(1)
Professor of Plastic Surgery, Director of Diabetic Wound Center, Director of Cell Therapy Laboratory, Korea University College of Medicine and Korea University Guro Hospital, Seoul, Republic of Korea (South Korea)
Abstract
Wound infections delay and may even prevent wound healing. Management of wound infection cannot be overstated for successful wound healing. To properly manage wound infections, there must first be accurate diagnosis as to what constitutes an infected wound. Various diagnostic methods for wound infection are presented in this chapter. After identifying the offending organism, the physician must determine the most appropriate intervention to reduce the wound’s bioburden. Available options include antimicrobial therapy, debridement, and adjunctive therapies. Debridement plays a vital role in the management of wound infections. Debridement of necrotic tissue and exudate helps to reduce wound bioburden and may also increase the effectiveness of topical antimicrobials and antibiotics. There are six primary types of debridements: autolytic, enzymatic, biological, mechanical, sharp, and surgical debridements. Surgical debridement is required for severe infection including osteomyelitis or sepsis and is usually followed by a course of antibiotics. Details of each debridement are described in this chapter. Biofilm is a relatively new concept in the fields of wound infection and healing. Although scientific research regarding wound biofilm is increasing, little is known about the effective management strategies. The author’s method to treat the wound biofilm is presented.
Keywords
Diagnosis of infectionAntimicrobial therapyDebridementBiofilmAn infection is defined as invasion and multiplication of microorganisms in body tissues, especially that causing local cellular injury. The infecting organism is called pathogen. Inflammation is the host’s response to infection.
The three key parameters determining the development of infection are the number of microorganisms, virulence of microorganisms, and host resistance. Host resistance is the most important factor for the development of infection. The skin has several built-in mechanisms to protect the body from infections. The slightly acidic pH of the skin discourages microbial growth. Epithelial cells and lipids form a protective barrier against microbial invasion. Immune cells (polymorphonuclear neutrophils, Langerhans cells, and macrophages) destroy pathogens. Resident microflora also protects the body from pathogenic organisms.
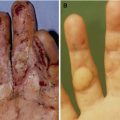
Factors that increase the risk of infection include a break in skin integrity, dry and cracked skin, alterations in barrier function, host characteristics (DM, nutrition, steroids, etc.), ischemia, and the presence of epidermal cysts or foreign debris (Fig. 7.1).
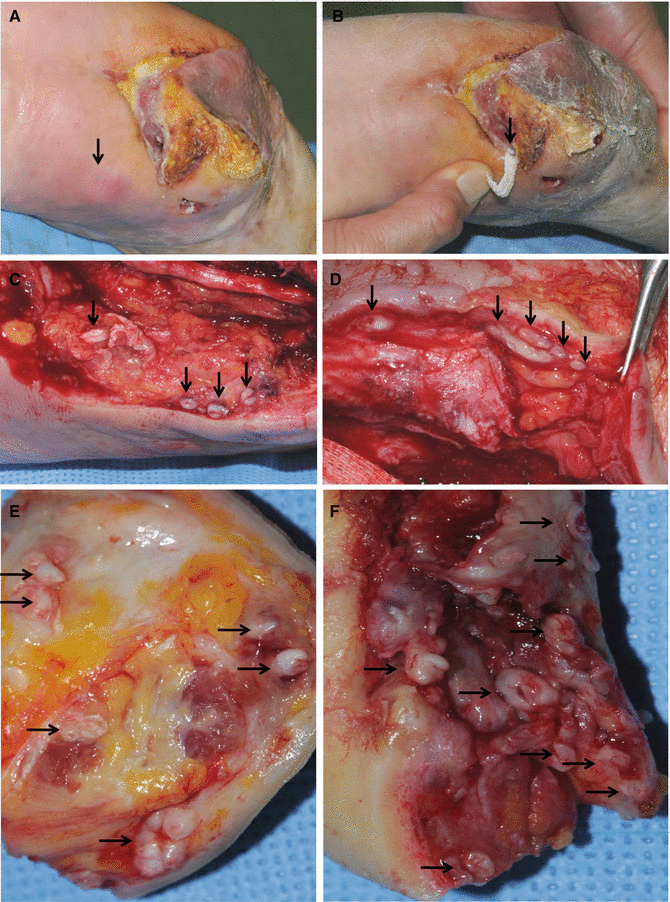

