following subtleties can indicate that a fracture has occurred: sudden change in the position of the implant, sudden lack of resistance to impaction, or a change in the audible pitch made when impacting the implant. Wedge-tapered stems have been found to have the highest rate of fracture when compared with metaphyseal or diaphyseal engaging stems or cemented stems.30 Cortical perforations can also occur during reaming, broaching, or implant insertion. The risk of perforation is significantly increased when exposure is not adequate (due to poor exposure by the surgeon and/or with morbidly obese or very muscular patients) and reaming or insertion is forced (FIG 5).
During surgery, a high index of suspicion and thorough exposure and inspection of the surrounding bone are fundamental to immediate diagnosis and treatment of intraoperative fractures. Fractures can occur during impaction or removal of implants or during reaming, broaching, or dislocation of the hip. During impaction of the implants, any of the following subtleties can indicate that a fracture has occurred: sudden change in the position of the implant, sudden lack of resistance to impaction, or a change in the audible pitch made when impacting the implant. Wedge-tapered stems have been found to have the highest rate of fracture when compared with metaphyseal or diaphyseal engaging stems or cemented stems.30 Cortical perforations can also occur during reaming, broaching, or implant insertion. The risk of perforation is significantly increased when exposure is not adequate (due to poor exposure by the surgeon and/or with morbidly obese or very muscular patients) and reaming or insertion is forced (FIG 5).
Treatment
 Appropriate treatment of recognized fractures can lead to very successful outcomes. Identifying the fracture, full exposure to determine its extent, and stable internal fixation are all imperative for appropriate treatment. If a fracture is suspected, remove the implant, broach, or reamer, and expose the fracture fully to obtain direct visualization. If there is any difficulty determining the full extent of propagation, fluoroscopy or radiographs should be used.
Appropriate treatment of recognized fractures can lead to very successful outcomes. Identifying the fracture, full exposure to determine its extent, and stable internal fixation are all imperative for appropriate treatment. If a fracture is suspected, remove the implant, broach, or reamer, and expose the fracture fully to obtain direct visualization. If there is any difficulty determining the full extent of propagation, fluoroscopy or radiographs should be used.
 Greater trochanteric fractures can be treated with trochanteric claws and/or cerclage cables. Nondisplaced linear fractures around the metaphysis can be stabilized with a cerclage cable and reinsertion of the stem. Excellent results have been shown with this technique, with 100% femoral component survival up to 16 years.4 A displaced fracture can be reduced and fixed with cerclage cables; however, a diaphyseal stem should be used. A diaphyseal fracture can be treated with standard reduction and internal fixation with a plate, screws, and cables if the stem is stable. However, if the stem is unstable, a long-stemmed implant and fixation is necessary. If the bone stock is poor, cortical allograft can be used as supplemental fixation.
Greater trochanteric fractures can be treated with trochanteric claws and/or cerclage cables. Nondisplaced linear fractures around the metaphysis can be stabilized with a cerclage cable and reinsertion of the stem. Excellent results have been shown with this technique, with 100% femoral component survival up to 16 years.4 A displaced fracture can be reduced and fixed with cerclage cables; however, a diaphyseal stem should be used. A diaphyseal fracture can be treated with standard reduction and internal fixation with a plate, screws, and cables if the stem is stable. However, if the stem is unstable, a long-stemmed implant and fixation is necessary. If the bone stock is poor, cortical allograft can be used as supplemental fixation.
 Cortical perforations can be identified with curettes, curved hemostats, or intraoperative fluoroscopy/radiographs. If a perforation is noted, full exposure of the defect is necessary to ensure no fracture line has propagated distally beyond the defect. A long-stem implant should be used to bypass the perforation by a distance of two cortical diameters. A cerclage cable can be placed around the bone distal to the perforation to ensure no propagation while reaming, broaching, and inserting the implant. If the perforation is substantial, consider augmenting the repair with a cortical strut graft placed over the defect.
Cortical perforations can be identified with curettes, curved hemostats, or intraoperative fluoroscopy/radiographs. If a perforation is noted, full exposure of the defect is necessary to ensure no fracture line has propagated distally beyond the defect. A long-stem implant should be used to bypass the perforation by a distance of two cortical diameters. A cerclage cable can be placed around the bone distal to the perforation to ensure no propagation while reaming, broaching, and inserting the implant. If the perforation is substantial, consider augmenting the repair with a cortical strut graft placed over the defect.
 Fractures can be missed intraoperatively. Therefore, all patients should receive postoperative radiographs in the recovery room. Besides evaluation of the implant for positioning, sizing, etc., these radiographs should be carefully scrutinized for the presence of a periprosthetic fracture. If a fracture line is seen on the postoperative radiograph, the fracture and stem are evaluated for stability. If both appear stable, protected weight bearing can be ordered. Close radiographic follow-up is necessary to monitor for displacement. If the fracture or implant are initially unstable or become unstable during follow-up, revision surgery should be performed.
Fractures can be missed intraoperatively. Therefore, all patients should receive postoperative radiographs in the recovery room. Besides evaluation of the implant for positioning, sizing, etc., these radiographs should be carefully scrutinized for the presence of a periprosthetic fracture. If a fracture line is seen on the postoperative radiograph, the fracture and stem are evaluated for stability. If both appear stable, protected weight bearing can be ordered. Close radiographic follow-up is necessary to monitor for displacement. If the fracture or implant are initially unstable or become unstable during follow-up, revision surgery should be performed.
Fractures of the Acetabulum
 Intraoperative periprosthetic acetabular fractures are uncommon; however, with increased use of press-fit cups, the incidence is increasing. To achieve adequate fixation and stability, acetabular components are designed to create plastic deformation of the host bone when impacted. Commonly, the components are slightly larger than the amount that is reamed (ie, under-reaming) and this can lead to fracture when impacting. Peterson and Lewallen39 classified periprosthetic acetabular fractures into two types: implant stable (type 1) and implant unstable (type 2).
