Key points
- •
Cutaneous microcirculation, a type of microvasculature situated just below the epidermis, is composed of major components of blood vessels (arterioles and venules) and lymphatic vessels.
- •
The cutaneous blood vessels are organized into a superficial plexus near the dermal-epidermal junction and a lower plexus near the dermal-subcutaneous junction. Cutaneous arteriole delivers oxygen and nutrients to the skin. It is also the conduit from which the immune system sent their armies of white blood cells to counter invasive microorganisms that enter into the skin. Importantly, the cutaneous arteriole is the key to mobilize immune components to the skin in case of allergic events, such as in atopic dermatitis. The most important segment in this regard is the postcapillary venule, site of leukocyte transmigration from vascular space into the tissue site.
- •
The cutaneous lymphatic system is comprised of larger draining vessels and is organized into two plexuses. The major function of the cutaneous lymphatic system is maintenance of interstitial fluid balance and immune recognition, but it can also become a pathway of cancer metastasis.
- •
Cutaneous microcirculation, due to its essential immunologic roles, plays an important part in the skin inflammatory process of atopic dermatitis. This specific role is illustrated in the keratin-14/IL4 transgenic mice, an animal model of atopic dermatitis in which cutaneous blood vessels exhibit angiogenesis and leakage, with resulting prominent skin infiltration of inflammatory cells. Similarly, the lymphatic vessels of this animal model exhibit prominent lymphangiogenesis and evidence of compromised integrity, providing another support in its role in atopic dermatitis development and sustainment.
- •
Understanding of the roles of cutaneous vasculature in the development of atopic dermatitis will pave the way to future targeted treatments.
Introduction
The cutaneous microvasculature situated at the dermis just below the epidermis is the major instrument for skin microcirculation. The cutaneous vasculature has three major components: arteriole, venule, and lymphatic vessels ( ). In the sections that follow, the basic structures and common functions of blood vessels and lymphatic vessels are delineated. Having described these functional structures, we then discuss the roles of cutaneous microvasculature played in the inflammatory skin disease atopic dermatitis.
Cutaneous blood vessels: Structures
Since there is a close relationship between cutaneous blood vessels and cutaneous lymphatic vessels, the structures of cutaneous blood vessels are better depicted in conjunction with cutaneous lymphatic vessels. Fig. 12.1 illustrates a schematic picture of the structural proximity between these vessels ( ). More details on the structures of cutaneous blood vessels are discussed in other publications ( ).
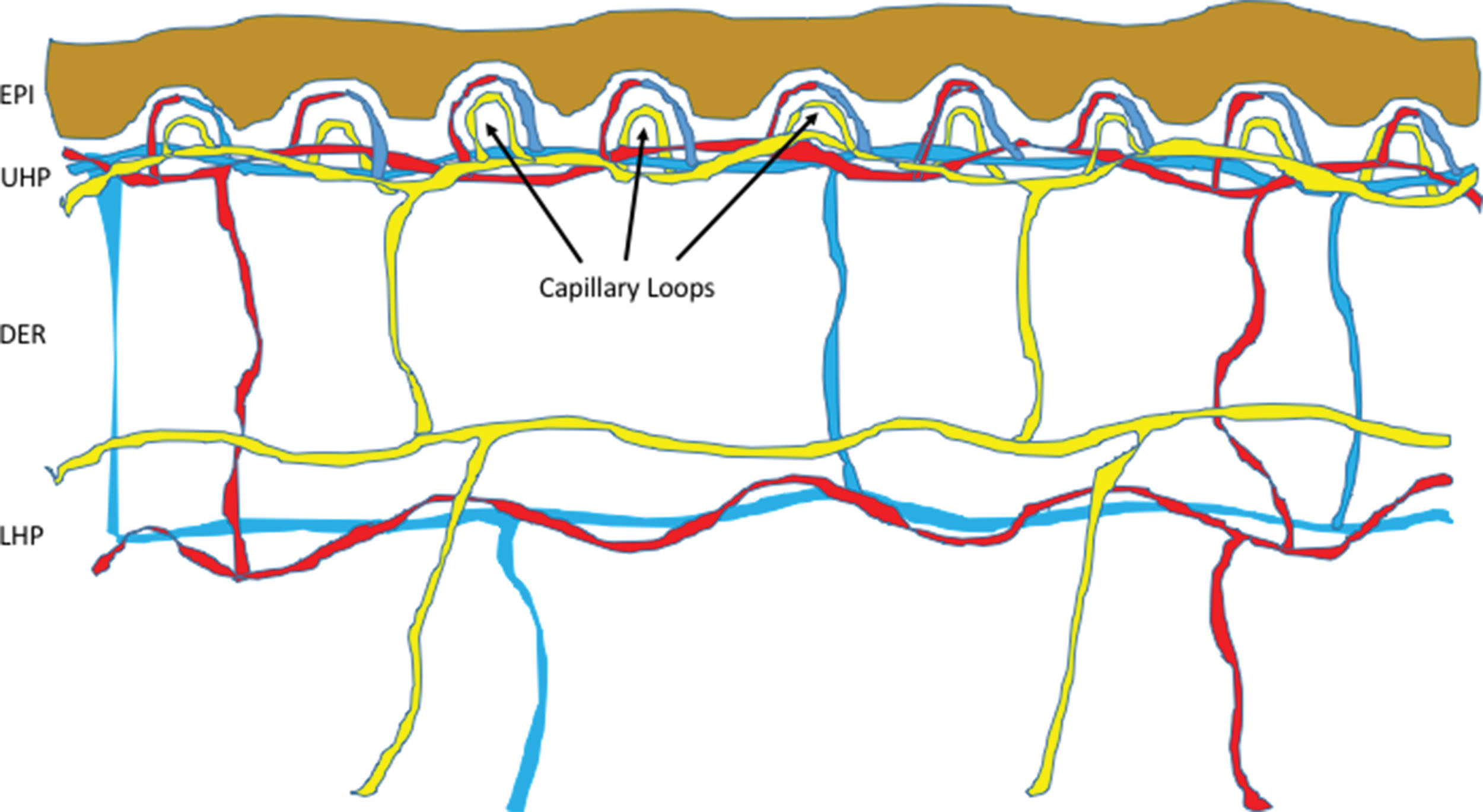
The cutaneous microcirculation is organized into two horizontal plexuses. Plexus , derived from a Latin word meaning “braid,” denotes a branching network of blood vessels or nerves. The upper plexus is located just 1 to 1.5 mm below the stratum corneum, whereas the lower plexus is situated along the dermal-subcutaneous junction. Connecting the upper and lower plexuses are the pairing ascending arterioles and the descending venules. Arising from the upper horizontal plexus are the capillary loops. From an ultrastructural perspective, arterioles distinguish from capillaries and venules by the presence of an internal elastic lamina, capillaries are unique for their thin vascular wall containing pericytes (a special type cell of vessel wall) on the outer surface, and venules are characterized by their thicker vessel walls without elastic fibers. The papillary dermal arterioles have an outer diameter ranging from 17 to 26 μm and vessel wall with two layers of smooth muscle cells surrounding the endothelial cells at the lumen, and elastic fibers. As the arterioles move toward the capillary loop, their diameters, smooth muscle, and elastic fibers all reduce in caliber. When the vessels reach a size of 15 μm in outer diameter, the smooth muscles, which are identified by their dense bodies and myofilaments, are no longer present. At the point where the blood