Fig. 7.1
(A) Wound infection (an arrow) spreads under the sebaceous skin area. (B) A cheese-like material (keratin) drained from the area by pressure. (C, D) After surgical debridement, multiple epidermal cysts (arrows) were exposed. (E, F) Close-up views
High concentrations of microbes adversely affect the host in four ways. First, the microbes compete with host cells for available oxygen and nutrients. Second, bacterial exotoxins may be cytotoxic, resulting in host cell dysfunction or death. Third, bacterial endotoxins may activate host inflammatory processes, increasing the production of matrix metalloproteinases. Fourth, wound infections delay and may even prevent wound healing.
Infection-causing microbes may be either endogenous, meaning that they are part of the normal microflora, or exogenous, meaning that they are from the environment. An acquired infection while an individual is hospitalized is called a nosocomial infection.
To properly manage wound infections, there must first be a consensus as to what constitutes an infected wound. There are some common concepts that explain the host–bacterial relationship related to wound infection. The presence of the nonreplicating microbes that make up the normal microflora of the skin is called contamination. Intact skin may be contaminated with a bacterial count of up to 103 microbes/g of tissue without any adverse tissue reaction. Wound colonization occurs if microflora adheres to the body surface and replicates to form colonies, but do not adversely affect the individual or cause a host response (Fig. 7.2). Critical colonization (subclinical infection or preinfection) is the theoretical turning point at which the number of bacteria becomes a bioburden and declines in wound status without the symptoms and signs of infection. A decline in wound status despite appropriate care is a signal of critical colonization (Figs. 7.3 and 7.4). Wound infection occurs when microorganisms multiply and invade viable body tissues. Wound infection generally occurs when 105 microbes/g of tissue is present (Fig. 7.5). However, bacterial quantity is only one of the three key pieces required, as described earlier. Certain types of bacteria are more detrimental than others, and their presence in any quantity warrants intervention for infection. Host resistance must be also considered. While contamination and colonization are normal status, critical colonization and infection are not.
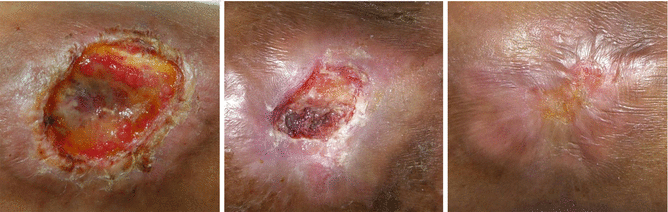
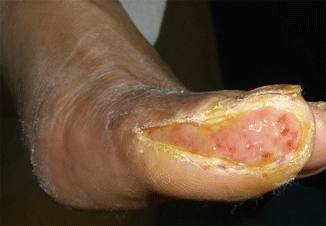
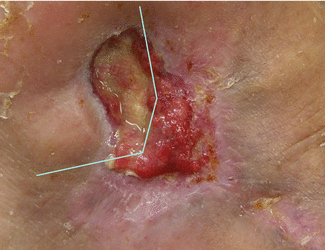
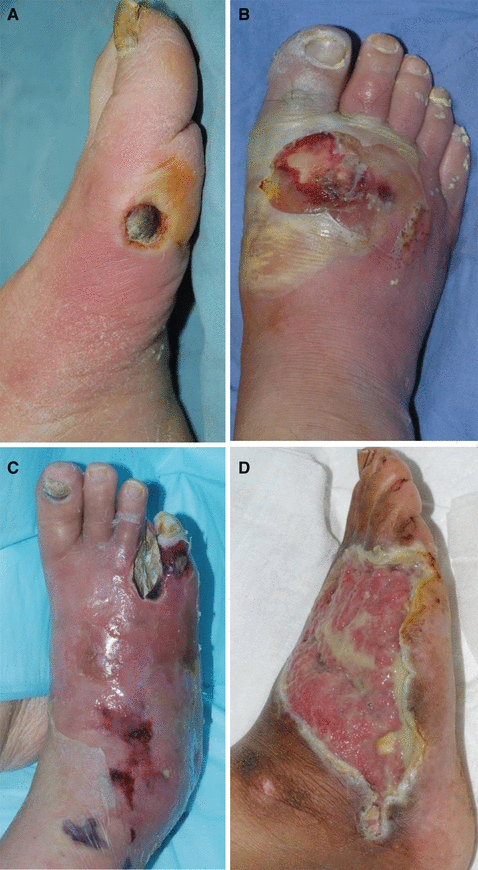

Fig. 7.2
A contaminated (or colonized) wound showing normal wound healing

Fig. 7.3
A critically colonized wound

Fig. 7.4
Some wound beds may be a mixture of the colonized and the critically colonized states. The left and the right sides of this wound demonstrate critically colonized and contaminated (or colonized) states, respectively

