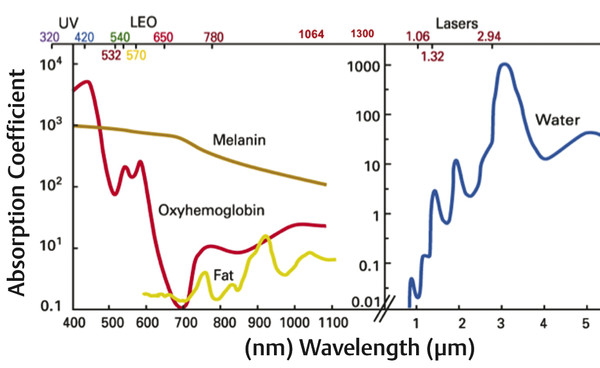
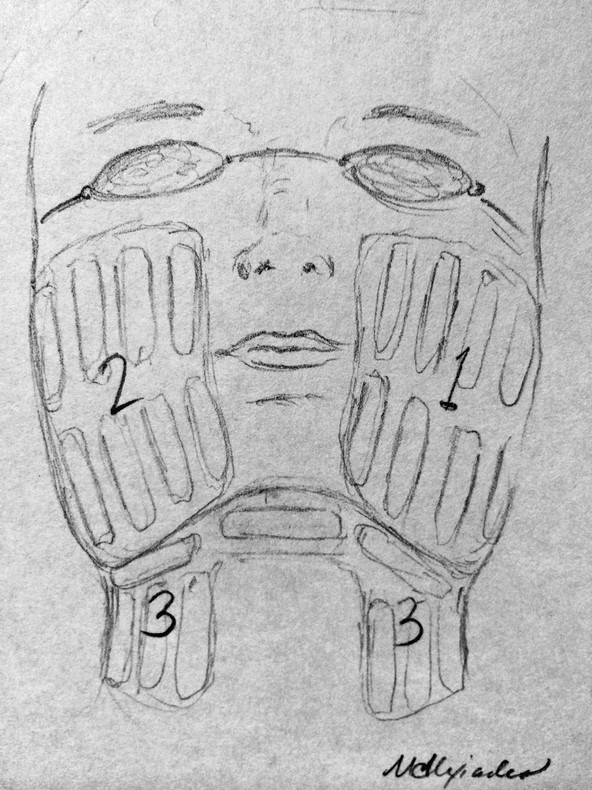
Quantitative comprehensive grading scale of rhytids, laxity, and photoaging Grading scale Descriptive parameters Categories of skin aging and photodamage Rhytids Laxity Elastosis Dyschromia Erythema/ Telangiectasia (E/T) Keratoses Texture 0.00 None None None None None None None None 1.00 Mild Wrinkles in motion, few, superficial Localized to nasolabial folds (NLFs) Early, minimal yellow hue Few (1–3) discrete small (< 5 mm) lentigines Pink E or few T, localized to single site Few Subtle irregularity 1.50 mild Wrinkles in motion, multiple, superficial Localized, NLFs and early melolabial folds (MLFs) Yellow hue or early, localized periorbital (PO) elastotic beads (EBs) Several (3–6), discrete small lentigines Pink E or several T localized two sites Several Mild irregularity in few areas 2.00 Moderate Wrinkles at rest, few, localized, superficial Localized, NLFs/MLFs, early jowls, early submental/submandibular (SM) Yellow hue, localized PO EBs Multiple (7–10), small lentigines Red E or multiple T localized to two sites Multiple, small Rough in few, localized sites 2.50 Moderate Wrinkles at rest, multiple, localized, superficial Localized, prominent NLFs/MLFs, jowls, and SM Yellow hue, PO and malar EBs Multiple, small and few large lentigines Red E or multiple T, localized to three sites Multiple, large Rough in several, localized areas 3.00 Advanced Wrinkles at rest, multiple, forehead, PO and perioral sites, superficial Prominent NLFs/MLFs, jowls and SM, early neck strands Yellow hue, EBs involving PO, malar and other sites Many (10–20) small and large lentigines Violaceous E or many T, multiple sites Many Rough in multiple, localized sites 3.50 Advanced Wrinkles at rest, multiple, generalized, superficial; few, deep Deep NLFs/MLFs, prominent jowls and SM, prominent neck strands Deep yellow hue, extensive EBs with little uninvolved skin Numerous (> 20) or multiple large with little uninvolved skin Violaceous E, numerous T with little uninvolved skin Little uninvolved skin Mostly rough, little uninvolved skin 4.00 Severe Wrinkles throughout, numerous, extensively distributed, deep Marked NLFs/MLFs, jowls and SM, neck redundancy and strands Deep yellow hue, EBs throughout, comedones Numerous, extensive, no uninvolved skin Deep, violaceous E, numerous T throughout No uninvolved skin Rough throughout Note: This 4-point grading scale, created by the author of this chapter, has been extensively tested and employed for evaluating laser and energy-based cosmetic treatments.1,2,3,5,6,24,56 Clinical improvement of facial rhytids following laser treatment was initially observed when treating other aspects of photoaging.7,8,9,10,11,12 This finding led to histological and clinical confirmation of neocollagenesis and rhytid reduction following treatment with lasers in the visible, near-infrared, and midinfrared spectrums.7,8,9,10,11,12 Among the early lasers and light sources, the potassium titanyl phosphate (KTP) laser (532 nm), pulsed dye laser (PDL) (585 and 595 nm), IPL (515–1,200 nm), neodymium:yttrium aluminum garnet (Nd:YAG) (1,064-nm Q-switched, 1,064-nm long pulse, 1,319, and 1,320 nm), diode (980 and 1,450 nm), and erbium glass (Er:glass) (1,540 nm) all demonstrated some degree of efficacy for facial nonablative resurfacing. However, midinfrared devices, owing to deeper penetration of longer wavelengths and lower absorption by epidermal melanin, were the most effective for deeper rhytids and acne scarring after a series of treatments.10,13,14,15,16,17,18,19,20,21,22,23,24 These findings were followed by the development of BBIR devices, which have delivered significant reductions in rhytids and skin laxity of the face and neck, and a histological correlation of both neocollagenesis and neoelastogenesis.5,25,26,27,28 In contrast, IPL established itself as a mainstay for the treatment of all categories of photoaging, most notably vascularity and dyspigmentation, and to a lesser degree, rhytids.4,29,30,31,32,33,34 The histological and ultrastructural effects of IPL include neocollagenesis, and effects on dermal fibroblasts, resulting in induction of collagen, matrix metalloproteinases (MMPs), and transforming growth factor (TGF) gene expression.4,30,32 The application of IPL (ranging between 500–1,300 