Intraoperative periprosthetic acetabular fractures are uncommon; however, with increased use of press-fit cups, the incidence is increasing. To achieve adequate fixation and stability, acetabular components are designed to create plastic deformation of the host bone when impacted. Commonly, the components are slightly larger than the amount that is reamed (ie, under-reaming) and this can lead to fracture when impacting. Peterson and Lewallen39 classified periprosthetic acetabular fractures into two types: implant stable (type 1) and implant unstable (type 2).
Surgical risks
 Screws are often used to augment fixation of cementless acetabular components. Due to concerns regarding fretting and osteolysis with screws, the use of oversized components that are press-fit into an under-reamed acetabulum can be used. This can lead to fracture during component insertion, especially in osteoporotic bone. Curtis et al9 found a significant risk of fracture when under-reaming a hemispherical, uncemented cup by 4 mm. Sharkey et al45 reported on 13 cases with acetabular fracture and found osteoporosis and component oversizing to be risk factors for fracture. The authors recommended line-to-line reaming in patients with osteoporosis. As with the femur, the risk of fracture is increased during revision surgery. Removal of a well-fixed cup can lead to damage of the surrounding bone stock. Also, impacting implants into bone that is significantly weakened by osteolysis can lead to fracture and loss of fixation.
Screws are often used to augment fixation of cementless acetabular components. Due to concerns regarding fretting and osteolysis with screws, the use of oversized components that are press-fit into an under-reamed acetabulum can be used. This can lead to fracture during component insertion, especially in osteoporotic bone. Curtis et al9 found a significant risk of fracture when under-reaming a hemispherical, uncemented cup by 4 mm. Sharkey et al45 reported on 13 cases with acetabular fracture and found osteoporosis and component oversizing to be risk factors for fracture. The authors recommended line-to-line reaming in patients with osteoporosis. As with the femur, the risk of fracture is increased during revision surgery. Removal of a well-fixed cup can lead to damage of the surrounding bone stock. Also, impacting implants into bone that is significantly weakened by osteolysis can lead to fracture and loss of fixation.
Treatment
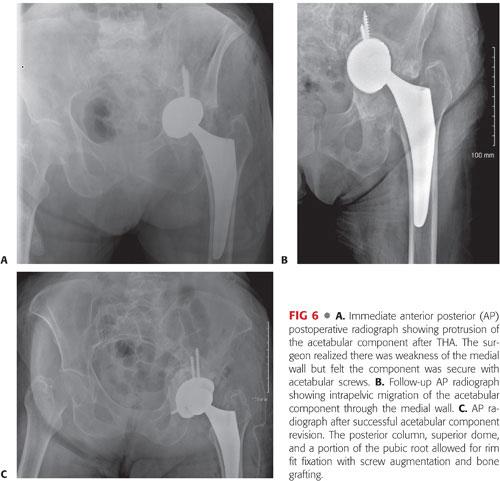
 The treatment of an intraoperative acetabular fracture is dependent on fixation of the implant. If a fracture is identified while impacting the component and the component is well fixed, the implant can remain in place and the fracture can be treated with protected weight bearing. If there are screw holes, screws can be added to augment fixation (FIG 6). If the implant is unstable, it must be removed and the surrounding bone stock assessed. Bone grafting of defects and impacting a multihole revision shell can achieve fixation in most fractures, but plate fixation prior to cup insertion may be necessary. Despite these recommendations, these patients need to be followed carefully. Peterson and Lewallen39 noted that even when the fracture heals, 80% of the cases in their series eventually required revision.
The treatment of an intraoperative acetabular fracture is dependent on fixation of the implant. If a fracture is identified while impacting the component and the component is well fixed, the implant can remain in place and the fracture can be treated with protected weight bearing. If there are screw holes, screws can be added to augment fixation (FIG 6). If the implant is unstable, it must be removed and the surrounding bone stock assessed. Bone grafting of defects and impacting a multihole revision shell can achieve fixation in most fractures, but plate fixation prior to cup insertion may be necessary. Despite these recommendations, these patients need to be followed carefully. Peterson and Lewallen39 noted that even when the fracture heals, 80% of the cases in their series eventually required revision.
Preventing Intraoperative Fractures
 As always, preventing intraoperative fractures from happening is the best course. Preoperative preparations, including patient evaluation for risk factors, templating of sizes, and determining the type of implant, will help avoid periprosthetic fracture. When faced with a high-risk patient with osteoporotic bone, cementing the femoral and/or acetabular components is a reliable alternative that has a much lower risk of fracture. If press-fit implants are used, performing line-to-line reaming of the acetabular component and avoiding under-reaming can lessen the risk of fracture.45 It is helpful to use multiple senses when inserting press-fit femoral implants. The use of auditory, visual, and tactile senses can ensure appropriate broaching and implantation of the implants. The force of impacting press-fit femoral stems and frequency analysis of hammering sound data have been studied. Finite element analysis has shown that a decrease in hammering sound frequency indicates adequate hammering has occurred and any further hammering risks fracture.42 Therefore, when the broach stops moving and a change in pitch is heard when impacting, no further impaction should occur.
As always, preventing intraoperative fractures from happening is the best course. Preoperative preparations, including patient evaluation for risk factors, templating of sizes, and determining the type of implant, will help avoid periprosthetic fracture. When faced with a high-risk patient with osteoporotic bone, cementing the femoral and/or acetabular components is a reliable alternative that has a much lower risk of fracture. If press-fit implants are used, performing line-to-line reaming of the acetabular component and avoiding under-reaming can lessen the risk of fracture.45 It is helpful to use multiple senses when inserting press-fit femoral implants. The use of auditory, visual, and tactile senses can ensure appropriate broaching and implantation of the implants. The force of impacting press-fit femoral stems and frequency analysis of hammering sound data have been studied. Finite element analysis has shown that a decrease in hammering sound frequency indicates adequate hammering has occurred and any further hammering risks fracture.42 Therefore, when the broach stops moving and a change in pitch is heard when impacting, no further impaction should occur.