Fig. 7.5
Infected wounds
Diagnosis
Signs and Symptoms
The signs and symptoms of infection are the results of a struggle between the body’s immune system and the invading organisms. This response is similar to the cardinal signs of inflammation (redness, heat, swelling, and pain), but these signs are typically excessive or disproportionate to the size and extent of the wound due to increasing wound bioburden. Because of the similarities in presentation, a normal inflammation is sometimes mistaken for a wound infection.
An inflamed wound has a well-defined erythemal border. In contrast, an infected wound has a poorly defined erythemal border, a disproportionate amount of erythema, and possible proximally directed erythemal streaking.
An inflamed wound has a localized increase in temperature. In a patient with an infected wound, this response is magnified. Warmth extends further away from the site of injury, and this patient may also present a systemic increase in body temperature (fever).
An inflamed wound has a small amount of edema proportional to the wound. In an infected wound, the amount of edema is disproportionate to the size of the wound. Tissue around the wound may be indurated.
An inflamed wound may be painful. However, an increased pain level is consistent with a wound infection.
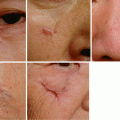
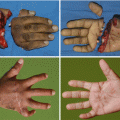
Sometimes an infection may be clinically silent or unapparent. Patients who are immunocompromised or who have inadequate perfusion are at a greater risk for silent infections (Figs. 7.6 and 7.7). An example of silent infection is a patient with severe arterial insufficiency. This patient may be unable to present sufficient response to an infection because of inadequate blood flow to the lesion.
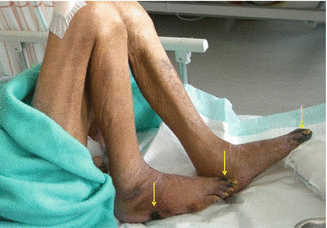
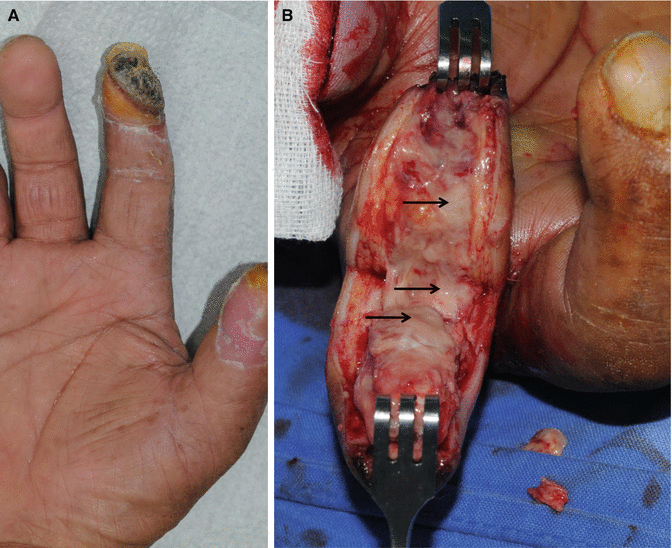

Fig. 7.6
Wounds (arrows) of silent infections. The wounds were finally diagnosed as infected by tissue biopsies, but there were no signs of inflammation

Fig. 7.7
Although the wound did not show inflammatory signs (A), there were several pus pockets (arrows) beneath the skin (B)
Tissue Biopsy
A wound culture confirms the presence or absence of infection. Tissue biopsy is the gold standard for wound culturing. Tissue biopsy samples should be obtained from a deep tissue since microbiology of the superficial and the deep tissues are commonly different in chronic wounds (Fig. 7.8).
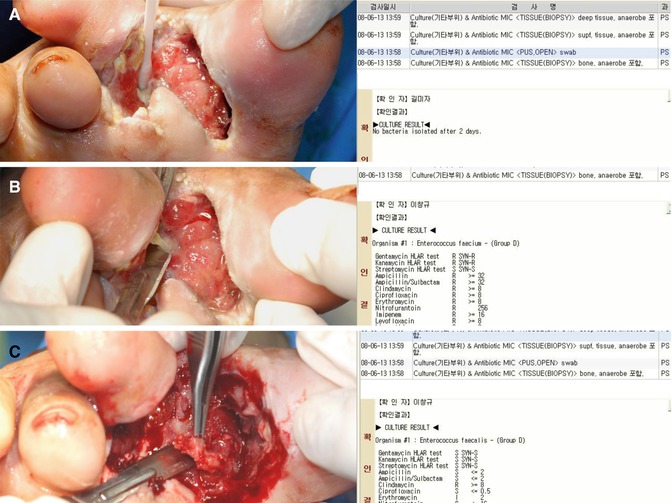