nm) for the treatment of skin aging has proven to be a first-line procedure for the reduction of the signs of photoaging and, to a lesser degree, rhytids.1,2,3,33 The advantage of IPL is its ability to target both melanin and hemoglobin, resulting in global improvement in dyspigmentation and vascularity. The term photorejuvenation was coined in describing the global improvement in multiple parameters of photoaging that is observed with the IPL.29,31,35,36 Filters may be placed to exclude shorter wavelengths, thereby preferentially targeting various chromophores. Its use for wrinkle reduction has been assessed with evidence of multiple histological changes after treatment.1,4,30,32 The patient perception of improvement is increased likely due to apparent decreases in dyspigmentation and vascularity, which are more easily detectable than mild changes in rhytids, therefore placing this device in the mainstream of nonablative resurfacing. The ideal patient for treatment with IPL is one who exhibits the signs of photoaging, namely dyspigmentation, vascularity, and mild-to-moderate rhytids of the facial skin (▶ Table 21.1). On physical examination, lentigines and telangiectasias should be present. In addition, fine rhytids and, to a lesser degree, coarse rhytids may respond but this varies from patient to patient. IPL is the treatment of choice for light-skinned patients with photoaging who present at any age with pigment, vascularity, and/or concomitant rhytids. This modality is appropriate for younger patients in their third to fourth decade who manifest early signs of photoaging and fine rhytids, and for whom standard ablative resurfacing may be considered too aggressive. Owing to the lack of thermal injury, patients may receive multiple treatments over the course of many years without developing the textural changes that may be a complication of repeated procedures with fractional laser resurfacing. Therefore it may be recommended in a series, followed by yearly maintenance treatment sessions. Patients less likely to respond to IPL are those lacking the features of photoaging (▶ Table 21.1). Patients lacking dyspigmentation or vascularity will unlikely be able to appreciate the benefits of IPL. Those presenting primarily with advanced-to-severe rhytids and/or laxity (▶ Table 21.1) are not appropriate candidates for IPL because this device has little-to-no efficacy in the treatment of skin laxity, such as jowels or folds, and limited efficacy on rhytids of an advanced severity. Additionally, patients with more advanced grades 3 to 4 rhytids (▶ Table 21.1) are better candidates for standard or fractional ablative laser resurfacing than IPL, and are less likely to attain appreciable improvement in their rhytids through IPL alone. Those whose primary concern is skin laxity are better candidates for BBIR, other skin tightening technologies, or the surgical facelift. The range of procedure options must be presented at time of consultation. Contraindications for treatment include: pregnancy, breast-feeding, history of isotretinoin use in the preceding 6 months, keloids, connective tissue disease, or a history of adverse events following IPL. Patients with a history of frequent, recurrent Herpes simplex and/or Staphylococcal infections should be premedicated with antimicrobial antibiotics such as famciclovir or dicloxacillin, respectively. Tanned or dark-skinned (Fitzpatrick skin type IV or V) patients should be treated only by a highly experienced clinician and with very conservative settings. Alternatively, tanned and/or darkly pigmented skin should be considered relative contraindications. Skin type VI patients are contraindicated because the vast majority of IPL technologies do not provide settings to safely treat such darkly pigmented skin. The ideal IPL patient presents with the findings of photoaging, manifested by lentigines and telangiectasias (▶ Table 21.1). Proper patient selection will ensure high patient satisfaction. The advantages of IPL include the nonablative aspect of the treatment, the absence of perioperative discomfort, and the immediacy of posttreatment recovery. No anesthesia is necessary because the treatment is typically painless or associated with minimal discomfort. Immediately following the procedure, the skin may appear slightly erythematous; however, this