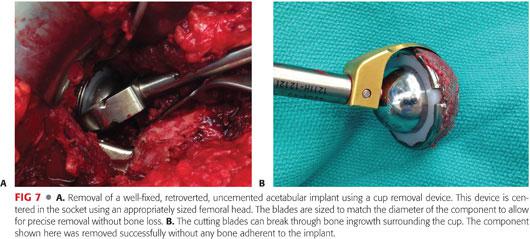
 During revision THA, removal of well-fixed cemented or cementless implants requires special preparation to avoid fracture. A review of these techniques is found in Chapter 24. Specialized instruments can be used such as cup-out systems that use a precisely curved blade to remove fixed, cementless acetabular components (FIG 7). On the femoral side, burrs, flexible osteotomes, and ultrasonic cement removal systems can help remove fixed stems. However, if a stem is well fixed, an extended trochanteric osteotomy should be performed. This allows for controlled removal of implants and cement and lessens the risk of a comminuted, unstable fracture.
During revision THA, removal of well-fixed cemented or cementless implants requires special preparation to avoid fracture. A review of these techniques is found in Chapter 24. Specialized instruments can be used such as cup-out systems that use a precisely curved blade to remove fixed, cementless acetabular components (FIG 7). On the femoral side, burrs, flexible osteotomes, and ultrasonic cement removal systems can help remove fixed stems. However, if a stem is well fixed, an extended trochanteric osteotomy should be performed. This allows for controlled removal of implants and cement and lessens the risk of a comminuted, unstable fracture.
 Recognition of fractures intraoperatively is paramount. During surgery, if a fracture is recognized, appropriate treatment can be taken to ensure the components are stable, whether it is on the femoral or acetabular side. A treated small, nondisplaced fracture will likely go on to heal without issue, but an unrecognized fracture could lead to unnecessary morbidity and additional surgery. If there is any suspicion for fracture, intraoperative fluoroscopy should be used, especially in high-risk patients.
Recognition of fractures intraoperatively is paramount. During surgery, if a fracture is recognized, appropriate treatment can be taken to ensure the components are stable, whether it is on the femoral or acetabular side. A treated small, nondisplaced fracture will likely go on to heal without issue, but an unrecognized fracture could lead to unnecessary morbidity and additional surgery. If there is any suspicion for fracture, intraoperative fluoroscopy should be used, especially in high-risk patients.
Periprosthetic Joint Infection
 PJI, an often-studied and dreaded complication of total joint arthroplasty (TJA), is addressed in Chapters 33 and 34. In conjunction with the Musculoskeletal Infection Society, an International Consensus Meeting was held in Philadelphia, Pennsylvania in 2013.34 The recommendations from this meeting were not level 5 expert advice; however, thousands of articles were studied, discussed, and agreed on by hundreds of experts from all over the world. The following is a list of the recommendations from this historical meeting that pertain specifically to preventing PJI.
PJI, an often-studied and dreaded complication of total joint arthroplasty (TJA), is addressed in Chapters 33 and 34. In conjunction with the Musculoskeletal Infection Society, an International Consensus Meeting was held in Philadelphia, Pennsylvania in 2013.34 The recommendations from this meeting were not level 5 expert advice; however, thousands of articles were studied, discussed, and agreed on by hundreds of experts from all over the world. The following is a list of the recommendations from this historical meeting that pertain specifically to preventing PJI.
Consensus Recommendations for Prevention of Periprosthetic Joint Infection34
 Avoid performing TJA in patients with an active infection. Active infection of the arthritic joint (septic arthritis); presence of septicemia; and/or presence of active local cutaneous, subcutaneous, or deep tissue infection are all significant risk factors predisposing patients to SSI or PJI and are contraindications to undertaking elective TJA.
Avoid performing TJA in patients with an active infection. Active infection of the arthritic joint (septic arthritis); presence of septicemia; and/or presence of active local cutaneous, subcutaneous, or deep tissue infection are all significant risk factors predisposing patients to SSI or PJI and are contraindications to undertaking elective TJA.
 Dental hygiene is important. All patients undergoing elective arthroplasty should be screened for evidence of active oral/dental infection. This may be performed by administration of a questionnaire or dental examination.
Dental hygiene is important. All patients undergoing elective arthroplasty should be screened for evidence of active oral/dental infection. This may be performed by administration of a questionnaire or dental examination.
 Consensus does not exist for routine screening for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) other than for high-risk patients. For those colonized, short-term nasal application of mupirocin is the most accepted current method of decolonization for MRSA and/or MSSA.
Consensus does not exist for routine screening for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) other than for high-risk patients. For those colonized, short-term nasal application of mupirocin is the most accepted current method of decolonization for MRSA and/or MSSA.
 Routine urine screening is not warranted for patients undergoing elective arthroplasty. Urine screening prior to elective arthroplasty should be reserved for patients with a present history or symptoms of a urinary tract infection.
Routine urine screening is not warranted for patients undergoing elective arthroplasty. Urine screening prior to elective arthroplasty should be reserved for patients with a present history or symptoms of a urinary tract infection.
 Disease-modifying agents (ie, antirheumatologic medications or immunosuppressants) should be stopped prior to elective TJA. The timing of drug discontinuation should be based on the specific medication and the individual patient. The cessation of immunosuppressant medications should be performed in consultation and under the direction of the treating physician.
Disease-modifying agents (ie, antirheumatologic medications or immunosuppressants) should be stopped prior to elective TJA. The timing of drug discontinuation should be based on the specific medication and the individual patient. The cessation of immunosuppressant medications should be performed in consultation and under the direction of the treating physician.
 All patients with prior septic arthritis should undergo evaluation by serology and aspiration of the joint whenever possible, prior to arthroplasty.
All patients with prior septic arthritis should undergo evaluation by serology and aspiration of the joint whenever possible, prior to arthroplasty.
Skin Preparation
 Preoperative cleansing of the skin with chlorhexidine gluconate (CHG) should be implemented. In the presence of sensitivity to CHG, or when it is unavailable, it is our consensus that antiseptic soap is appropriate.
Preoperative cleansing of the skin with chlorhexidine gluconate (CHG) should be implemented. In the presence of sensitivity to CHG, or when it is unavailable, it is our consensus that antiseptic soap is appropriate.
 We recommend that whole body skin cleansing should start at least the night prior to elective arthroplasty. It is a consensus that after bathing, patients are advised to sleep in clean garments and bedding without the application of any topical products.
We recommend that whole body skin cleansing should start at least the night prior to elective arthroplasty. It is a consensus that after bathing, patients are advised to sleep in clean garments and bedding without the application of any topical products.