vessels turn into 10- to 12-μm outer diameter in size, all elastic fibers disappear, and this segment is now the beginning of arterial capillary. In the capillaries, pericytes, which also have contractile functions, replace smooth muscles and form tight junctions with endothelial cells. They have an important role in maintaining capillary integrity ( ). The next segment is venous capillary, which has one layer of pericytes around the vessel wall. Venous capillary then connects to the next segment of the postcapillary venule, which usually has an external diameter ranging from 18 to 23 μm at the papillary dermis level, a thicker wall of 3.5 to 5 μm, and two to three layers of pericytes around the vessel wall. The papillary dermal blood vessels are composed entirely of terminal arterioles, arterial and venous capillaries, and postcapillary venules, with the latter being the major component. The vessel sizes are generally larger at the lower part of the dermis. At the lower third of the dermis, arterioles and collecting venules can reach external diameter ranging from 40 to 50 μm, with vessel wall thickness ranging from 10 to 16 μm, and four to five layers of pericytes surrounding the vessel wall ( ).
Cutaneous blood vessels: Functions
One of the important functions of a cutaneous blood vessel is to provide nutrients to the skin. The capillary loops arise from the upper horizontal plexus, which is the primary avenue for nutrient delivery. The lower horizontal plexus, however, is responsible to supply life-sustaining substances for the hair bulbs, sweat glands, and other dermal glands ( ). The control of cutaneous blood flow is achieved by vasodilatation (increase) and vasoconstriction (decrease) through the smooth muscles directed by the sympathetic branch of the central nervous system ( ). Vasodilation, itself, also serves as an effective mechanism for thermal regulation (i.e., heat loss) ( ). The upper horizontal plexus acts as a thermal radiator ( ). Interestingly, physical exercise training can modify cutaneous microvascular reactivity to different stimuli ( ).
Another major function of cutaneous blood vessel is to provide immune support to the skin. As such, the microvascular system is always involved when there is an inflammatory event ( ). From a physiologic perspective, the postcapillary venules are the most important as they are the primary sites where inflammatory cells transmigrate from the vascular space into the target tissue sites. It is in this vessel segment that endothelial cells, in response to inflammatory signals, commonly develop intercellular gaps leading to increased vascular permeability ( ). Endothelial cells in the cutaneous blood vessel, when encountering appropriate triggers, such as stimulation by immunoglobulin-1 (IL1), tumor necrosis factor-α (TNF-α), or B-spectrum ultraviolet light, upregulate expressions on their cell surfaces of several adhesion molecules that facilitate the binding and the subsequent transportation of inflammatory cells out of the cutaneous blood vessels into the tissue location of the inflammatory targets. These adhesion molecules include intercellular adhesion molecule-1 (ICAM-1) (CD54), vascular cell adhesion molecule-1 (VCAM-1) (CD106), endothelial leukocyte adhesion molecule-1 (ELAM-1) (new term E-selectin, CD62E), and P-selectin (CD62P) ( ). The inflammatory cells, with the adhesion molecules such as cutaneous lymphocyte antigen (CLA), the very late antigen-4 (VLA-4, integrin α4β1) β1 subunit (CD29), or lymphocyte function-associated antigen-1 (LFA-1) α subunit (CD11a) expressed on their surfaces, can interact with ICAM-1, VCAM-1, or ELAM-1 and thus help to facilitate the movement into the tissue inflamed sites ( ). In addition, junctional adhesion molecules (JAMs), the proteins located at the borders of endothelial cells that take part in the final step of leukocyte extravasation, are also important for the transmigration of leukocytes from cutaneous blood vessels into the inflamed skin sites, although their expressions are unaltered by the inflammatory process ( ). Moreover, the expression of CD40 in endothelial cells, a costimulatory protein typically found in antigen-presenting cells, raises another possible role of cutaneous blood vessels in the skin inflammation ( ).