resolves within minutes. No erythema, blistering, wounding, or obvious crusting should be observed posttreatment, obviating any need for wound care. For these reasons, IPL is considered a “no downtime” process, with patients returning to normal activities immediately following treatment. The other key advantages are the visible improvements that are appreciated by the patient within weeks and engender a high degree of patient satisfaction. The disadvantages of IPL include the need for multiple treatments, the subtlety of reduction to rhytids, the incompleteness of removal of dyspigmentation/vascularity, and the risk of adverse events. In years past, IPL instruments typically required five to six monthly treatments in order to achieve appreciable results. However, recent technological advancements have boosted treatment efficacy, resulting in shorter required treatment cycles of three monthly sessions. Despite a relatively high level of efficacy in the treatment of photoaging (see section 21.4.3), complete clearance of all lentigines and telangiectasias is unlikely. This must be made clear to the patient at the outset. In addition, IPL is less effective on rhytids, with minimal improvement expected. Another important disadvantage of IPL is the unpredictability of adverse events in certain skin types. For example, patients with actinic bronzing, or excessively photoaged skin on sun-exposed areas, may develop crusting throughout the sun-damaged areas of the skin following a session at conservative settings. Similarly, patients who tan between sessions and do not report this to the clinician may crust following treatment despite relatively low settings. Unexpected crusting typically heals with areas of temporarily decreased pigment levels as compared to surrounding bronzed or tanned skin, causing an uneven color complexion to the skin. This adverse event typically resolves over time without scarring; however, it is very disconcerting to the patient and clinician and must be treated promptly. In the event of crusting, topical corticosteroid and/or silver sulfasalazine ointment and close follow-up should be prescribed. In light of such adverse events, IPL―often given to a physician-extender to perform―can be unpredictable and requires a great deal of oversight and experience. Always review history and technique settings on every patient prior to treating with IPL. If the patient has had sun exposure in the preceding 6 weeks, decrease the power or energy output on the mechanism or defer treatment until the tan has faded to avoid the pitfall of unexpected crusting posttreatment. Instruct patients to avoid sun exposure prior to and between treatment sessions. Sessions should not be scheduled if the patient has had extensive sun exposure or tanning in the preceding 6 weeks. Patients should immediately report to the clinician performing the procedure any incidence of discomfort during the session and any crusting posttreatment. Patients should be instructed that lentigines form superficial crusts and flake off within 4 to 7 days. Therefore, the patient should not schedule any important social events within 1 week to 10 days following a procedure. Anesthesia is unnecessary for IPL. Premedication with antimicrobials is also unnecessary, except in the aforementioned instances (see section 21.2.2). Each IPL tool will have different cutoff filters for various applications (▶ Table 21.2). Make certain that the photorejuvenation filter is employed. This typically provides a cutoff at or approximately 500 to 560 nm depending upon the technology. The upper wavelength output on IPL instruments is typically around 1,200 nm. Each device provides guidelines for power or energy output and pulse duration for each skin type. The highest starting fluences and the shortest pulse durations are employed on lighter skin types. Darker skin types and tanned skin require lower fluences and longer pulse durations to avoid thermally heating the melanosomes in the epidermis. See ▶ Table 21.2 for the recommended settings on several IPL devices on the market. IPL device Manufacturer Spectral output (nm; filtered) Pulse duration (ms) Fluence (J/cm2) Spot size (mm) Harmony Alma 420–950 3–50 1–30 40 × 16 30 × 10 Skinstation Radiancy 400–1,200 10 3–7 36 × 12 Aurora Syneron 580–980 20–200 10–30 12 × 25 BBL