 Clipping, as opposed to shaving, is the preferred method for hair removal at the surgical site. If necessary, hair removal should be performed as close to the time of the surgical procedure as possible.
Clipping, as opposed to shaving, is the preferred method for hair removal at the surgical site. If necessary, hair removal should be performed as close to the time of the surgical procedure as possible.
 Elective arthroplasty should not be performed in patients with active ulceration of the skin in the vicinity of the surgical site. It is our consensus that incisions should not be placed through active skin lesions. For certain lesions such as those due to eczema and psoriasis, surgery should be delayed in these patients until their lesions have been optimized.
Elective arthroplasty should not be performed in patients with active ulceration of the skin in the vicinity of the surgical site. It is our consensus that incisions should not be placed through active skin lesions. For certain lesions such as those due to eczema and psoriasis, surgery should be delayed in these patients until their lesions have been optimized.
 The surgeon and operating room personnel should mechanically wash their hands with an antiseptic agent for a minimum of 2 minutes for the first case. A shorter period may be appropriate for subsequent cases. There is no clear difference among various antiseptic agents for hand washing.
The surgeon and operating room personnel should mechanically wash their hands with an antiseptic agent for a minimum of 2 minutes for the first case. A shorter period may be appropriate for subsequent cases. There is no clear difference among various antiseptic agents for hand washing.
Perioperative Antibiotics
 We support the surgical checklist protocol as beneficial to patient safety and specifically as it applies to correct administration of prophylactic antibiotics.
We support the surgical checklist protocol as beneficial to patient safety and specifically as it applies to correct administration of prophylactic antibiotics.
 The preoperative dose of antibiotics should be administered within 1 hour of surgical incision; this can be extended to 2 hours for vancomycin and fluoroquinolones. Postoperative antibiotics should not be administered for greater than 24 hours after surgery.
The preoperative dose of antibiotics should be administered within 1 hour of surgical incision; this can be extended to 2 hours for vancomycin and fluoroquinolones. Postoperative antibiotics should not be administered for greater than 24 hours after surgery.
 A first- or second-generation cephalosporin (cefazolin or cefuroxime) should be administered for routine perioperative surgical prophylaxis. Isoxazolyl penicillin is used as an appropriate alternative.
A first- or second-generation cephalosporin (cefazolin or cefuroxime) should be administered for routine perioperative surgical prophylaxis. Isoxazolyl penicillin is used as an appropriate alternative.
 Currently, teicoplanin and vancomycin are reasonable alternatives when routine antibiotic prophylaxis cannot be administered.
Currently, teicoplanin and vancomycin are reasonable alternatives when routine antibiotic prophylaxis cannot be administered.
 In a patient with a known anaphylactic reaction to penicillin, vancomycin or clindamycin should be administered as prophylaxis. Teicoplanin is an option in countries where it is available.
In a patient with a known anaphylactic reaction to penicillin, vancomycin or clindamycin should be administered as prophylaxis. Teicoplanin is an option in countries where it is available.
 Preoperative antibiotics have different pharmacokinetics based on patient weight and should be weight-adjusted.
Preoperative antibiotics have different pharmacokinetics based on patient weight and should be weight-adjusted.
 For current MRSA carriers, vancomycin or teicoplanin is the recommended perioperative antibiotic prophylaxis.
For current MRSA carriers, vancomycin or teicoplanin is the recommended perioperative antibiotic prophylaxis.
 Patients with prior history of MRSA should be rescreened preoperatively. If patients are found to be negative for MRSA, we recommend routine perioperative antibiotic prophylaxis.
Patients with prior history of MRSA should be rescreened preoperatively. If patients are found to be negative for MRSA, we recommend routine perioperative antibiotic prophylaxis.
 The type of preoperative antibiotic administered to a patient with prior septic arthritis or PJI should cover the previous infecting organism of the same joint.
The type of preoperative antibiotic administered to a patient with prior septic arthritis or PJI should cover the previous infecting organism of the same joint.
 An additional dose of antibiotic should be administered intraoperatively after two half-lives of the prophylactic agent. The general guidelines for frequency of intraoperative antibiotic administration are cefazolin every 2 to 5 hours,4 cefuroxime every 3 to 4 hours, clindamycin every 3 to 6 hours, isoxazolyl penicillin every 3 hours, and vancomycin every 6 to 12 hours.
An additional dose of antibiotic should be administered intraoperatively after two half-lives of the prophylactic agent. The general guidelines for frequency of intraoperative antibiotic administration are cefazolin every 2 to 5 hours,4 cefuroxime every 3 to 4 hours, clindamycin every 3 to 6 hours, isoxazolyl penicillin every 3 hours, and vancomycin every 6 to 12 hours.
 We recommend that redosing of antibiotics be considered in cases of large blood volume loss (>2000 mL) and fluid resuscitation (>2000 mL). As these are independent variables, redosing should be considered as soon as the first of these parameters are met.
We recommend that redosing of antibiotics be considered in cases of large blood volume loss (>2000 mL) and fluid resuscitation (>2000 mL). As these are independent variables, redosing should be considered as soon as the first of these parameters are met.
Operating Environment
 We believe that arthroplasty surgery may be performed in operating theaters without laminar flow. Laminar flow rooms and other strategies that may reduce particulates in operating rooms would be expected to reduce particulate load. Studies have not shown lower SSI in laminar flow rooms, and some cases are associated with increased rates of SSI.
We believe that arthroplasty surgery may be performed in operating theaters without laminar flow. Laminar flow rooms and other strategies that may reduce particulates in operating rooms would be expected to reduce particulate load. Studies have not shown lower SSI in laminar flow rooms, and some cases are associated with increased rates of SSI.
 We recommend that operating room traffic should be kept to a minimum.
We recommend that operating room traffic should be kept to a minimum.
 We agree that ultraviolent light environments can lower infection rates but recognize that this can pose a risk to operating room personnel.
We agree that ultraviolent light environments can lower infection rates but recognize that this can pose a risk to operating room personnel.
 We recommend that all personnel wear clean theater attire, including disposable head covering, when entering an operating room. Garments worn outside of the hospital should not be worn during TJA.