Fig. 7.8
The microbiologic results of tissue cultures can be different according to the sites of biopsy sampling. (A) A result of a swab culture. (B) A result of a superficial tissue culture. (C) A result of a deep tissue culture
Some wound beds are not of uniform depth and form sinuses or tunnels that invade to deeper tissues. The specimens for sinus wounds should be obtained from the deepest areas (Figs. 7.9 and 7.10). Bone biopsy is the gold standard in the diagnosis of osteomylitis.
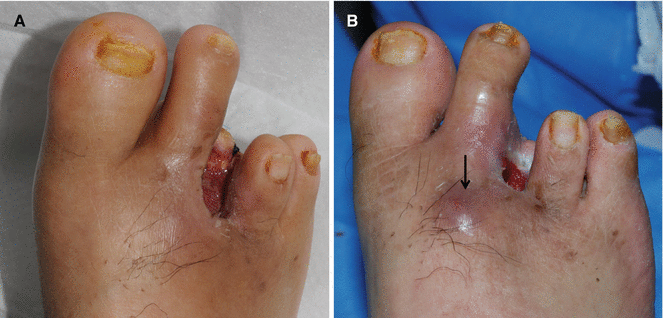
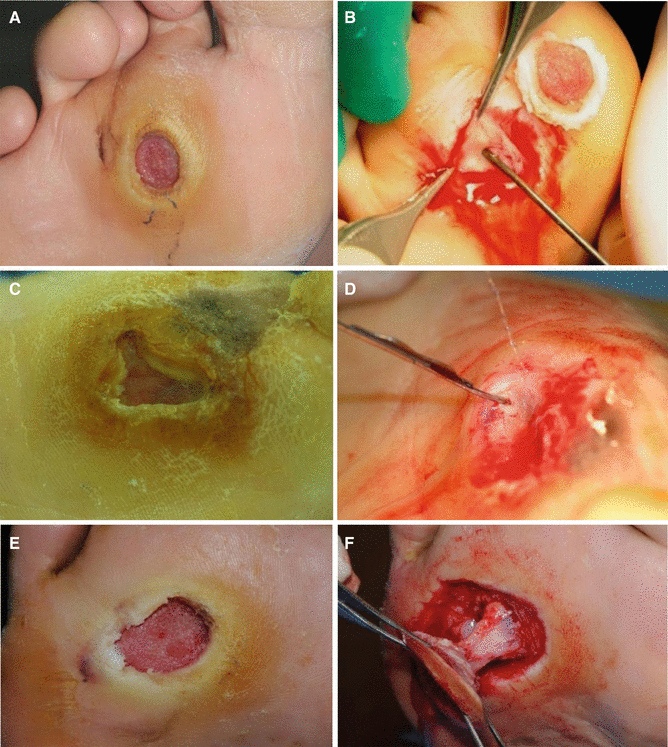

Fig. 7.9
(A) An open chronic wound. (B) One month later, wound infection developed at a site away from the original wound by tunneling of pathogens under the skin (an arrow)