Another inflammation-related function of cutaneous blood vessels is documented in an animal model of wound healing. Mice lacking in both P- and E-selectins by genetic knockout showed a substantial reduction of inflammatory cell (neutrophils and macrophages) recruitment into the wounding site, resulting in impairment of wound closure ( ).
Cutaneous blood vessels can be examined in skin frozen sections by labeling with antibodies against common endothelial cell markers such as E-selectin or PAL-E ( ). Cutaneous blood vessels and blood flow can be assessed by different noninvasive methods, including laser-based techniques such as laser Doppler flowmetry or laser speckle contrast imaging (LSCI), and more recently by optical coherence tomography and photoacoustic imaging methodologies ( ).
Cutaneous blood vessels: Roles in atopic dermatitis
It is intuitively obvious that blood vessel is involved in transporting those inflammatory cells into the site of skin inflammation. Atopic dermatitis, a chronic inflammatory skin disease, is no exception. Next, we will discuss the roles of angiogenesis in atopic dermatitis as documented in a mouse model and in human patients affected by this disease.
Cutaneous blood vessels in the keratin-14/IL4 transgenic mice
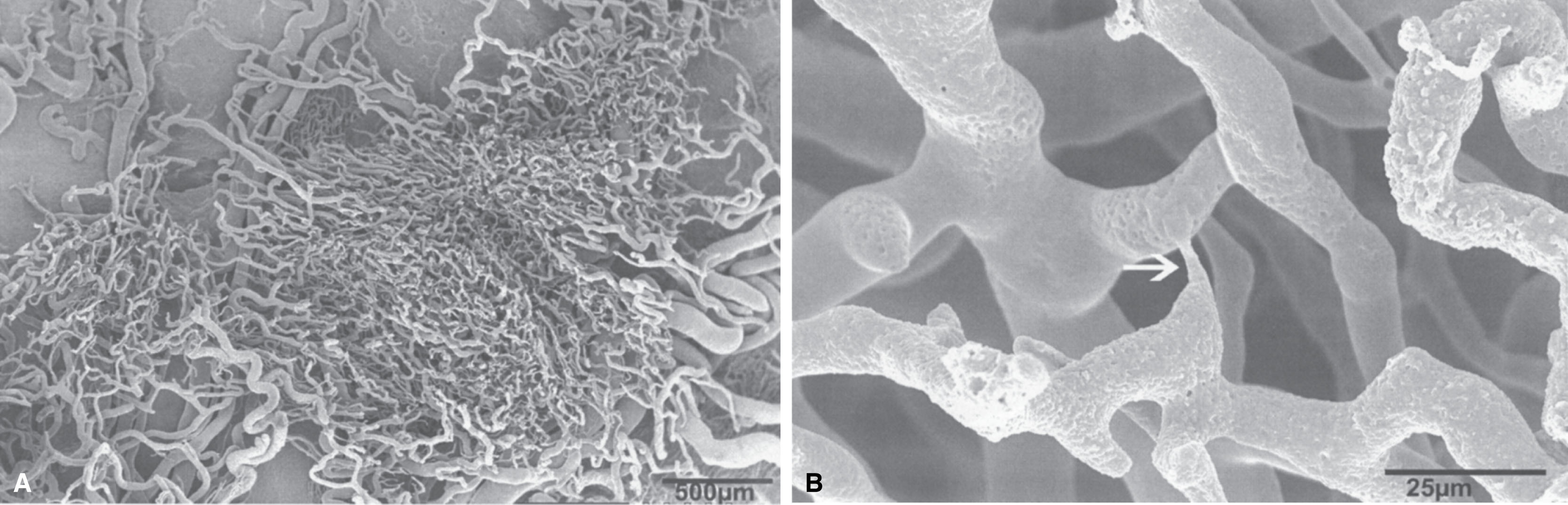
To generate a mouse model of atopic dermatitis, a prominent Th2 cytokine IL4 that is upregulated in human atopic dermatitis skin was artificially expressed in the basal layer of epidermis through a keratin-14 promoter. The IL4 transgenic mice showed substantial changes in cutaneous blood vessels, in parallel with prominent dermal inflammatory cell infiltration ( ; ). All the keratin-14/IL4 transgenic mice, but none of nontransgenic littermates, developed chronic inflammatory skin lesions primarily on their hairless skin surfaces, ears, face, neck, tails, and periorbital areas (see Fig. 11.5 ). Heavy infiltrations of mononuclear cells, mast cells, and eosinophils were present under light microscopy on the lesional skin of the IL4 transgenic mice (see Fig. 11.6 ) ( ). These substantial infiltrations of inflammatory cells would logically require the support of cutaneous microcirculation for delivering these immune cells to the site of inflammation. The findings of clinical disease progression (from before disease onset), to early skin lesion (within 1 week of onset), to chronic skin lesion (>3 weeks of onset), paralleled with progressive increase in the amount of inflammatory cell infiltration (CD3+, CD4+, CD8+, IA-IE+, CD11a+, and CD23+ cells) in the transgenic mouse skin, provided another indirect evidence of cutaneous microcirculation involvement in delivering these inflammatory cells to the target sites ( ). To obtain direct evidence for the roles of cutaneous circulation, particularly the blood vessels, in the development and progression of atopic dermatitis, blood vessels and their promoting factors in these IL4 transgenic mice skin were investigated in greater details ( ). Skin samples were obtained from nontransgenic mice, the transgenic mice at different disease stages: before onset, early skin lesion, and late skin lesion. When transmission and scanning electron microscopy methods were used to visualize the cutaneous blood vessels in these mice, substantial ultrastructural changes were present in the transgenic mice by electron microscopy, providing solid evidences of the occurrence of angiogenesis ( Fig. 12.2 ) ( ).