Sciton 560–1,400 Up to 200 1–40 16 × 46 Xeo Cutera 500–1,100 Automatic 3–35 10 × 30 PhotoLight Cynosure 400–1,200 5–80 3–30 46 × 10 StarLux Cynosure (formerly Palomar) 500–1,200 0.5–600 Up to 50 12 × 12 Abbreviations: BBL, broadband light; IPL, intense pulsed light. Note: The standard photorejuvenation settings for several IPL devices are shown. Aqueous gel is applied to the skin surface. Initially, a few test pulses should be performed. Ask patients if they feel any discomfort. The pulse may cause a pinching or fleeting smarting, but should not feel hot or burning. Importantly, a patient’s skin should not feel a burning sensation after the pulse has concluded. If this is the case, lower the fluence and lengthen the pulse duration. Pulses are administered in rows across the forehead, followed by each cheek. Great care is taken to ensure that ample aqueous gel is applied so that the IPL handpiece tip makes full contact with the gel and skin. Pulses are administered in passes across each anatomical area. Additional care is taken around the nose to provide a great deal of aqueous gel such that full contact of the laser tip is made. Typically, two to three full-face passes are administered per treatment. The author has found it helpful to administer extra pulses to darker lentigines in order to achieve adequate clinical response. Immediately following a procedure, lentigines should appear to have a slightly erythematous ring surrounding them and slight edema; these findings correlate with their eventual crusting and desquamation. Immediately following treatment, minimal transient erythema is present. The patient should be given an instruction sheet detailing what to expect. Although lentigines will form crusts over the ensuing days and peel off, the remaining skin should not blister or scab. Patients should be told to notify the office immediately if blistering or crusting occurs. If there is vesiculation or crusting following the procedure, wound-care management should be implemented as soon as possible. The patient should be prescribed a silver preparation such as silver sulfasalazine and a topical corticosteroid such as fluocinolone acetonide 0.025% ointment to be applied twice daily. In the case of swelling, edema, or hiving, antihistamine should also be prescribed. Immediately upon identifying vesiculation, prolonged erythema, or thermal burns, ice-cold compresses should be applied for approximately 1 to 3 hours. Following this time period, prolongation of the cold compresses is of diminishing returns. At this point, attention should be turned to topical therapy. It is prudent to bring the patient back to the office the following day to monitor progress and to assist in wound care. Typically, if wound-care instructions are followed, the crusts heal with a transient period of relative hypopigmentation as compared to the surrounding photoaged skin; however, they eventually heal with even pigmentation upon further treatment. In darker skin types, postinflammatory hyperpigmentation may be observed; this eventually resolves without treatment over the course of several months. Topical antipigment agents such as glycolic acid or hydroquinone may speed its resolution. Should irregularity of pigment occur posttreatment, additional IPL treatments serve to even out the pigmentation. The broad range of wavelengths emitted from IPL devices (ranging from 500–1,300 nm) accounts for the ability of these tools to effectively target both melanin and hemoglobin in the skin (▶ Fig. 21.1).33 The high absorption of IPL wavelengths by melanin and hemoglobin correlates with the utility of IPL for improving the dyspigmentation and vascularity that characterize photoaged skin (▶ Fig. 21.1).34,35 As a result, the use of IPL has been consistently demonstrated to result in dramatic improvement in pigment and vascular abnormalities of photodamaged skin.1,2,4,29,30,31,32,33,34,35,36,37 The author conducted a clinical trial of a combination IPL and radiofrequency technology for the treatment of rhytids, laxity, and photoaging in 28 subjects, employing a quantitative 4-point grading scale of skin aging (▶ Table 21.1).2,3 In that trial, quantitative analysis demonstrated a mean 10.9% grade reduction per treatment across all categories of skin aging, and a mean 25% grade reduction across all categories of