Fig. 7.10
Examples of the sinus tracts or tunneling in chronic wounds. The left photographs are original wounds before debridement, and the right photographs are wounds after surgical debridement showing the sinus tracts of the wounds
Swab Culture
Swab cultures can be used to quantify the number and type of bacteria present in the wound. The benefits of swab culture over tissue culture are simplicity, lack of trauma, and avoidance of a surgical procedure. In addition, swab cultures can be performed by nurses and physical therapists. It is important to understand, however, that the results of swab cultures may only reflect surface contamination or colonization rather than the infecting microorganism.
Fluid Aspiration
Wound fluid can be drawn up into a needle for analysis. The benefit of fluid aspiration is that this procedure assesses bacteria within the tissue rather than on the surface. The dangers of this procedure include potential spread of infection, potential fistula formation, and damage to underlying structures.
Blood Tests
Serology involves identifying microbes by the increase of inflammatory markers or presence of serum antibodies within the infected individual. Inflammatory blood markers, such as white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) are commonly used to help diagnose the infection.
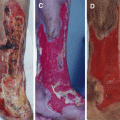
The author has carried out a study to evaluate the diagnostic usefulness of WBC, ESR, and CRP in the detection of diabetic foot infection. Peripheral blood samples were taken from 113 patients with diabetic foot ulcers. Diabetic foot infection was diagnosed according to the microbiological culture from soft tissue and bone specimens. Reference values of the tests were 4500–11,000/μl for WBC count, 0–20 mm/h for ESR, and 0–5 mg/ℓ for CRP. Sensitivities, specificities, and positive and negative predictive values of laboratory tests were calculated and compared. The results demonstrated that there was a significant difference in WBC, ESR, and CRP between the infection group and the noninfection group. The sensitivity of WBC, ESR, and CRP was 30 %, 96 %, and 84 %, respectively. The specificity was 86 %, 14 %, and 50 % for WBC, ESR, and CRP, respectively. The positive predictive values were 88 %, 78 %, and 84 %, and the negative predictive values were 28 %, 50 %, and 50 %, respectively. Based on the results of this study, it can be concluded that CRP is a more useful method to predict and diagnose infection than WBC and ESR in diabetic foot ulcer patients (Figs. 7.11 and 7.12).
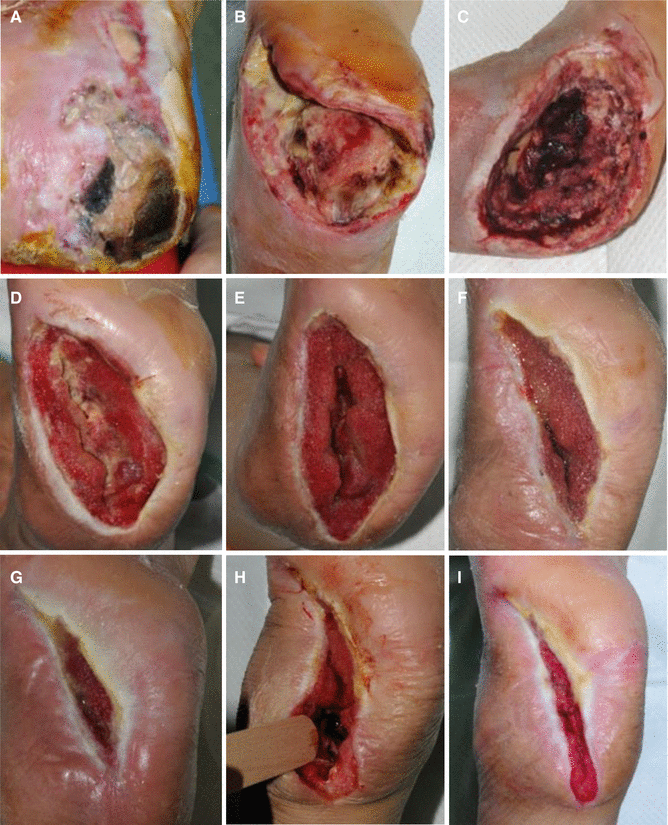
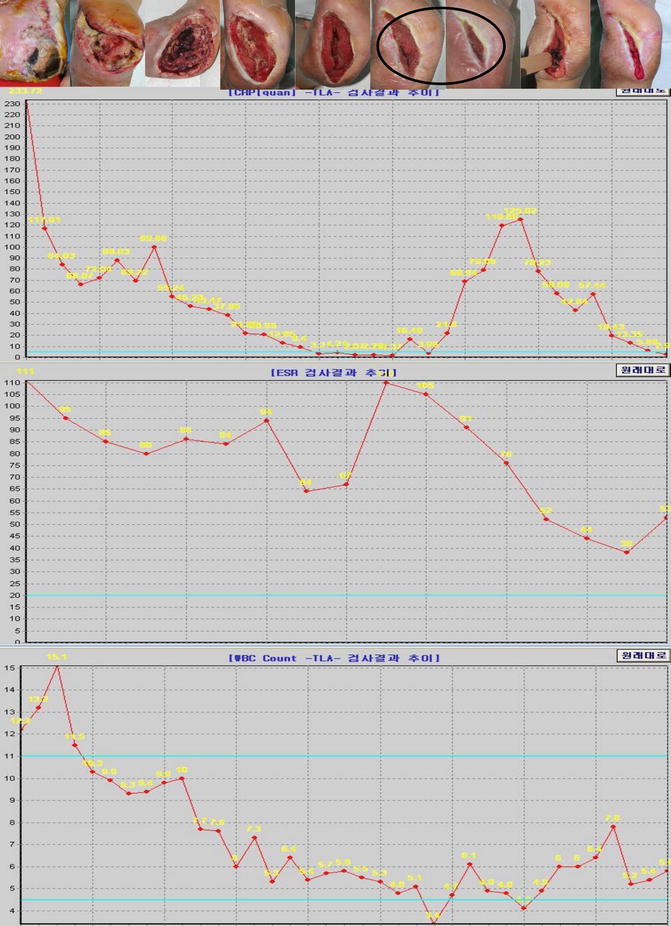

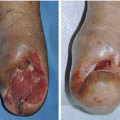
Fig. 7.11
(A) A severely infected wound of the foot. (B–F) Wound infection was gradually improved by antimicrobial therapy and surgical debridement. (G) However, the wound infection became worse again. (H, I) After another surgical debridement, the wound infection subsided