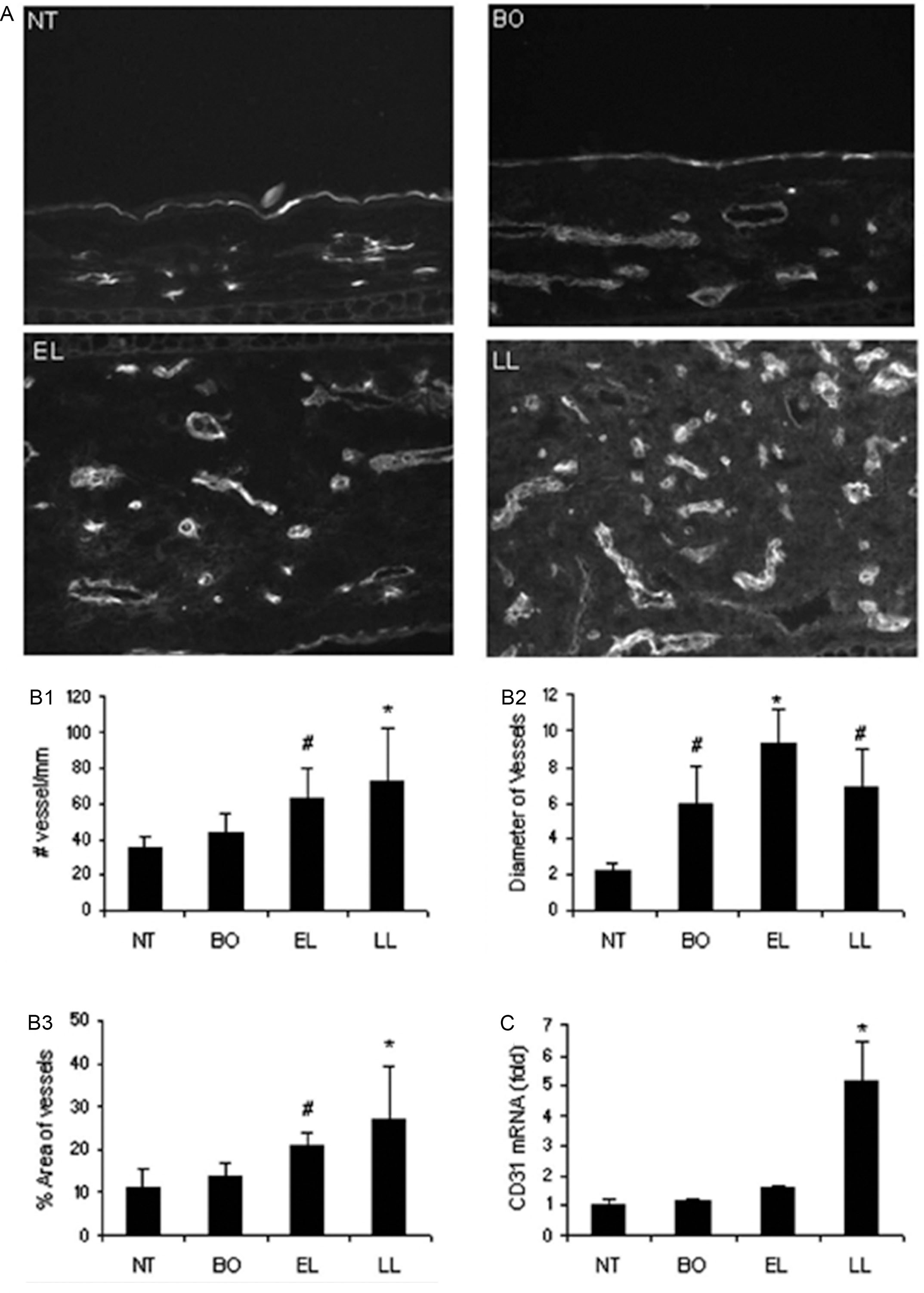
When the frozen sections of these skin samples were labeled with vascular endothelial cell marker CD31 and analyzed by computer-assisted photometric analysis, a significant increase of the number of blood vessels and area occupied by dermal vessels were present, compared to the nontransgenic mice. In addition, the diameters of the dermal blood vessels, as determined by the positive CD31 labeling, also increased in the transgenic mice skin. When these skin samples were extracted for RNA and reverse transcription real-time polymerase chain reaction (PCR) was performed, we found an upregulation of the CD31 mRNAs in the transgenic mice skin, compared to that of nontransgenic mice ( Fig. 12.3 ) ( ). Thus we documented that in this animal model of human atopic dermatitis, blood vessels increased in number and diameter, suggesting the occurrence of an angiogenesis process ( ). To delineate the possible involvement of an angiogenic process, these skin sections were labeled by an antibody against vascular endothelial growth factor receptor-2 (VEGFR2), the receptor on the endothelial cell surfaces responsible for receiving signal from vascular endothelial growth factor (VEGF), a potential angiogenic factor, and for initiating cell proliferation and migration ( ). The increasingly positive VEGFR2 protein labeling and mRNA expression, not surprisingly, paralleled the increase in CD31 labeling and had a general trend of progressive increase, as the disease progressed from before disease onset to early skin lesion, and then to late skin lesion ( ). Thus the determined parallel increase of VEGFR2 and CD31+ vessels and disease progression further supports a role of angiogenesis in atopic dermatitis. These parallel events of increased inflammation and increased blood vessel density are consistent with the findings that occurred in other cutaneous inflammatory processes ( ).