We recommend that all personnel wear clean theater attire, including disposable head covering, when entering an operating room. Garments worn outside of the hospital should not be worn during TJA.
 We recommend that a coordinated effort be made to minimize the duration of surgery without technical compromise of the procedure. Increased surgical time is a risk factor for PJI.
We recommend that a coordinated effort be made to minimize the duration of surgery without technical compromise of the procedure. Increased surgical time is a risk factor for PJI.
 We recognize the significance of patient normothermia and the data from nonorthopaedic procedures. We support general recommendations from the general surgery literature and identify this as a field that requires further research.
We recognize the significance of patient normothermia and the data from nonorthopaedic procedures. We support general recommendations from the general surgery literature and identify this as a field that requires further research.
 We recommend double gloving and recognize the theoretical advantage of triple gloving.
We recommend double gloving and recognize the theoretical advantage of triple gloving.
 We recognize the advantage of glove changes at least every 90 minutes or more frequently and the necessity of changing perforated gloves.
We recognize the advantage of glove changes at least every 90 minutes or more frequently and the necessity of changing perforated gloves.
 We recommend that the timing of opening trays should occur as close to the start of the surgical procedure as possible with the avoidance of any delays between tray opening and the start of surgery.
We recommend that the timing of opening trays should occur as close to the start of the surgical procedure as possible with the avoidance of any delays between tray opening and the start of surgery.
 We recognize high contamination rates in studies of scalpel blades that have been used for the skin incision and recommend changes after skin incision.
We recognize high contamination rates in studies of scalpel blades that have been used for the skin incision and recommend changes after skin incision.
 We recommend changing suction tips every 60 minutes based on studies showing higher rates of contamination.
We recommend changing suction tips every 60 minutes based on studies showing higher rates of contamination.
 We recommend against the use of fluid-filled basins that sit open during the surgery.
We recommend against the use of fluid-filled basins that sit open during the surgery.
 We recognize the theoretical basis for irrigation to dilute contamination and nonviable tissue and that a greater volume of irrigation would be expected to achieve greater dilution. We recognize advantages and disadvantages of different methods of delivering fluid but make no recommendations of one method over another.
We recognize the theoretical basis for irrigation to dilute contamination and nonviable tissue and that a greater volume of irrigation would be expected to achieve greater dilution. We recognize advantages and disadvantages of different methods of delivering fluid but make no recommendations of one method over another.
 We recognize the mechanical advantage of irrigation but that conflicting evidence exists supporting the use of one agent over the other and make no recommendation regarding type of solution.
We recognize the mechanical advantage of irrigation but that conflicting evidence exists supporting the use of one agent over the other and make no recommendation regarding type of solution.
 Antibiotic-impregnated polymethylmethacrylate cement reduces the incidence of PJI following TJA and should be used in patients at high risk for PJI following elective arthroplasty.
Antibiotic-impregnated polymethylmethacrylate cement reduces the incidence of PJI following TJA and should be used in patients at high risk for PJI following elective arthroplasty.
 In a patient with prior septic arthritis or PJI, we recommend the use of antibiotic-impregnated cement if a cemented component is used.
In a patient with prior septic arthritis or PJI, we recommend the use of antibiotic-impregnated cement if a cemented component is used.
 Antibiotic should be added to cement in all patients undergoing cemented or hybrid fixation as part of revision arthroplasty.
Antibiotic should be added to cement in all patients undergoing cemented or hybrid fixation as part of revision arthroplasty.
 If possible, avoid allogeneic blood transfusions as they are associated with an increased risk of SSI/PJI.
If possible, avoid allogeneic blood transfusions as they are associated with an increased risk of SSI/PJI.
 Compared to general anesthesia, neuraxial anesthesia reduces the amount of blood loss during THA.
Compared to general anesthesia, neuraxial anesthesia reduces the amount of blood loss during THA.
 Avoid metal-on-metal bearing surfaces as observational data suggest that these bearings may be associated with a higher risk of PJI.
Avoid metal-on-metal bearing surfaces as observational data suggest that these bearings may be associated with a higher risk of PJI.
Dislocation
 Although dislocation is not considered an intraoperative complication during THA, it deserves special consideration as it can have a devastating impact on the outcome of patients. The incidence of dislocation is approximately 1% to 10% after primary THA and as high as 27% after revision THA.2,15,21 An epidemiologic study found that dislocation was the number one cause for THA revision.6 The purpose of this section is to focus on preventing dislocation through appropriate patient selection and surgical technique.
Although dislocation is not considered an intraoperative complication during THA, it deserves special consideration as it can have a devastating impact on the outcome of patients. The incidence of dislocation is approximately 1% to 10% after primary THA and as high as 27% after revision THA.2,15,21 An epidemiologic study found that dislocation was the number one cause for THA revision.6 The purpose of this section is to focus on preventing dislocation through appropriate patient selection and surgical technique.
Risk Factors
 There are many factors that increase the risk of dislocation including patient characteristics, implant design, and surgical-related factors. Patient characteristics that may lead to higher rates of dislocation are female, age older than 80 years, previous surgery, rheumatoid arthritis, hip trauma, developmental dysplasia of the hip, neuromuscular disorders, dementia, alcoholism, and psychosis. These factors are not adjustable. Surgeon experience has a positive impact on reducing dislocation, and an inexperienced low-volume surgeon should consider obtaining assistance when treating the high-risk patient.
There are many factors that increase the risk of dislocation including patient characteristics, implant design, and surgical-related factors. Patient characteristics that may lead to higher rates of dislocation are female, age older than 80 years, previous surgery, rheumatoid arthritis, hip trauma, developmental dysplasia of the hip, neuromuscular disorders, dementia, alcoholism, and psychosis. These factors are not adjustable. Surgeon experience has a positive impact on reducing dislocation, and an inexperienced low-volume surgeon should consider obtaining assistance when treating the high-risk patient.