Fig. 7.12
Three graphs showing the changes of CRP, ESR, and WBC over time for the Fig. 7.11 wound. A circle represents the time when the wound infection worsened. The graphs demonstrate that CRP is more reliable to predict and diagnose infection than WBC and ESR
Imaging Tests
Imaging tests such as plain radiography, CT scan, bone scan, and MRI can be useful to detect the presence and the extent of soft tissue infection or osteomyelitis. In particular, MRI is useful to diagnose osteomyelitis, which is notorious for its chronicity and difficult eradication (Figs. 7.13, 7.14, and 7.15). Once a bone is infected, inflammatory cells enter the infected area, engulf the infectious organisms, and release enzymes that lyse the bone. The resultant bone debris and pus spread into the blood vessels in the bone, impair blood flow, and finally form areas of devitalized infected bone, known as sequestra. Therefore, early diagnosis of osteomyelitis is crucial in successful management of chronic wound infection.
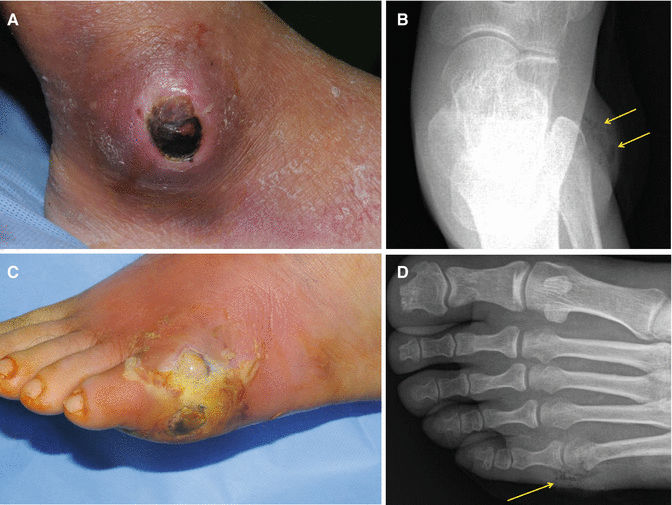
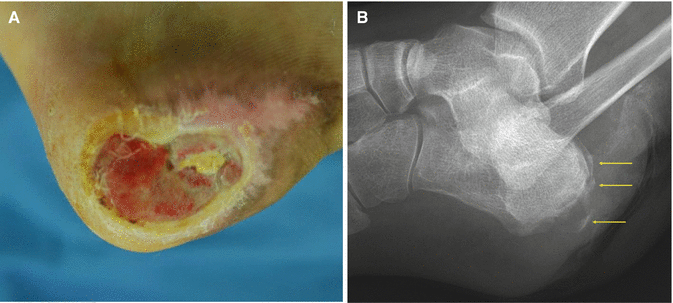
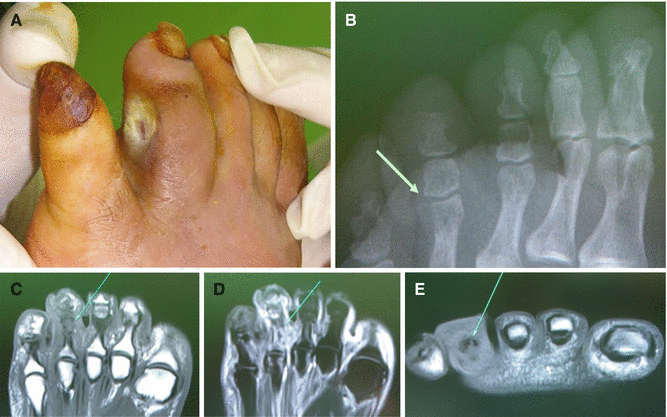

Fig. 7.13
Gross and plain radiologic findings of gas-forming bacterial infection (arrows). (A, B) An infected wound on the lateral malleolus. (C, D) An infected wound on the 5th toe

Fig. 7.14
Gross and plain X-ray findings of osteomyelitis (arrows)