skin aging following an average of 2.4 treatments.2 Another clinical study calculated high but incomplete clearance rates of lentigines and telangiectasias ranging from 62.5 to 82% following three monthly treatments.37 Significant descriptive improvements in lentigines have been demonstrated in Caucasian38 and Asian skin.39 Additional clinical studies of IPL added to the findings of significant modest-to-moderate reductions in facial telangiectasias.40,41 Fig. 21.1 Absorption curves of chromophores in human skin: melanin, hemoglobin, and tissue water. The wavelengths emitted by intense pulsed light (IPL), ranging between 500 and 1,200 nm, are strongly absorbed by melanin and hemoglobin as shown by their absorption curves, resulting in improvements in dyspigmentation and vascularity (left-hand curve). In addition, the near-infrared wavelengths are absorbed by tissue water, which likely accounts for the dermal remodeling that is observed. Broadband infrared (BBIR) light (1,100–1,800 nm) wavelengths, which are more deeply penetrating, are strongly absorbed by tissue water, which accounts for their ability to achieve deep dermal heating and resulting improvements in skin rhytids and laxity (right-hand curve). (Courtesy of Alma Lasers, Caesarea, Israel.) Whereas the efficacy of IPL has been well established in the treatment of dyspigmentation and vascularity, studies conflict on the clinical efficacy of this modality for rhytids. Several split-face randomized trials comparing treatment with IPL alone to IPL in combination with other rejuvenating procedures have documented variable amounts of improvement in fine rhytids after IPL monotherapy.42,43 In addition, partial improvement after IPL has been detected in several uncontrolled studies.44,45,46 Other randomized, controlled, split-face trials with IPL showed improvement in dyspigmentation and vascularity, but no change in rhytids.34,47 For example, one of these was a randomized, split-face trial in which 32 women with mild-to-moderate rhytids received three once-monthly treatments with filtered IPL (530–750 nm, 7.5–8.5 J/cm2, 2 × 2.5-ms pulses, 10-ms interpulse delay) to one side of the face and no treatment to the other side. Greater improvements in telangiectasias, pigmentation, and skin texture were shown on the IPL side, but there was no significant difference in the effect on rhytids.34 Variability in the parameters across studies (e.g., fluence, pulse durations, and filtered wavelengths) may account for the inconsistency on the efficacy of IPL for rhytids. Importantly, among the trials that found IPL was ineffective for rhytids, the devices filtered the longer, more deeply penetrating wavelengths of IPL (750–1200 nm), a factor that in the author’s opinion likely compromised treatment efficacy.34,47 These clinical findings have been accompanied by histological changes indicative of a dermal remodeling effect, but most recently of a more youthful genetic expression program. In a number of studies with histological analysis of skin samples following IPL treatment, increases in extracellular matrix proteins and neocollagenesis have been reported.4,30,32,48 In contrast, some investigators failed to detect collagen or elastin fiber changes.49 Recently, the gene expression patterns of IPL-treated skin have been shown to reflect a younger gene expression pattern.50 In this study, investigators obtained skin samples from younger female volunteers, age < 30, and from site-matched untreated and treated skin of older female volunteers, age > 50―the latter after three monthly IPL treatments. The researchers studied the messenger ribonucleic acid (mRNA) transcript levels of 3,530 genes and identified those genes whose average expression level in older treated skin was closer to younger rather than older untreated skin. Mean gene expression levels in the treated older group were subtracted from mean gene expression levels in the untreated younger group, as well as from the untreated older group. If the difference in gene expression level was less with the untreated younger group compared with the difference with the untreated older group, the gene was operationally defined as “rejuvenated.” A total of 1,293 transcripts qualified as “rejuvenated genes.” Hierarchical clustering showed that the gene expression pattern of treated older