 From a surgical standpoint, it is imperative to use appropriate implants, position them appropriately, and ensure the soft tissues are tensioned properly.14 It has been shown that implants designed to maximize the head–neck ratio (ie, larger head with smaller neck) provide a greater impingement-free range of motion. Larger diameter heads also increase the jump distance necessary for the femoral head to disengage from the liner. Avoiding skirts on the femoral head will minimize the neck size and the chance for impingement. A larger head–neck ratio, combined with an increased jump distance, gives greater range of motion without impingement, therefore reducing dislocation rates.23 However, very large heads (>36 mm) have shown no increased benefits for stability versus their smaller counterparts and can cause iliopsoas impingement, leading to postoperative groin pain. Additional stability can be obtained with dual mobility bearing surfaces, especially in challenging revision THA procedures.40
From a surgical standpoint, it is imperative to use appropriate implants, position them appropriately, and ensure the soft tissues are tensioned properly.14 It has been shown that implants designed to maximize the head–neck ratio (ie, larger head with smaller neck) provide a greater impingement-free range of motion. Larger diameter heads also increase the jump distance necessary for the femoral head to disengage from the liner. Avoiding skirts on the femoral head will minimize the neck size and the chance for impingement. A larger head–neck ratio, combined with an increased jump distance, gives greater range of motion without impingement, therefore reducing dislocation rates.23 However, very large heads (>36 mm) have shown no increased benefits for stability versus their smaller counterparts and can cause iliopsoas impingement, leading to postoperative groin pain. Additional stability can be obtained with dual mobility bearing surfaces, especially in challenging revision THA procedures.40
 Exposure to the hip is an important factor for hip stability. Visualizing all areas of the surgical field is critically important to place the implants appropriately. It is well known that dislocation rates differ for the various approached to the hip; however, surgeons must be comfortable that the joint can be exposed properly to ensure appropriate implant positioning. Morrey’s classic meta-analysis revealed the following dislocation rates based on the approach: 3.2% posterior, 0.55% direct lateral, 2.2% anterolateral, and 1.3% transtrochanteric.32 Repairing the posterior capsule reduces the incidence of dislocations performed via the posterior approach.48
Exposure to the hip is an important factor for hip stability. Visualizing all areas of the surgical field is critically important to place the implants appropriately. It is well known that dislocation rates differ for the various approached to the hip; however, surgeons must be comfortable that the joint can be exposed properly to ensure appropriate implant positioning. Morrey’s classic meta-analysis revealed the following dislocation rates based on the approach: 3.2% posterior, 0.55% direct lateral, 2.2% anterolateral, and 1.3% transtrochanteric.32 Repairing the posterior capsule reduces the incidence of dislocations performed via the posterior approach.48
 Once the hip is exposed, appropriate implant orientation and positioning are crucial. A safe zone for the acetabulum has been defined as 40 ± 10 degrees of cup inclination or abduction and 15 ± 10 degrees of anteversion.25 Excessive anteversion can lead to anterior dislocations, whereas retroversion can lead to posterior dislocations. Excessive abduction can lead to dislocation when the hip is adducted, whereas a horizontal cup lacking abduction can cause an impingement-related dislocation. Also, acetabular osteophytes can lead to impingement and should be removed.
Once the hip is exposed, appropriate implant orientation and positioning are crucial. A safe zone for the acetabulum has been defined as 40 ± 10 degrees of cup inclination or abduction and 15 ± 10 degrees of anteversion.25 Excessive anteversion can lead to anterior dislocations, whereas retroversion can lead to posterior dislocations. Excessive abduction can lead to dislocation when the hip is adducted, whereas a horizontal cup lacking abduction can cause an impingement-related dislocation. Also, acetabular osteophytes can lead to impingement and should be removed.
 Normal femoral version is 10 to 15 degrees and restoration of this is important in achieving hip stability. Rarely is femoral version in itself an issue with stability during primary THA but careful attention to version is necessary during revision THA and patients with dysplasia. Characteristics of the femur have more to do with soft tissue tensioning (ie, offset and length are important elements to restore for hip stability). Increased lateral offset tensions the abductor muscles. Conversely, the lack of offset results in abductor weakness, possible impingement, and loss of soft tissue tension, resulting in increased risk of dislocation. Offset and length need to be checked carefully so as to not overlengthen the limb. Also, excessive lateral offset can lead to lateral hip pain. Tensioning can be assessed by distracting the limb distally and ensuring the femoral head does not disengage from the acetabular liner (Shuck test).
Normal femoral version is 10 to 15 degrees and restoration of this is important in achieving hip stability. Rarely is femoral version in itself an issue with stability during primary THA but careful attention to version is necessary during revision THA and patients with dysplasia. Characteristics of the femur have more to do with soft tissue tensioning (ie, offset and length are important elements to restore for hip stability). Increased lateral offset tensions the abductor muscles. Conversely, the lack of offset results in abductor weakness, possible impingement, and loss of soft tissue tension, resulting in increased risk of dislocation. Offset and length need to be checked carefully so as to not overlengthen the limb. Also, excessive lateral offset can lead to lateral hip pain. Tensioning can be assessed by distracting the limb distally and ensuring the femoral head does not disengage from the acetabular liner (Shuck test).
Preventing Hip Dislocation
 Appropriate implant positioning and soft tissue balancing are the key surgical principles of performing a stable THA. A systematic approach should start with preoperative planning. Start by templating the size, position, and offset of the components, restoring leg lengths and femoral offset. Choose an implant that is unlikely to impinge and sizes that give a high head–neck ratio. In challenging cases, be prepared to use modular components and dual mobility bearings. Choose the surgical approach to the hip that gives you the best exposure and comfort with dissection. If a posterior approach is used, repair of the posterior capsule is imperative.
Appropriate implant positioning and soft tissue balancing are the key surgical principles of performing a stable THA. A systematic approach should start with preoperative planning. Start by templating the size, position, and offset of the components, restoring leg lengths and femoral offset. Choose an implant that is unlikely to impinge and sizes that give a high head–neck ratio. In challenging cases, be prepared to use modular components and dual mobility bearings. Choose the surgical approach to the hip that gives you the best exposure and comfort with dissection. If a posterior approach is used, repair of the posterior capsule is imperative.