Fig. 7.15
(A) A chronic diabetic ulcer on the 4th toe. (B) This wound did not show any abnormal findings (an arrow) including bony destruction on plain X-ray. (C–E) However, osteomyelitis of the phalangeal bones of the 4th toe (arrows) was diagnosed by MRI
Treatment
After identifying the offending organisms, the physician must determine the most appropriate intervention to reduce the wound’s bioburden. Available options include antimicrobial therapy, debridement, and negative pressure wound therapy (NPWT).
Antimicrobial Therapy
An antimicrobial agent is a substance that is able to destroy microorganisms. They include antibacterial agents that are effective against bacteria and antifungal agents that destroy yeasts and molds.
Bacteria that are unable to grow in the presence of a certain antimicrobial agent are considered sensitive to the drug. Bacteria that continue to multiply in the presence of a drug are considered resistant to the drug. Bacterial resistance may be natural or acquired.
Premature cessation of antimicrobials and misuse/overuse of antibiotics increase the possibility of resistance. The two most prevalent strains of resistant bacteria are MRSA and VRE. In general, MRSA infections may be nosocomial or community acquired, whereas VRE infections are more commonly seen in surgical wounds and urinary tract infections. Although resistant strains have become more prevalent, they have not become more resistant.
Topical Antimicrobial Therapy
Topical antimicrobials are applied to the wound surface and reapplied regularly. Solutions, ointments, and creams are available. Several types of antiseptic-impregnated wound dressings, such as iodine and silver, are also currently available. Silver is a broad-spectrum antimicrobial with rare resistance. Some problems with these dressings are cost and their cytotoxicity that retard wound healing (details in Chap. 2). Overuse of broad-spectrum topical antibiotics may possibly contribute to the development of resistant bacteria. In addition, if a dressing does not stay in contact with the wound bed, the effectiveness is decreased.
Advantages of topical antimicrobial therapy include lower cost than systemic therapy and their effectiveness in treating wounds with compromised circulation. Disadvantages include higher cost than non-antimicrobial agents, need for frequent applications, sensitivity or allergic reactions, and potential for resistance.
Systemic Antimicrobial Therapy
Systemic antibiotics may be prescribed for sepsis and signs of advancing infection, with or without topical antimicrobials. They may be administered orally, intramuscularly, or intravenously. Advantages of systemic antibiotics include effective reduction in bacterial load in deep tissue infection and ease of application for oral medications. Disadvantages include more severe adverse reactions, development of resistant bacterial strains, problems with missed doses, higher cost, and disruption in lifestyle due to intravenous administration.
Adverse Reactions
Generally, adverse reactions are infrequent and more severe in systemic antimicrobials compared to topical ones. Reactions vary from mild skin reactions to hives, difficulty in breathing, and anaphylactic shock. Common sensitivities are to penicillin and sulfa drugs.
Debridement
Debridement plays a vital role in the management of wound infections. Regular debridement of necrotic tissue and exudate helps to reduce wound bioburden (Fig. 7.16). Regular debridement may also increase the effectiveness of topical antimicrobials and antibiotics. Surgical debridement is required for osteomyelitis or sepsis and is usually followed by a course of antibiotics.
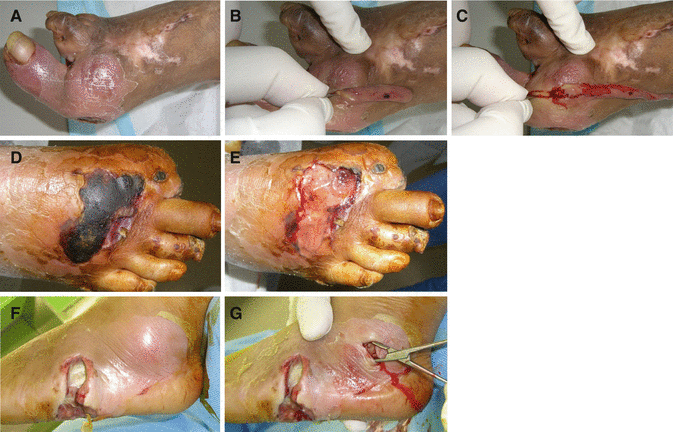