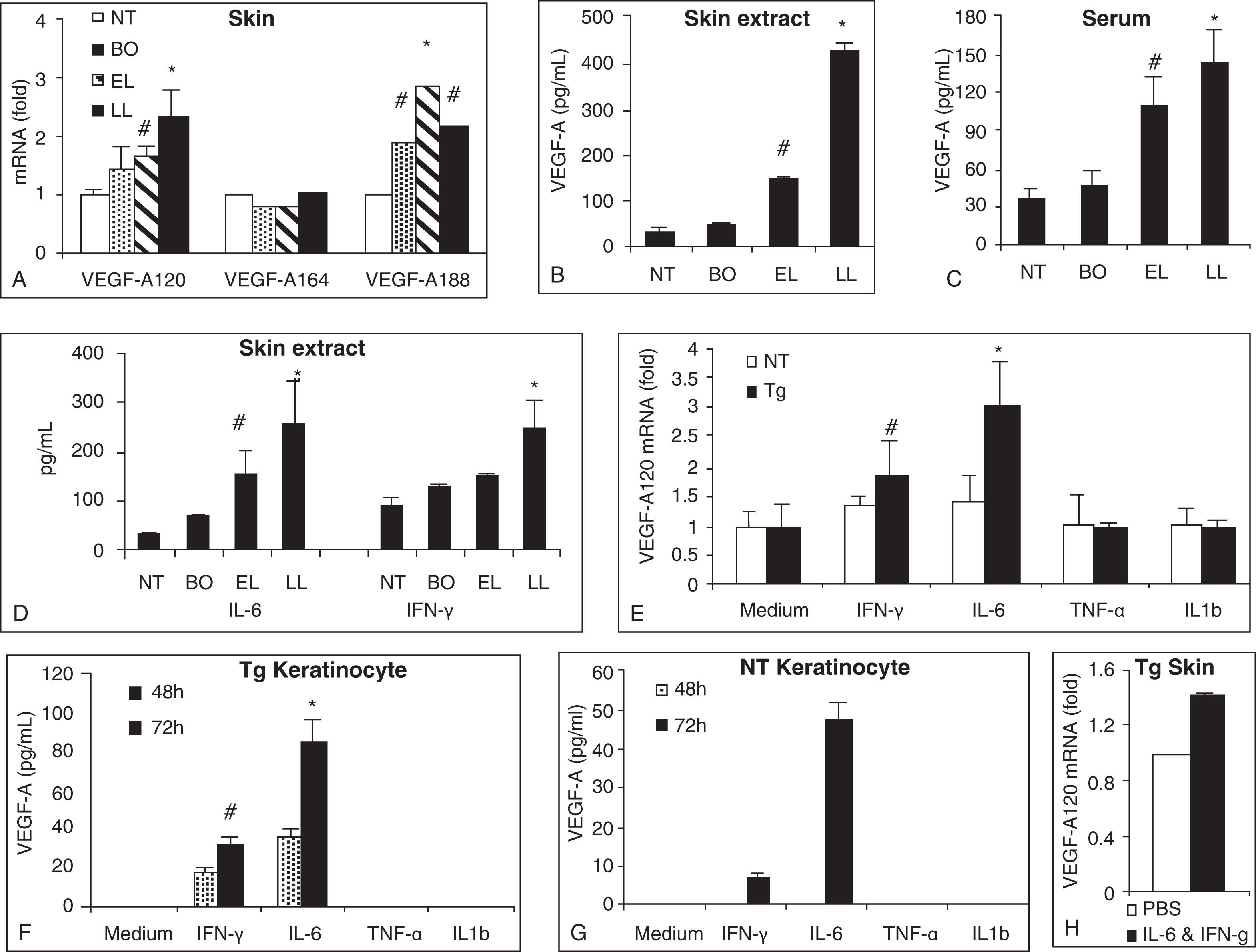
To access the underlying mechanism of the angiogenic process, we examined proangiogenic factors known to take part in angiogenesis. Consistent with the progressive increase in skin endothelial cell marker CD31 and VEGFR2, there were also progressive increases of a potent proangiogenic factor VEGF-A skin mRNAs, skin-extracted proteins, and serum proteins ( Fig. 12.4 ) ( ). On the skin mRNA level, the prominent upregulations were detected for VEGF-A120 and VEGF-A188 isoforms. VEGF-A120 isoform and the VEGF-A164 isoform are the two predominant forms, capable of inducing proliferation of endothelial cells and in vivo angiogenesis process ( ). In the skin and serum protein levels, the immunoreactive VEGF-A concentrations progressively elevated as the disease progressed from before onset stage, to early lesion stage, and then to late lesion stage (see Fig. 12.4 ). The upregulation of IL6 and interferon-gamma (IFN-γ) (both known to promote VEGF-A) in the protein and mRNA levels in the skin of transgenic mice supported the angiogenic role of these cytokines on atopic skin. In addition, primary keratinocytes cultured from skin of nontransgenic and transgenic mice exhibited upregulation of VEGF-A protein and mRNA expressions upon stimulation by IL6 and INF-γ, with transgenic keratinocytes showing greater enhancement. Moreover, VEGF-A120 mRNA was upregulated in the skin of transgenic mice upon intradermal administration of IL6 and IFN-γ (see Fig. 12.4 ) ( ).
To determine if proangiogenic factors other than VEGF-A might also be involved in the process of atopic dermatitis, we analyzed mRNA levels of the skin of both nontransgenic mice and transgenic mice in different disease stages for these angiogenic factors: VEGF-B, VEGF-D, VEGFR1, VEGFR3, VE-Cadherin, angiopoietin-1 (Ang-1), Ang-2, Tie-2 (endothelium-specific receptor tyrosine kinase, a ligand for which Ang-1), and guanylate-binding protein-1 (GBP-1). We found that there were substantial upregulations on VE-cadherin, Ang-1, Ang-2, and GBP-1, especially at the late lesion stage of disease in transgenic mice compared to nontransgenic mice. Similarly, in the same samples we also demonstrated increased protein levels of Ang-1 and GBP-1 in the skin of transgenic mice compared to that of nontransgenic mice by Western blot analysis ( Fig. 12.5 ) ( ).
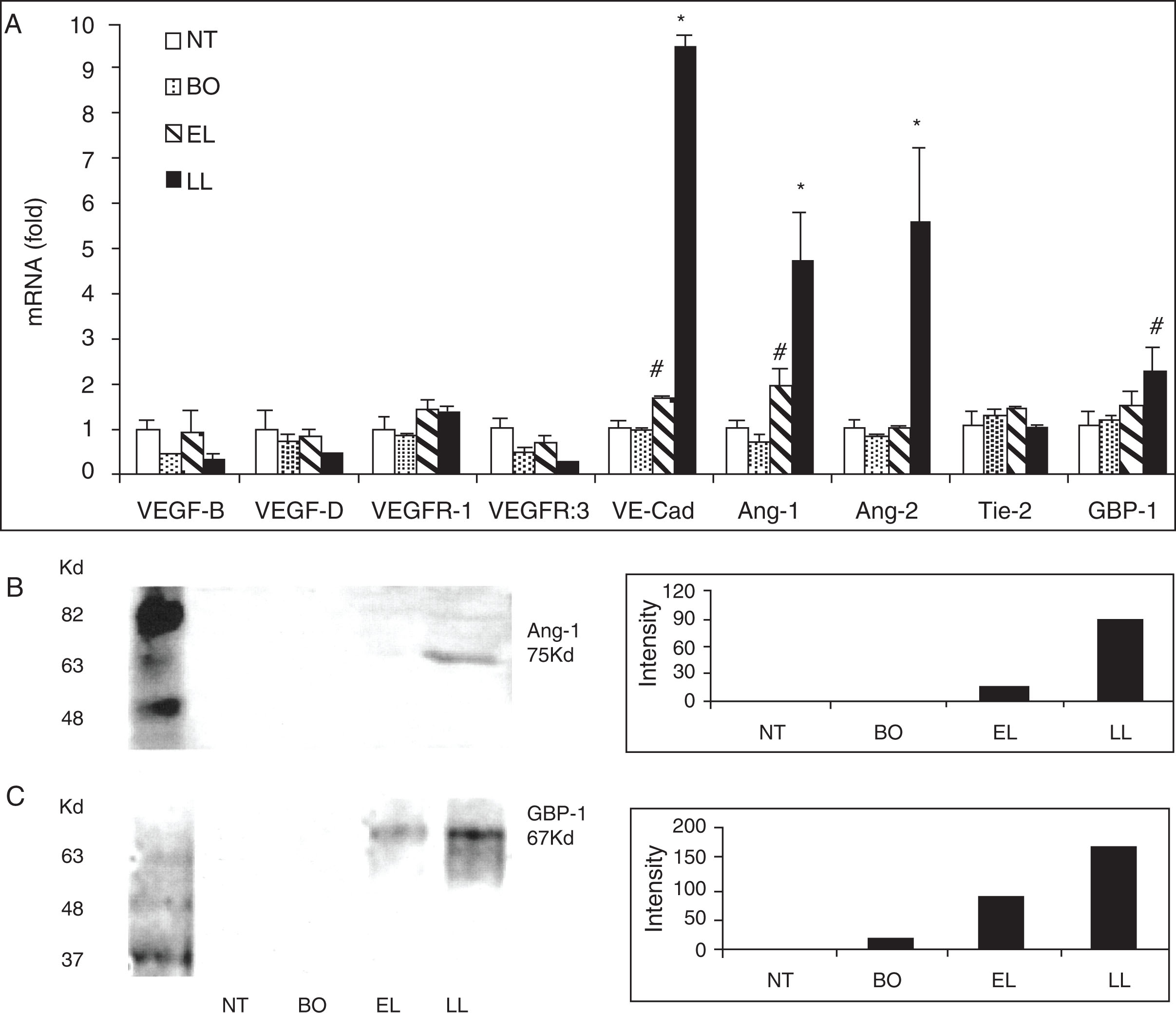
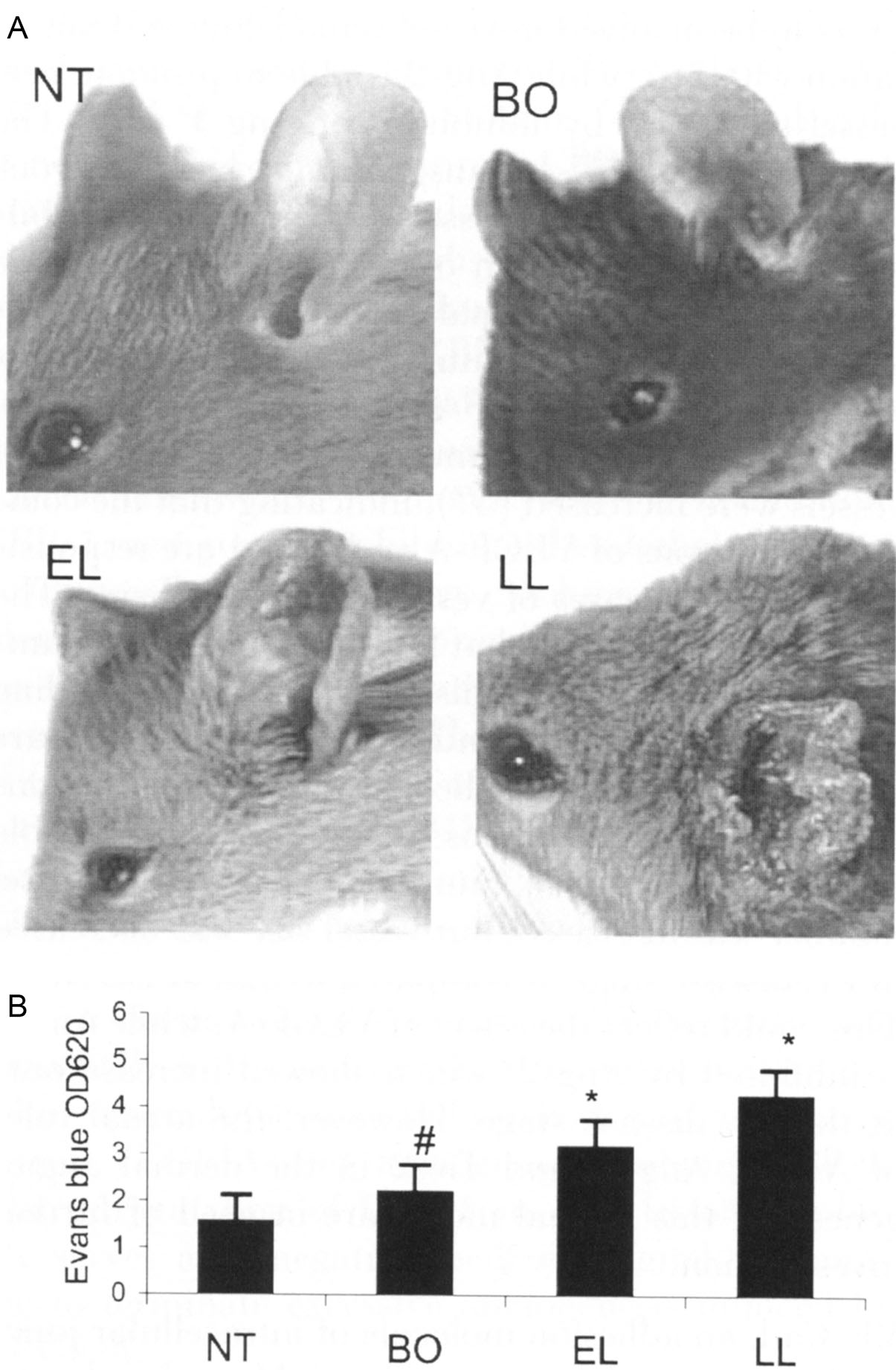
To evaluate if the prominent angiogenesis of the blood vessels in the mice affected by atopic dermatitis negatively modify the function of these blood vessels, we performed leakage study to answer this question. In parallel to the increase of skin inflammation in the transgenic mice, there was a progressive increase of leakage of intravenous administrated Evans blue dye into the skin matrices of transgenic mice, as determined by the increased blue intensities in the ear by standardized exposure photographs and by the higher concentrations of Evans blue by unit weight ( Fig. 12.6 ) ( ).
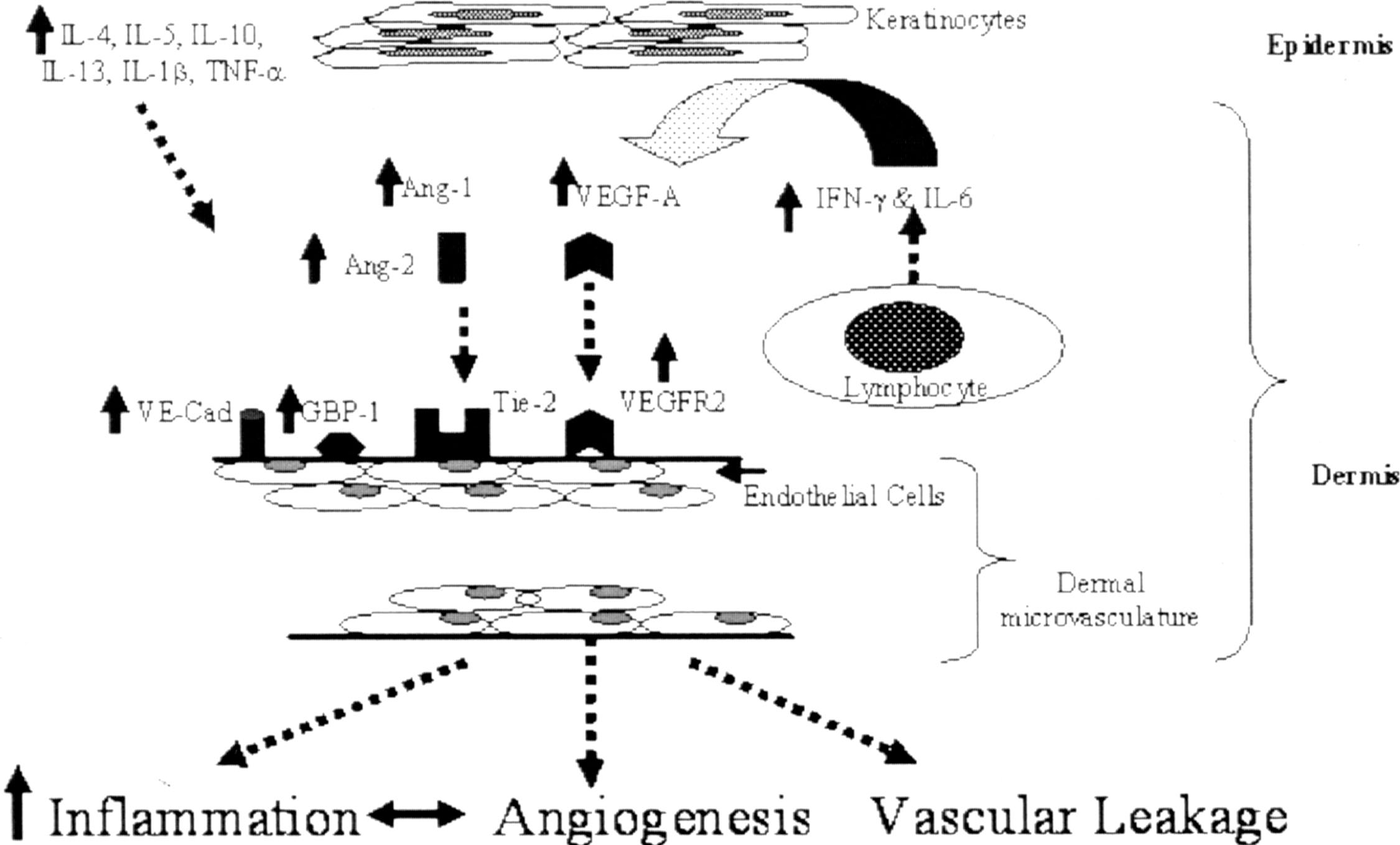
More recently, the important atopic dermatitis–related cytokine IL4 has shown its direct effect on cutaneous blood vessel by its upregulating of VEGF expressions in keratinocyte ( ). Together, the study results of this keratin-14/IL4 transgenic mouse model of atopic dermatitis establish a powerful link between cutaneous microvascular angiogenesis and atopic dermatitis. Next we illustrate the proposed immunologic mechanism of angiogenesis involvement in skin inflammation of this atopic dermatitis model ( Fig. 12.7 ).
Cutaneous blood vessels in human patients of atopic dermatitis
As in other inflammatory skin diseases, a histology picture in an atopic dermatitis skin lesion is characterized by the presence of inflammatory cell infiltrate and some features of angiogenesis ( ). Skin lesion of atopic dermatitis is characterized by a high level of vascular endothelial growth factor alpha (VEGF-A) corresponding to disease severity ( ). Since angiogenic factor-producing cells, such as lymphocytes, eosinophils, and mast cells, are typically present in the lesional skin of atopic dermatitis, the angiogenesis in atopic dermatitis is likely linked to the inflammatory process ( ). Consistent with the skin upregulation of VEGF-A level, the serum of patients affected by atopic dermatitis also have a significant increase of VEGF-A ( ).
In human patients affected by atopic dermatitis, their lesional skin extracts have revealed upregulation of VEGF proteins ( ). In addition, the patients’ skin dermal vascular expressions of E-selectin were found to be especially prominent ( ). Recently, some abnormalities of cutaneous blood vessels were also revealed in the erythematous skin of human patients affected by atopic dermatitis. Specifically, researchers, using 2-photon microscopy in living tissues, found that skin from patients affected by atopic dermatitis exhibits “thickened, flexuous” blood vessels, suggesting increased blood flow in the erythematous skin areas ( ). In addition, the prominent presence of mast cells, which has documented effects on angiogenesis and vascular