 Careful positioning of the implants is crucial. Place the acetabular component at 40 ± 10 degrees of cup inclination or abduction and 15 ± 10 degrees of anteversion. Maintain the femoral anteversion of 15 degrees. Combined anteversion of the acetabulum and femur should be approximately 35 to 45 degrees in females and 25 to 35 degrees in males. Ensure that there is no Shuck. Take the limb through a full range of motion while monitoring the implants for impingement or frank dislocation. If these occur, build offset or length (ie, tension the soft tissues) and reevaluate. Verify that the leg has not been inappropriately lengthened. If the leg is long, carefully reevaluate the position and orientation of the acetabulum. Frequently, the leg can be lengthened indirectly in an attempt to tighten the soft tissues when the acetabulum version is incorrect.36 Reposition and reorient the acetabulum if this is the case. High wall liners can be placed to assist in stability; however, these will reduce the available range of motion and can lead to impingement. If necessary, intraoperative radiographs could be obtained to evaluate positioning of the implants. Postoperatively, hip precautions can be ordered, especially if there is any concern for instability. However, it has been shown that hip precautions are not necessary when a THA has been performed in a stable fashion via the direct lateral approach.37
Careful positioning of the implants is crucial. Place the acetabular component at 40 ± 10 degrees of cup inclination or abduction and 15 ± 10 degrees of anteversion. Maintain the femoral anteversion of 15 degrees. Combined anteversion of the acetabulum and femur should be approximately 35 to 45 degrees in females and 25 to 35 degrees in males. Ensure that there is no Shuck. Take the limb through a full range of motion while monitoring the implants for impingement or frank dislocation. If these occur, build offset or length (ie, tension the soft tissues) and reevaluate. Verify that the leg has not been inappropriately lengthened. If the leg is long, carefully reevaluate the position and orientation of the acetabulum. Frequently, the leg can be lengthened indirectly in an attempt to tighten the soft tissues when the acetabulum version is incorrect.36 Reposition and reorient the acetabulum if this is the case. High wall liners can be placed to assist in stability; however, these will reduce the available range of motion and can lead to impingement. If necessary, intraoperative radiographs could be obtained to evaluate positioning of the implants. Postoperatively, hip precautions can be ordered, especially if there is any concern for instability. However, it has been shown that hip precautions are not necessary when a THA has been performed in a stable fashion via the direct lateral approach.37
PEARLS AND PITFALLS | |
Vascular injury |
|
| |
| |
| |
| |
| |
Peripheral nerve injury |
|
| |
| |
| |
| |
| |
Intraoperative periprosthetic fracture |
|
| |
| |
| |
| |
| |
| |
Periprosthetic joint infection |
|
| |
| |
| |
| |
| |
| |
| |
Dislocation |
|
| |
| |
| |
| |
| |
| |
CONCLUSION
 THA has revolutionized the management of patients crippled with arthritis, providing excellent long-term results. The effectiveness of the procedure and the durability of implants have been superb. Despite the evolution in techniques for THA, complications can still occur. These complications can range from minor inconveniences to extremely serious, life- and/or limb-threatening injuries.
THA has revolutionized the management of patients crippled with arthritis, providing excellent long-term results. The effectiveness of the procedure and the durability of implants have been superb. Despite the evolution in techniques for THA, complications can still occur. These complications can range from minor inconveniences to extremely serious, life- and/or limb-threatening injuries.
 Complications after THA can have a profound effect on patients’ lives. Similar to all complications, it is best to prevent them from happening rather than treat them after they have occurred. Understanding the anatomy surrounding the hip, diligent preoperative planning, and meticulous surgical technique are required to avoid these potentially tragic complications. Prompt recognition of neurovascular injuries and swift action can be the difference between life and death. Recognition and stabilization of intraoperative fractures can ensure an excellent outcome and avoid the need for further surgery. Proper preparation and precaution can help to prevent PJI. Precise technique can avoid an unstable THA.
Complications after THA can have a profound effect on patients’ lives. Similar to all complications, it is best to prevent them from happening rather than treat them after they have occurred. Understanding the anatomy surrounding the hip, diligent preoperative planning, and meticulous surgical technique are required to avoid these potentially tragic complications. Prompt recognition of neurovascular injuries and swift action can be the difference between life and death. Recognition and stabilization of intraoperative fractures can ensure an excellent outcome and avoid the need for further surgery. Proper preparation and precaution can help to prevent PJI. Precise technique can avoid an unstable THA.
REFERENCES
1. Aleto T, Ritter MA, Berend ME. Case report: superficial femoral artery injury resulting from cerclage wiring during revision THA. Clin Orthop Relat Res 2008;466(3):749–753.
2. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br 1981;63(2):214–218.
3. Barrack RL. Neurovascular injury: avoiding catastrophe. J Arthroplasty 2004;19(4 suppl 1):104–107.
4. Berend KR, Lombardi AV Jr, Mallory TH, et al. Cerclage wires or cables for the management of intraoperative fracture associated with a cementless, tapered femoral prosthesis: results at 2 to 16 years. J Arthroplasty 2004;19(7 suppl 2):17–21.
5. Berry DJ, Lewallen DG, Hanssen AD, et al. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am 1999;81(12): 1692–1702.
6. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am 2009;91:128–133.
7. Butt AJ, McCarthy T, Kelly IP, et al. Sciatic nerve palsy secondary to postoperative haematoma in primary total hip replacement. J Bone Joint Surg Br 2005;87(11):1465–1467.
8. Calligaro KD, Dougherty MJ, Ryan S, et al. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg 2003;38:1170–1177.
9. Curtis MJ, Jinnah RH, Wilson VD, et al. The initial stability of uncemented acetabular components. J Bone Joint Surg Br 1992;74(3): 372–376.
10. Davidson D, Pike J, Garbuz D, et al. Intraoperative periprosthetic fractures during total hip arthroplasty. Evaluation and management. J Bone Joint Surg Am 2008;90:2000–2012.
11. Davis ET, Gallie PA, James SL, et al. Proximity of the femoral neurovascular bundle during hip resurfacing. J Arthroplasty 2010;25(3): 471–474.
12. Dehart MM, Riley LH Jr. Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg 1999;7:101–107.
13. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop Relat Res 1987;(218):136–141.
14. Eftekhar NS. Dislocation and instability complicating low friction arthroplasty of the hip joint. Clin Orthop Relat Res 1976;(121):120–125.