skin more closely resembled that of untreated younger skin than untreated older skin from the same individuals. A twofold to fourfold increase in transcripts among markers typically expressed in younger untreated skin was observed.49 Thus histological findings are now being accompanied by genetic analysis to identify the proteins involved in youthful skin and support the role of IPL in turning on a more youthful genetic program. With the antecedent application of 5-amino levulinic acid (5-ALA) prior to the administration of IPL treatment, greater pigmentary and vascular enhancement is achieved while increasing the degree of improvement of fine wrinkles.1 The term photodynamic photorejuvenation has been applied to the use of IPL in the treatment of actinic keratosis (AK) and photodamage.50 Among all the light sources, IPL combined with ALA photodynamic therapy (PDT) has been the most extensively studied for use in photorejuvenation, largely stemming from the fact that IPL has independently been shown to rejuvenate skin while spanning wavelengths that activate the downstream photosensitizer of 5-ALA, protoporphyrin IX (PPIX). A randomized, split-face design clinical study comparing ALA IPL to IPL alone demonstrated greater improvement on the ALA side in erythema, dyspigmentation, and fine rhytids following two monthly treatments.43 Another IPL following a 1- to 2-hour incubation of topical ALA resulted in crusting when fluences above a certain threshold were delivered.51 ALA IPL varies in both clinical response and side-effect profile, likely due to the variability of different IPL devices in wavelength irradiances (▶ Table 21.2). It should be noted, however, that IPL-mediated PDT typically causes photosensitivity for at least 48 hours posttreatment or longer depending upon the duration of 5-ALA incubation and degree of photodamage and AK. Therefore, proper preoperative and postoperative instructions must be administered to the patient in order to avoid phototoxicity. The author recommends a 1-hour incubation and IPL at photorejuvenation settings with the longest pulse duration, followed by strict bright-light avoidance for 48 hours posttreatment. In a patient with photoaging and AK, application of 5-ALA followed by IPL should be considered in an effort to optimize clinical outcomes. However, strict bright-light avoidance posttreatment is necessary to avoid phototoxic reactions. The application of BBIR to the treatment of facial and neck skin rhytids and laxity has contributed greatly to the laser, light, and energy-based technologies designed to treat skin aging.1 When applied and used properly, BBIR-emitting wavelengths ranging between 800 to 1,600 nm, depending upon the device, yield consistent and reproducible refinements in skin rhytids and laxity to the face and neck without recovery time, side effects, or significant complications.5,6,52 Skin laxity on the face and neck is manifested by progressive loss of skin elasticity, loosening of connective tissue framework, deepening and redundancy of skin folds, and progressive prominence of submandibular and submental tissues. A classification scale of skin laxity has been validated that categorizes progressive appearance of hallmark clinical findings into clinical laxity grades (▶ Table 21.1).2,3 Intrinsic genetic factors and extrinsic factors, such as photoaging, contribute to skin laxity, as evinced by genetic skin disorders with mutations in filaggrin and other elastin genes, and the histopathology findings of solar elastosis of photoaged skin, respectively.53,54 Infrared wavelengths were first employed for volumetric heating and treatment of skin laxity with the introduction of the 1,100 to 1,800-nm infrared light device (Titan, Cutera, San Francisco, California), which was Food and Drug Administration (FDA)-approved for deep dermal heating in 2006.5,55 Soon after, a variable-depth targeting infrared laser (1,310 nm, Candela Wayland, Massachusetts) was also shown to effectively treat skin laxity.24 Thus, infrared wavelengths were found to be effective for the treatment of skin laxity, although until now reserved by FDA approval to the treatment of rhytids and/or deep dermal heating. As BBIR technologies are applied on the skin surface, a major limiting factor in energy delivery to dermal targets is the heating of the