leakage, in skin lesions of atopic dermatitis, may also contribute to the overall inflammatory process of the disease ( ). Overall, the findings in human atopic dermatitis patients are consistent with that found in the keratin-14/IL4 transgenic mouse, an animal model of the disease.
Cutaneous lymphatic vessels: Structures
Several publications have depicted details of general and cutaneous lymphatic structures ( ). Although it has originally been viewed as a passive channel for fluid and cellular transport, lymphatic vessels are now recognized as an active participant of homeostasis and physiologic functions, including inflammation, and lymphatic vessels are organ- specific ( ). Lymphatic vessels are absent from certain tissues such as cornea, lens, and cartilage but are abundantly present in skin (other than epidermis) and mucous membranes (other than epithelium). Like cutaneous blood vessels, the cutaneous lymphatic system also organizes in two plexuses. The superficial lymphatic plexus locates around the subpapillary arterial network and extends into the dermal papillae. In these areas, the lymphatic vessels have smaller diameters and no valves. Branching vessels from the superficial plexus drain vertically down to larger vessels in the lower dermis and the superficial zone of subcutaneous tissue. The lower lymphatic plexus locates below the second arterial network and contains larger lymphatic vessels with valves. In general, the lymphatic vessels have a uniform shape in firm and thick skin areas but have a variable shape in thin and loose skin areas ( ). Lymphatic capillaries, the likely segment of lymphatic vessels where the resorption of fluid and macromolecules takes place, have several distinct features in comparison to arterial capillaries, and these differences are stated in Table 12.1 .