15. Ekelund A, Rydell N, Nilsson OS. Total hip arthroplasty in patients 80 years and older. Clin Orthop Relat Res 1992;(281):101–106.
16. Feugier P, Fessy MH, Carret JP, et al. Total hip arthroplasty. Risk factors and prevention of iatrogenic complications [in French]. Ann Chir 1999;53:127–135.
17. Goulding K, Beaulé PE, Kim PR, et al. Incidence of lateral femoral cutaneous nerve neurapraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res 2010;468:2397–2404.
18. Graf R, Azizbaig-Mohajer M. Minimally invasive total hip replacement with the patient in the supine position and the contralateral leg elevated. Oper Orthop Traumatol 2006;18:317–329.
19. Graw BP, Woolson ST, Huddleston HG, et al. Minimal incision surgery as a risk factor for early failure of total hip arthroplasty. Clin Orthop Relat Res 2010;468(9):2372–2376.
20. Johanson NA, Pellicci PM, Tsairis P, et al. Nerve injury in total hip arthroplasty. Clin Orthop Relat Res 1983;(179):214–222.
21. Kavanagh BF, Fitzgerald RH Jr. Multiple revisions for failed total hip arthroplasty not associated with infection. J Bone Joint Surg Am 1987;69(8):1144–1149.
22. Kennedy WF, Byrne TF, Majid HA, et al. Sciatic nerve monitoring during revision total hip arthroplasty. Clin Orthop Relat Res 1991;264:223–227.
23. Krushell RJ, Burke DW, Harris WH. Range of motion in contemporary total hip arthroplasty. The impact of modular head-neck components. J Arthroplasty 1991;6(2):97–101.
24. Lewallen DG. Neurovascular injury associated with hip arthroplasty. Instr Course Lect 1998;47:275–283.
25. Lewinnek GE, Lewis JL, Tarr R, et al. Dislocations after total hip replacement arthroplasties. J Bone Joint Surg Am 1978;60(2):217–220.
26. Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury 2007;38:651–654.
27. Mahadevan D, Challand C, Keenan J. Cement extrusion during hip arthroplasty causing pain and obturator nerve impingement. J Arthroplasty 2009;24(1):158.
28. Mallory TH. Sciatic nerve entrapment secondary to trochanteric wiring following total hip arthroplasty. A case report. Clin Orthop Relat Res 1983;(180):198–200.
29. Masri BA, Meek RM, Duncan CP. Periprosthetic fractures evaluation and treatment. Clin Orthop Relat Res 2004;(420):80–95.
30. Mayle RE, Della Valle CJ. Intra-operative fractures during THA: see it before it sees us. J Bone Joint Surg Br 2012;94(11 suppl A):26–31.
31. Meek RM, Garbuz DS, Masri BA, et al. Intraoperative fracture of the femur in revision total hip arthroplasty with a diaphyseal fitting stem. J Bone Joint Surg Am 2004;86(3):480–485.
32. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am 1992;23(2):237–248.
33. Nachbur B, Meyer RP, Verkkala K, et al. The mechanisms of severe arterial injury in surgery of the hip joint. Clin Orthop Relat Res 1979;141:122–133.
34. Parvizi J, Gehrke T, et al. Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection. Brooklandville, MD: Data Trace Publishing, 2013:1–140.
35. Parvizi J, Pulido L, Slenker N, et al. Vascular injuries after total joint arthroplasty. J Arthroplasty 2008;23(8):1115–1121.
36. Parvizi J, Sharkey PF, Bissett GA, et al. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am 2003;85(12):2310–2317.
37. Peak EL, Parvizi J, Ciminiello M, et al. The role of patient restriction in reducing the prevalence of early dislocation following total hip arthroplasty. A randomized, prospective study. J Bone Joint Surg Am 2005;87(2):247–253.
38. Pekkarinen J, Alho A, Puusa A, et al. Recovery of sciatic nerve injuries in association with total hip arthroplasty in 27 patients. J Arthroplasty 1999;14(3):305–311.
39. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am 1996;78(8): 1206–1213.
40. Philippot R, Adam P, Reckhaus M, et al. Prevention of dislocation in total hip revision surgery using a dual mobility design. Orthop Traumatol Surg Res 2009;95(6):407–413.
41. Porter SS, Black DL, Reckling FW, et al. Intraoperative cortical somatosensory evoked potentials for detection of sciatic neuropathy during total hip arthroplasty. J Clin Anesth 1989;1:170–173.
42. Sakai R, Kikuchi A, Morita T, et al. Hammering sound frequency analysis and prevention of intraoperative periprosthetic fractures during total hip arthroplasty. Hip Int 2011;21(6):718–723.
43. Satcher RL, Noss RS, Yingling CD, et al. The use of motor-evoked potentials to monitor sciatic nerve status during revision total hip arthroplasty. J Arthroplasty 2003;18:329–332.
44. Schmalzreid TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. J Bone Joint Surg Am 1991;73:1074–1080.
45. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular component insertion: a report of 13 cases. J Arthroplasty 1999;14(4):426–431.
46. Sharma DK, Kumar N, Mishra V, et al. Vascular injuries in total hip replacement arthroplasty: a review of the problem. Am J Orthop 2003;32:487–491.
47. Sheth NP, Brown NM, Moric M, et al. Operative treatment of early peri-prosthetic femur fractures following primary total hip arthroplasty. J Arthroplasty 2013;28(2):286–291.
48. Suh KT, Park BG, Choi YJ. A posterior approach to primary total hip arthroplasty with soft tissue repair. Clin Orthop Relat Res 2004;418:162–167.
49. Wasielewski RC, Cooperstein LA, Kruger MP, et al. Acetabular anatomy and the transacetabular fixation of screws in total hip arthroplasty. J Bone Joint Surg Am 1990;72:501–508.
50. Yacoubian SV, Sah AP, Estok DM II. Incidence of sciatic nerve palsy after revision hip arthroplasty through a posterior approach. J Arthroplasty 2010;25:31–34.
< div class='tao-gold-member'>





