epidermis and dermoepidermal junction (DEJ). The risk of thermal injury was particularly prohibitive of fluence delivery when a stationary technique of pulse application was used; this risk has been greatly diminished by the mobile technique developed by the author.5,56 The mobile protocol was first developed for a radiofrequency technology in an effort to increase the speed and efficiency of radiofrequency energy delivery to dermal structures, while allowing for cooling of epidermal and DEJ structures.56 Higher fluence delivery was achieved by moving the handpiece on the skin in a continuous motion, which was shown to render the treatment painless and virtually eliminate the risk of burns or complications.56 In that publication, the author put forth the calculation that the thermal relaxation time of the cutaneous pain sensory nerve fibers was substantially shorter than that of the dermal collagen fibers.56 Mobile delivery therefore allowed for cooling of these superficial sensory afferents while continuing to deposit energy into the large dermal targets, thereby precluding the firing of pain afferents and rendering the process painless.56 The mobile energy delivery approach was subsequently applied to BBIR 1,100 to 1,800 nm (Titan, Cutera) in a successful effort to augment fluence delivery, patient tolerance of high fluences, and to increase safety.5 As was the case for mobile radiofrequency delivery, the mobile delivery of BBIR precluded the need for topical anesthesia, rendered the treatment painless, and safely allowed for a 30% increase in fluence dosage delivered per pulse, as well as an increase in the pass count to eight passes.5 This increased energy delivery correlated with higher efficacy and response rates. Careful patient selection is paramount to attaining meaningful improvements and in meeting patient expectations with BBIR. The classification of neck laxity and photoaging into quantitative grades has been previously published and evaluated for clinical trial use by the author (▶ Table 21.1). Treatment with BBIR is indicated for patients with a baseline skin laxity of 2 to 3 on the 4-point quantitative laxity grading scale (▶ Table 21.1). In contrast, patients with severe skin laxity of grades 3.5 to 4 are not proper candidates for BBIR because the degree of improvement is unlikely to yield patient satisfaction. Patients over age 65 are less likely to respond to skin-surface applied nonablative procedures, and should be considered for this treatment approach on a case-by-case basis. Patients with a history of thyroid or parathyroid disease or neoplasia are contraindicated. The consultation should entail the presentation of all treatment options, including the surgical facelift. Patients should be offered all the treatment options for treating their skin rhytids and laxity, including but not limited to injectables, alternative laser and light-based treatments, surgical options, and no treatment. The rare but real risk of a thermal burn and scar should be discussed during informed consent, in addition to the usual and customary risks and complications of any medical procedure. Premedication and anesthesia are not necessary. BBIR treatments are FDA-approved for deep dermal heating and the application for the treatment of rhytids and skin laxity are off-label. Proper patient selection is critical to achieving patient satisfaction, with laxity grades 2 to 3 being the ideal candidates and grades 3.5 to 4 being poor candidates for meaningful improvements (▶ Table 21.1). Provide patients with a full understanding of treatment options and level of expected efficacy in order to avoid the pitfall of not meeting patient expectations. The advantages of BBIR for the treatment of skin rhytids and laxity include the excellent safety profile, lack of downtime, and natural aesthetic outcome. The mobile technique has been associated with no thermal burns to date and no or transient discomfort. Patients report a sense of warmth, but no burning sensation with this treatment approach. As a result, the procedure requires no anesthetic. Whereas transient perioperative and postoperative erythema are desired clinical end points, the erythema resolves within minutes. Therefore, there is no recovery time necessary for the procedure. Adverse events are very rare, particularly with the mobile protocol. Finally, the aesthetic outcomes are exceedingly natural. Because the clinical improvements are due to neocollagenesis and neoelastogenesis without vaporization or coagulation of tissue, the tissue-tightening effect follows the natural vectors to the skin and the texture or color of the skin is not altered as may sometimes be seen with ablative or fractional nonablative technologies. The disadvantages of this approach include the need for multiple treatments, the need for numerous passes during procedures, the modest efficacy per treatment, and the sometimes-subtle clinical outcomes. For these reasons, the guidelines for the patient consultation and patient selection previously described must be adhered to. Patients should be educated that a range of two to four monthly treatments is necessary for significant improvement. In addition, it is imperative to take baseline digital photographs of all patients so as to track progress with each treatment. It is of paramount importance that the clinician delivers an adequate number of passes in order to attain and maintain target temperature for enough time to induce clinical changes. The number of pulses and/or total energy delivery targets must be followed in order to attain a clinical response. It is advised to review the level of response after the first session with clinical pretreatment and posttreatment photographs. In the author’s experience, the patient and clinician should appreciate progress at 1 month following the first procedure, or an alternative treatment course should be considered. Baseline and follow-up digital photographs are strongly advised at each visit. Progress should be observed at 1 month following the first treatment as a predictor for significant clinical outcome from a treatment series. If the patient does not observe an improvement after the first treatment, a review of baseline and follow-up photographs can serve to prevent the pitfall of patient dissatisfaction. Photography: Baseline and follow-up photography front view, three-quarter views, and from both side views, are necessary. Patients should be advised that clinical results will typically start to manifest at 2 weeks following the first treatment and the results continue to accumulate to approximately 6 months follow-up. It should be explicitly stated that two to four monthly treatment sessions are required for meaningful improvements to be appreciated for patients with baseline laxity grades of 2 to 3. Photographs should be repeated at each treatment and follow-up visit. Patient preparation: No topical anesthetic is needed for the procedure. A thin 1-mm layer of aqueous ultrasound gel is applied. The typical treatment areas includes the lower face and neck, excluding the thyroid region (▶ Fig. 21.2). Maintain complete contact of the treatment tip with the aqueous gel and skin surface throughout each pulse. Fig. 21.2 Anatomical treatment areas for application of broadband infrared (BBIR) light. Using the mobile application protocol, procedure should commence on one of the two lower cheek areas. Treatment pulses should be applied sequentially, as described in the treatment protocol, until confluent erythema and heating are attained in a treatment area. The handpiece should be moved to the next procedure area until target temperature and confluence of erythema are achieved. Once all treatment areas are equivalently heated and erythematous, maintenance pulses are administered throughout until total energy delivery, as detailed in the protocols, is reached.
21.2 Intense Pulsed Light
21.2.1 Background
21.2.2 Patient Selection: Indications and Contraindications
Pearls and Pitfalls
21.3 Advantages and Disadvantages
21.3.1 Pearls and Pitfalls
21.4 Preoperative Instructions
21.4.1 Treatment Protocol
21.4.2 Postoperative Care/Preventing and Managing Adverse Events
21.4.3 Clinical, Histological, and Genetic Results
21.4.4 IPL-Mediated Photodynamic Therapy
21.4.5 Pearls and Pitfalls
21.5 Broadband Infrared Light
21.5.1 Background
21.5.2 Patient Selection: Indications and Contraindications
Pearls and Pitfalls
21.5.3 Advantages and Disadvantages
Pearls and Pitfalls
21.6 Preoperative Instructions
Light Therapy for Aging Facial Skin: Intense Pulsed Light and Infrared Broadband Light
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