Fig. 7.16
Severely infected wounds should be first surgically debrided to remove infected tissues, bacteria, and toxins and to prevent growth of infection
Details of debridement will be discussed later.
NPWT
NPWT, also called negative pressure therapy (NPT) or a vacuum-assisted closure (VAC), is application of negative pressure from a vacuum and a tube computerized device that is connected to a dressing.
There are several benefits associated with this modality. NPWT provides a closed moist wound healing environment, helps to reduce contamination from outside bacteria, removes excess fluid from the wound and surrounding tissues, reduces cell death caused by dehydration, and improves blood flow to the wound. Therefore, NPWT can result in reducing wound bioburden. More details will be discussed in Chap. 8.
Hyperbaric Oxygen, Photodynamic Therapy, and Ultraviolet Light
Hyperbaric oxygen therapy is administration of 100 % oxygen to patients within an airtight vessel under increased atmospheric pressure. Hyperbaric oxygen therapy may help to reduce bacterial load, increase angiogenesis, and reduce edema. Details of hyperbaric oxygen therapy will be discussed in Chap. 11. Photodynamic therapy and ultraviolet light therapy may also be used for the treatment of infection. However, a significant controversy exists regarding the effectiveness of these modalities at present.
Debridements
Wound bed preparation is needed to optimize the wound environment for optimal healing by promoting a clean, moist, warm, granular wound bed, and protecting the periwound and the intact skin. Debridement, bacterial control, and exudate management are all part of wound bed preparation. This chapter will provide an in-depth analysis of wound debridement.
Debridement is the removal of necrotic tissue, foreign material, and debris from the wound bed. Debridement plays a vital role in wound management. Purposes of debridement are to decrease bacterial concentration, increase the effectiveness of topical treatments, improve the activity of leukocytes, shorten the inflammatory phase, free up energy for wound healing, remove the barrier for healing, and decrease wound odor.
Debridement is indicated in the presence of necrotic tissues, foreign materials, or debris within a wound bed. Large blisters and calluses should also be debrided.
There are three main contraindications to debridement. First, red granular wounds should not be debrided. Second, noninfected ischemic wounds are contraindicated. Third, current guidelines suggest that stable heel ulcers with dry eschar should only be debrided if they have edema, erythema, fluctuance, or drainage.
There are six primary types of debridements. These are autolytic, enzymatic, biological, mechanical, sharp, and surgical debridements.
Autolytic Debridement
Autolytic debridement uses the body’s endogenous enzymes to digest necrotic tissues with a moisture-retentive dressing.
Autolytic debridement provides moist wound healing. It is considered the most conservative, least invasive, and least painful method of debridement. Autolytic debridement is also easy to teach to patients and caregivers. It may reduce the long-term cost of treatment. However, autolytic debridement requires time for the body to debride tissue and does not allow frequent visualization of the wound bed.
Autolytic debridement is indicated for noninfected wounds with necrotic tissue, patients who cannot tolerate other forms of debridement, and home or long-term care settings.
Autolytic debridement is not appropriate for infected or deep cavity wounds and wounds that require sharp or surgical debridement. If necrotic tissue fails to decrease in the expected amount of time, other types of debridements should be considered.
For autolytic debridement, eschar should be crosshatched, moisture-retentive dressings should be 2 cm larger than the wound, and periwound area must be protected. Signs and symptoms of infection should be observed.
Enzymatic (Chemical) Debridement
Enzymatic or chemical debridement refers to the use of a topical enzyme to remove devitalized tissue. Enzymatic debridement is a form of selective debridement. The three main types of enzymes used for enzymatic debridement are proteolytics, fibrinolytics, and collagenases.
Enzymatic debridement requires less advanced technique than sharp or surgical debridement. It is less painful than other methods except for autolytic debridement and easy to instruct patients and caregivers to perform. However, enzymatic debridement can be expensive and may require dressing changes up to three times a day.
Enzymatic debridement is appropriate for those who cannot tolerate sharp debridement, home or long-term care settings, and infected and uninfected wounds with necrotic tissue.
Enzymatic debridement is not appropriate for facial wounds, calluses (since enzymes cannot debride calluses), wounds free of necrotic tissue, and wounds with exposed deep tissues including tendons, blood vessels, and ligaments. If necrotic tissue fails to decrease in the expected amount of time, other types of debridements should be considered.
For enzymatic debridement, eschar should be crosshatched prior to application, and moist environment should be maintained.
Biological Debridement
Biological debridement is debridement of necrotic tissue using a live medical device such as maggots. Maggot therapy assists with wound debridement in two ways. Larvae produce and release enzymes that degrade and/or liquefy necrotic tissue without harming viable tissue. They also ingest necrotic tissue and bacteria (Figs. 7.17 and 7.18).
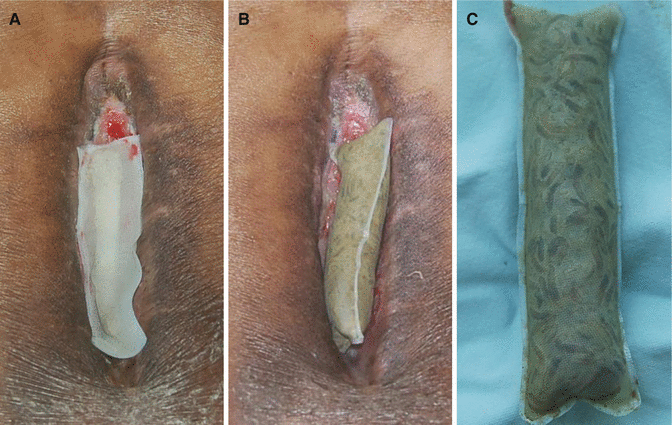

Fig. 7.17




A biologic dressing using maggots. (A) Immediate after application of the maggot dressing. Maggots are contained within a sealed pouch and placed on top of the wound. The dressing must be kept air permeable because maggots require oxygen to live. (B) Three days after the dressing. When maggots are satiated, they become substantially larger and leave the site of the wound. (C) A close-up view of the maggots inside the pouch
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree