Inherited ichthyoses
Non-syndromic
Mode of inheritance
Genes
Common ichthyoses
Ichthyosis vulgaris
Autosomal semi dominant
FLG
Autosomal recessive congenital ichthyosis
Harlequin ichthyosis
Autosomal recessive
ABCA12
Lamellar ichthyosis
Autosomal recessive
TGM1/NIPAL4/ALOX12B/ABCA12
Congenital ichthyosiform erythroderma
Autosomal recessive
ALOXE3/ALOX12B/ABCA12/CYP4F22/NIPAL4/TGM1/CerS3/PNPLA1/LIPN
Keratinopathic ichthyoses
Epidermolytic ichthyosis
Autosomal dominant
KRT1/KRT10
Superficial epidermolytic ichthyosis
Autosomal dominant
KRT2
Other forms
Loricrin keratoderma
Autosomal dominant
LOR
Erythrokeratodermia variabilis; Progessive symmetric Erythrokeratodermia
Autosomal dominant
GJB3/GJB4/GJA1
Peeling skin syndrome
Autosomal dominant
CHST8 (Type A); CDN (Type B); TGM5 or CSTA (acral type)
Keratosis linearis- ichthyosis Congenita- keratoderma (KLICK) syndrome
Autosomal recessive
POMP
Syndromic
X-linked ichthyosis syndromes
Recessive X-linked ichthyosis
X-linked recessive
STS
Ichthyosis follicularis atrichia photophobia (IFAP) syndrome
X-linked recessive
MBTPS2
Conradi-hunermann-happle Syndrome
X-linked dominant
EBP
Autosomal ichthyosis syndromes with
Prominent hair abnormalities:
Netherton syndrome
Autosomal recessive
SPINK5
Ichthyosis hypotrichosis syndrome
Autosomal recessive
ST14
Ichthyosis hypotrichosis sclerosing Cholangitis syndrome
Autosomal recessive
CLDN1
Trichothiodystrophy
Autosomal recessive
ERCC2/XPD ERCC3/XPB GTF2H5/TTDA
Prominent neurologic signs:
Sjogren-larsson syndrome
Autosomal recessive
ALDH3A2
Refsum syndrome
Autosomal recessive
PHYH/ PEX7
Mental retardation- enteropathy-deafness- neuropathy-ichthyosis- keratoderma (MEDNIK) syndrome
Autosomal recessive
AP1S1
Often fatal disease course:
Gaucher syndrome type 2
Autosomal recessive
GBA
Multiple sulfatase deficiency
Autosomal recessive
SUMF1
Cerebral digenesis- neuropathy-ichthyosis-palmoplantar keratoderma (CEDNIK) syndrome
Autosomal recessive
SNAP29
Arthrogryphosis-renal dysfunction-cholestasis (ARC) syndrome
Autosomal recessive
VPS33B
Other syndromic forms:
Keratitis-ichthyosis-deafness (KID) syndrome
Autosomal dominant
GJB2
Neutral lipid storage disease with ichthyosis
Autosomal recessive
ABHD5
lchthyosis prematurity syndrome
Autosomal recessive
SLC27A4
Management Strategies for DOC
Investigations for Diagnosis
Formal diagnostic guidelines for the DOC have not yet been established. Questions about history should ascertain prematurity and perinatal complications, presentation during the neonatal period (e.g., as a collodion baby, with erythroderma but not collodion membrane, blistering), course of the disorder, and experience with interventions. A pedigree should be obtained to suggest pattern of inheritance. Review of systems may reveal involvement of other organs (eyes, neurologic, etc.). Physical examination is crucial to define the cutaneous manifestations (character, severity and distribution of scaling, thickening and inflammation; presence of blisters or erosions; ectropion, eclabium, alopecia, and other hair changes) and observe any extracutaneous manifestations (see Table 5.1).
In some cases, diagnosis can be made prenatally. For example, pregnant mothers who carry a fetus with X-linked ichthyosis often have low serum unconjugated estriol levels during the second trimester, and FISH analyses can show the deletion in the fetal X-chromosome. Ultrasonographic findings may also suggest a diagnosis of ichthyosis, such as in Conradi syndrome (limb anomalies) or harlequin ichthyosis [3, 4]. If families have a risk of an affected fetus and the DNA mutation has been detected, prenatal diagnosis can be performed by amniocentesis or chorionic villus sampling to determine if the fetus is affected. Amniotic fluid itself may have a turbid appearance because of the large amount of desquamated skin floating in the amniotic cavity (the “snowflake sign”) [5]. Preimplantation diagnosis is a technique using in vitro fertilization that enables only unaffected blastomeres to be implanted; preimplantation has been performed for an at-risk family for Netherton syndrome [6].
In addition to DNA analysis for a specific genotype, laboratory testing can aid in the clinical diagnosis of several forms of DOC. Routine assessment of skin sections is often nonspecific, but may show specific histological features, such as the orthokeratosis and epidermolysis of epidermolytic ichthyosis and the suprabasal acantholysis, corps rond, and grains of Darier disease. Biochemical testing may also be helpful, such as in detecting high serum cholesterol sulfate levels in recessive X-linked ichthyosis and increased levels of phytanic acid in the cerebrospinal fluid of individuals with Refsum disease [7]. Simply observing blood smears will demonstrate the lipid-containing vacuoles in circulating white blood cells of neutral lipid storage disease [8]. Another relatively simple diagnostic tool is a hair mount; observation of broken hairs may demonstrate the characteristic trichorrhexis invaginata (“bamboo hair”) of Netherton syndrome, trichorrhexis nodosa (nodes along the hair shaft) or, under polarized light, the “tiger tail” patterning of trichothiodystrophy.
Genetic testing for mutation analysis is becoming more accessible as technology progresses, and can be a useful diagnostic tool for patients and families [9]. The more recent availability of amplicon-based tools for genotyping major forms of ichthyoses and of whole exome sequencing to find rare mutations in known genes and discover new genes that underlie DOC (for example, GJA1 mutations in a form of erythrokeratodermia variabilis) [10] have increased our understanding of these disorders and will drive pathogenesis-based personalized therapy.
General Approach to Therapy in Children
There is no cure presently for any of the DOC discussed in this chapter. The goals of therapy for DOC in infants and children are to: (i) preserve function; (ii) maximize the quality of life by improving itch, sleep, and appearance; and (iii) prevent complications such as infection, dehydration, and poor growth. For all children with DOC the mainstay of therapy is emollient application and bathing. Families spend hours a day on skin-directed therapy. In general, we take care not to subject young children to uncomfortable treatments for cosmesis alone. For children with widespread disease, specialists in the management of eye and ear complications should be engaged. Range-of-motion of fingers and toes should also be monitored carefully. Topical and systemic therapies, in addition to occupational and physical therapy, can be implemented to prevent or treat contractures. Because of their impaired barrier, infants and children with DOC lose water and heat through their skin, have greatly increased caloric needs, and can suffer from constipation. Their thicker stratum corneum and tendency keep their skin covered can lead to vitamin D deficiency and rickets [11]. Patients with DOC may also have micronutrient and vitamin deficiencies via other mechanisms as well [12]. In infancy and early childhood, weight and height should be measured frequently, and referrals to specialists in nutrition and gastroenterology should be considered for poor growth. Lastly, families and children can benefit from supportive networks of other families with similar conditions. The Foundation for Ichthyosis and Related Skin Types is an example of a wonderful patient and family support group (www.firstskinfoundation.org).
Specific Therapies
Emollients
Emollients (also called moisturizers) are considered a critical component of care for individuals with DOC. Emollients fill the empty spaces between the cells of the stratum corneum, resulting in a smoother and shinier skin surface [13]. There are two types of emollients: occlusives and humectants. Occlusives prevent transepidermal water loss (TEWL) and trap moisture derived from the deeper dermis and epidermis in the stratum corneum. Examples of occlusives include mineral oil, lanolin, and silicones. Petroleum jelly is by far the most effective occlusive, decreasing TEWL by 98 % when applied properly [13]. Humectants attract moisture from the environment when humidity is greater than 70 %. In less humid environments, humectants attract water molecules from the deeper layers of the skin. Examples of humectants include glycerin, propylene glycol, and alpha-hydroxy acids [14]. When humectants are used in isolation, they can actually increase TEWL, emphasizing the importance of use of humectants in conjunction with an occlusive moisturizer to retain skin hydration [15]. Hydration of the skin affects its barrier function, thus playing a role in both the integrity of the stratum corneum and influencing penetration through the upper layers of the epidermis [14]. Emollients are thought to decrease TEWL, creating an optimal environment for repair of the stratum corneum and its associated lipids. Emollients also play a role in lipid regeneration and maintenance, can decrease inflammation, and help to rebuild a defective barrier. However, a recent study showed that emollients (with both occlusive and humectants) improved skin dryness in individuals with RXLI, but had no impact on TEWL, pH, or gene expression [16], suggesting that more study is needed to understand the role of emollients in the ichthyoses.
Keratolytics
DOC are often associated with hyperkeratosis, which is an increase in the thickness of the stratum corneum. Substances used to treat hyperkeratosis are termed keratolytics. Several types of keratolytics are used to decrease the scaling of these disorders:
Lactic acid (LA) and glycolic acid (GA) are alpha-hydroxy acids. They decrease the hyperkeratosis associated with DOC by degrading corneodesmosomes, which promotes detachment of keratinocytes and accelerates stratum corneum turnover. Lactic and glycolic acids are commonly used for the treatment of DOC in combination with a petrolatum, cream, or lotion base and can be compounded or purchased over-the-counter in concentrations up to 12 %. Side effects include stinging, irritant contact dermatitis, and photosensitivity [13–15].
Salicylic acid (SA), a beta-hydroxy acid, is used in concentrations of 1–6 %. The proposed mechanism of action for SA is to reduce corneocyte adhesion. Systemic absorption, leading to salicylism, is a risk and metabolic acidosis, especially in children. SA is toxic to the CNS at concentrations >35 mg/dL. Tinnitus is an early warning sign, as it is correlates with plasma salicylate concentration [17]. Despite this, SA is safe in children as long as it is used on a limited area of involved skin.
Urea is both a keratolytic and a humectant. It causes physical alteration in the stratum corneum, leading to desquamation of corneocytes. Urea at a concentration of 10–25 % acts as a humectant and a mild keratolytic; concentrations of 40–50 % are required for hygroscopic keratolytic effects. Mild stinging and irritation are usually minimal, especially at lower concentrations [18].
Ten percent N-acetylcysteine can be added to low concentrations of urea (5 %) and used to treat widespread areas of lamellar ichthyosis. Mild stinging and irritation are reported side effects. Rosemary oil can be added to mask the smell of sulfur [19]. N-acetylcysteine may work via inhibition of keratinocyte proliferation [20].
Tar is both anti-inflammatory and anti-proliferative, causing progressive thinning of the stratum corneum. Although efficacious, tar can be difficult for patients to use due to its odor and color. Other side effects include phototoxicity, allergy, and possible carcinogenicity.
Propylene glycol can act as a keratolytic at concentrations of 10–20 %. It is a strong contact allergen sensitizer, and must be used carefully in patients who already have disturbed barrier function.
Antimicrobials
Bacterial colonization and infection, especially with S. aureus, and dermatophyte infection is not uncommon in the retained scale and fissured skin of individuals with DOC. In particular, overgrowth of bacterial organisms contribute to the skin odor and discomfort of children, such as with EI, the lamellar phenotype of ARCI, Netherton syndrome and keratitis-ichthyosis-deafness (KID) syndrome. Prophylactic use of antibiotics should be avoided, especially in neonates, in order to prevent the development of resistance and creation of a growth environment favoring Pseudomonas aeruginosa. Although chronic or repeated administration of antibiotics should be avoided, systemic intervention is necessary for infection. Cephalexin (10–15 mg/kg/dose given three times daily for 10 days) is most commonly administered for staphylococcal infection, although intervention should be based on resistance patterns if MRSA infection. Very localized bacterial infection can be managed by topical antibiotics. Bacterial infection tends to be characterized by pustules, abscesses, crusting, or increased erythema and swelling, and can be associated with discomfort. Maintenance intervention with antiseptics in the bath and antibacterial soaps (see section on “Baths”, below) are beneficial for colonization that leads to odor in the absence of infection [21].
Individuals with ichthyosis are also predisposed to the development of secondary fungal infections. Diagnosis of dermatophyte infection can be particularly tricky, given the generalized scaling (including of the scalp) and often associated inflammation of the ichthyosis. An annular patterning of the scaling and recalcitrance to therapy may be clues, but fungal infection may present only as worsening of itch and desquamation. The threshold for performing potassium hydroxide mount evaluation and fungal culture should be low in patients. Standard doses of griseofulvin (20 mg/kg/day divided into two doses; requires 2–4 weeks longer than terbinafine and other interventions), terbinafine (62.5 mg/day for 10–20 kg, 125 mg/day for 20–40 kg and 250 mg/day for more than 40 kg; given daily for 2–4 weeks for tinea corporis and 4–6 weeks for tinea capitis), itraconazole (5 mg/kg/day), or fluconazole (6 mg/kg/day). The dermatophyte infection in ichthyosis may be more persistent than in normal skin and require prolonged systemic antifungals courses [22].
Baths
Bathing should be encouraged to be a part of every ichthyosis patient’s daily regimen. Many patients with ichthyosis find that soaking for long periods (even an hour) hydrates the skin and facilitates scale removal. Bath additives can be soothing, antiseptics, and may promote desquamation. Use of oils or lanolin in the bathtub [23] should be avoided with young children, because of the risk of increased slipperiness. Addition of two cups of baking soda to a full standard tub [24] has been suggested. Baking soda increases the pH of tap water to an alkaline range, which may increase protease activity and promote scale desquamation. Adding salt to bath water may also be soothing and lead to decreased pruritus [25]. Adding bleach (sodium hypochlorite) at a concentration of 0.005 % (one half-cup to a full standard bath; 1 cc per L) to the bath water can be a convenient and affordable measure to minimize bacterial overgrowth. Although no studies of its effect in ichthyosis have been performed, in individuals with atopic dermatitis, at least twice-weekly dilute bleach baths have been shown to decrease disease activity, including inflammation and scaling [26]. Recently, sodium hypochlorite has been shown to inhibit NF-kB signaling and reduce radiation dermatitis in a murine model [27], suggesting a direct anti-inflammatory effect. Hydrotherapy treatments showed to have short- and medium-term efficacy, but are only available in specific centers [28]. Application of emollient immediately after bathing and while the skin is well hydrated is essential (see section on “Emollients”).
Patients with ichthyosis are more susceptible to overheating from hypohidrosis. Although occlusion of eccrine ducts by scaling has been blamed, many affected individuals continue to have issues with heat intolerance and inadequate sweating after therapeutic response to intervention, suggesting a developmental abnormality. Keeping a temperature-controlled environment throughout the year, wetting the skin with spray bottles of water or wet towels, and wetting clothing during outdoor activities in warm weather, or even using cooling suits, are highly recommended.
Physical Treatment
Soaking in water for prolonged periods softens the stratum corneum and allows for removal of the thickened skin with rough-textured sponges or abrasives [7]. The repetitive mechanical procedure may be physically and emotionally exhausting, but rewarding both cosmetically and symptomatically. Earwax from excessive desquamation in the ear canal can lead to conductive hearing loss. Earwax softeners (e.g., carbamide peroxide drops) can facilitate scale removal, which should be performed up to quarterly, as needed, by a trained professional. Although in general home earwax removal is discouraged, a flexible plastic loop with a safety stop (Ear-Wiz®) allows removal of excess wax without touching the eardrum. For patients with palmoplantar keratoderma, focal soaks and paring excess skin with a sharp hand tool is the most effective way to decrease thickening that interferes with performance of daily activities [29]. Surgical approaches, including CO2 laser treatments and full thickness excision and grafting, have anecdotally been described for adult patients [30, 31]. Constrictive skin bands in neonates with harlequin ichthyosis may lead to contractures, compartment syndrome, circulatory compromise, and autoamputation; releasing the skin bands with a 2 mm curette [9], an 11 blade [32], or with a linear band incision technique [33] should be done by a skilled dermatologist, plastic surgeon, or orthopedist. Patients with localized problematic ichthyotic areas of CHILD syndrome may benefit from excision and grafting from the contralateral, unaffected donor region [34].
Retinoids
Both topical and oral retinoids are used to treat various DOC [35]. Their primary effect is to normalize epidermal differentiation, which improves epidermal thickness and hyperkeratosis. In general, topical retinoids are used for milder localized disease and oral retinoids are used for more severe, widespread disease.
Topical Retinoids
Adapalene, tretinoin, and tazarotene have all been used for DOC, although tazarotene is more widely used because of its greater efficacy [35, 36]. Both normalize epidermal thickness via effects on epidermal transglutaminase and other mediators. Topical retinoids have a better safety profile than oral retinoids and do not require monitoring, but can be onerous to apply to large areas. Tazarotene can be absorbed systemically at low levels, but is rapidly degraded and excreted [37]. Adverse effects include erythema, dermatitis, pruritus, and burning. Tazarotene is pregnancy category X.
Topical tazarotene 0.1 % gel and cream have been reported to be effective in treating localized disease associated with lamellar ichthyosis including ectropion [38] and digital contractures of a collodion baby [39]. Topical retinoids are also effective in treating palmoplantar keratodermas [40]. Frequency of application can be decreased to once every few days if there is excessive irritation, or started twice a week and increased as tolerated.
Oral Retinoids
Oral retinoids have been used for many years in the treatment of children with inherited DOC [41], primarily to decrease scale and hyperkeratosis. Oral acitretin and isotretinoin are the most widely used forms. Oral retinoids are used for lamellar ichthyosis, Darier disease, Sjogren-Larsson Syndrome, and palmoplantar keratodermas. When used in congenital ichthyosiform erythroderma (CIE) and epidermolytic ichthyosis (EI), retinoids can lead to increased fragility and erythema. Therefore, starting at a low dose and escalating as tolerated is recommended. The risk of irritation is greater with either topical or oral retinoids when the skin is close to normalizing. Doses of acitretin used in children are often close to 0.5 mg/kg/day [42]. Doses of isotretinoin under 1 mg/kg/day, and sometimes substantially lower, tend to be effective. Prolonged courses of treatment are the norm when used for DOC, but some patients take “drug holidays” periodically, especially to minimize potential irritation. Patients should be counseled that hyperkeratosis and scaling return within months of discontinuing treatment. Other topical treatments such as emollients and keratolytics should be continued while on retinoid therapy, if tolerated, because they often have synergistic effects [41].
Acitretin and isotretinoin have similar toxicities, including mucocutaneous, metabolic, and bone toxicities. Mucocutaneous toxicities include xerosis, cheilitis, and dry nose and eyes. Laboratory abnormalities such as lipid derangements, transaminitis, and abnormalities in the complete blood count (CBC) can be seen. Baseline tests should include CBC, chemistry panel, liver function tests, fasting triglyceride and cholesterol panel, and pregnancy testing for women of childbearing age. These should then be followed periodically. Enrollment in the FDA-mandated I-pledge program is required for prescribers and patients using isotretinoin. All oral retinoids are teratogenic, and women must not become pregnant while initiating or taking a retinoid and for a period of time after using systemic retinoids. For isotretinoin, women should wait one month after cessation of therapy before becoming pregnant. Because acitretin can be re-esterified to etretinate when taken with alcohol and persist in the fat for prolonged periods of time, the current FDA recommendation is that women wait 3 years after cessation of therapy before conceiving [41]. The risk with pregnancy makes acitretin an impractical treatment option for older girls and teenagers, but the onerous requirements for use of isotretinoin make acetretin the drug of choice otherwise.
Because retinoids are often used for prolonged periods of time by people with DOC, the skeletal side effects bear consideration. After long-term treatment hyperostosis formation, analogous to diffuse idiopathic skeletal hyperostosis (DISH), is seen in most patients. Hyperostoses are duration-dependent and are much more common in adults. Premature epiphyseal closure has been reported in children taking either isotretinoin or etretinate for prolonged courses (i.e., 4.5–6 years). In general, higher doses and longer courses of treatment seem to increase risk for these toxicities, but they can be seen with short courses and low doses [43]. There are no clear recommendations for monitoring bony toxicities, but many clinicians will obtain baseline bone surveys and then repeat at intervals of 1–3 years [41].
Retinoic Acid Metabolism Blocking Agent (RAMBA)
Liarozole, an imidazole derivative, is a RAMBA that has orphan drug status for topical and oral use for congenital ichthyosis in Europe, but is not available in the United States. Liarozole inhibits the oxidative metabolism of retinoic acid, leading to higher levels of retinoic acid in the plasma and skin, which then normalizes keratinocyte differentiation and proliferation. Via CYP inhibition, liarozole can affect synthesis of estrogen, androgens, and cortisone, but these effects do not appear to be clinically significant. Liarozole appears to have similar clinical efficacy to retinoids, with fewer retinoid side effects. Topical use did lead to systemic absorption of varying degrees, which led to a decrease in estradiol concentrations, but not to any significant systemic side effects [44, 45].
Targeted Therapy
The progress of understanding the genetic basis and the pathogenesis of many of the ichthyoses has enabled more targeted therapy. Approaches may be gene-based or pathway-based pharmacologic. Gene-based therapy requires knowing the specific gene and/or mutation of the patient to replace, correct, or modulate the expression of the mutant gene. At this time, gene-based therapy has yet to be performed for the ichthyoses, but transduction of the normal SPINK5 gene using a viral vector normalized LEKTI expression in Netherton syndrome keratinocytes and restored normal skin architecture after transplantation; [46] phase I clinical trials are beginning using transplantation. Gene insertion also corrected the TGM1 mutation in a mouse model of lamellar ichthyosis [47, 48] and the restored lipid secretion in lamellar granules of ABCA12-mutated harlequin ichthyosis keratinocytes [49].
An example of pathway-based pharmacologic therapy is correction of a deficient end product (cholesterol) and blockade of accumulation of toxic metabolites (by a statin) in CHILD syndrome. The clinical and histological cutaneous abnormalities are reversed after application of 2 % cholesterol and 2 % lovastatin [50]. Systemic administration of orlistat, an intestinal lipase inhibitor, has led to significant reduction of phytanic acid levels and improved the neurological and dermatological symptoms of two siblings with Refsum’s disease [51].
Distinctive Features and Therapeutic Ladders for Selected DOC
Non-Syndromic Disorders: Autosomal Recessive Congenital Ichthyosis (ARCI), Epidermolytic Ichthyosis (EI), and Erythrokeratodermia Variabilis (EKV)
Epidermolytic Ichthyosis (EI)
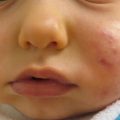
Epidermolytic ichthyosis (Fig. 5.1) is a keratinopathic ichthyosis that results from mutations in one of three keratins: KRT1, 2, and 10. The vast majority of affected individuals have a dominant negative mutation on one allele (autosomal dominant), but rare recessive and mosaic forms (epidermolytic epidermal nevi) have been described. More than half of the cases are due to a de novo mutation and occur sporadically. Clinically, EI is a spectrum of diseases differing in distribution and severity. Patients present with erythroderma, generalized blisters, and denuded skin at birth. Later, blisters become focal and the epidermis becomes hyperkeratotic with dark thick scales, often with a corrugated or ridged pattern. Some patients experience palmoplantar keratoderma, which predicts a KRT1 mutation.
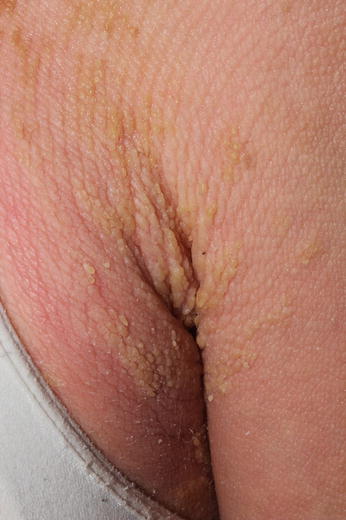

Fig. 5.1
Epidermolytic ichthyosis
Management Strategies
The approach to EI treatment is complicated by the skin fragility. All patients should use topical emollients and be monitored for skin infections, particularly Staphylococcus aureus. Topical and oral retinoids can decrease the hyperkeratosis, but risk increasing discomfort and skin fragility.
Investigations Recommended
Skin biopsy
Genetic testing, if available
Bacterial and fungal cultures as needed to consider secondary infection
Table 5.2
First line therapy
Emollients |
Topical or systemic antimicrobials as needed |
Table 5.3
Second line therapy
Topical retinoids |
A 14-year-old boy, treated with topical adapalene gel 0.1 % for his facial lesions, sustained good results during 2 years of usage [36].
Table 5.4
Third line therapy
Systemic retinoids |
Topical vitamin D analogs ± topical corticosteroids |
- 1.
Four children, ages 4–13, treated with acitretin 0.77–1.07 mg/kg/day, showed improvement in >90 % of skin lesions with sustained effect for 14–24 months. No severe side effects were noted, and there was no adverse effect on growth and development [52].
- 2.
A 7-year-old boy was treated with etretinate 0.75 mg/kg/day. The hyperkeratosis resolved in 2 months. The dosage was reduced to 0.5 mg/kg/day and continued intermittently for about 2 years. The hyperkeratosis was controlled well. No significant adverse effect of etretinate was observed [53].
- 3.
A 9-year-old boy was treated with four different topical preparations—one on each limb: (1) calcipotriol ointment (50 mg/g) on the left leg; (2) tretinoin ointment 30 mg/100 g the right leg; (3) ointment containing urea (10 %), lactic acid (5 %) and glycerol (5 %) on the right arm; (4) ointment containing urea (10 %) and sodium chloride (10 %) on the left arm. Topical calcipotriol twice daily was most effective, reducing scaling, itching, and skin tenderness in 3 weeks. Treatment was continued for 3 years with sustained results. Serum and urine calcium were within normal limits throughout this treatment [54].
- 4.
A 2-year-old boy with linear EI (epidermal nevus type) used maxacalcitol ointment (25 μg/g) twice daily. After 2 months he showed improvement, and after 2 years the affected skin became depigmented with normal texture. Serum calcium remained within normal limits throughout the treatment [55].
- 5.
A 5-year-old boy was treated with calcipotriol/ betamethasone propionate combination ointment once daily. After 2 months, the lesions became thinner and less erythematous [56].
- 6.
Thirteen patients (75 % younger than 20 years old) with generalized epidermolytic hyperkeratosis were treated with either topical or oral retinoids. Retinoids were particularly effective in patients with KRT10 mutations [40].
Autosomal Recessive Congenital Ichthyosis (ARCI)
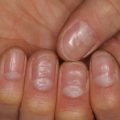
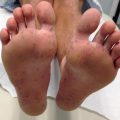
ARCI is a group of disorders that most commonly presents at birth as a collodion baby. It encompasses a spectrum of ichthyosis phenotypes, ranging from mild to severe and from congenital ichthyosiform erythroderma (CIE) to lamellar ichthyosis (LI) (Fig. 5.2). All forms are autosomal recessive, although rare autosomal dominant cases of ARCI have been described. Mutations in at least nine genes have been found to underlie ARCI (Table 5.1).
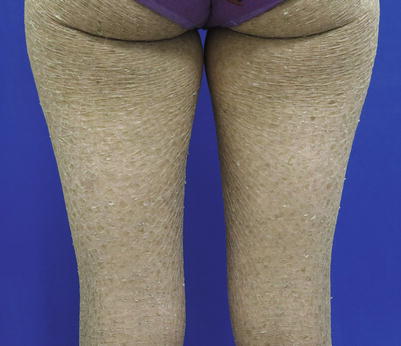

Fig. 5.2
Lamellar Ichthyosis phenotype of ARCI
The collodion baby shared phenotype (Fig. 5.3) presents at birth as encasement in shiny thickened skin, often with associated ectropion (eversion of the upper and lower eyelids) and eclabium (lip eversion), exposing the ocular and oral mucosae, respectively. Despite the thickening, the skin barrier of collodion babies is impaired. Transepidermal loss of water and electrolytes is increased, the skin is more permeable to topically applied agents, and there is a heightened risk of temperature instability and bacterial and fungal infection. The collodion membrane tends to be shed during the first month of life and the phenotype is revealed in the subsequent months.
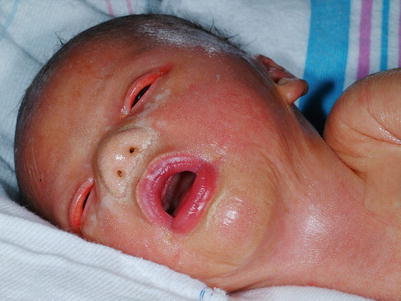

Fig. 5.3
Collodion baby
As discussed under interventions above, individuals with ARCI often have severe pruritus and, especially with the thick scaling of lamellar ichthyosis, an odor in association with overgrowth of skin bacteria (see increased risk of bacterial and fungal infections, above). Ectropion and extensive scaling of the ear canals lead to issues that may require special expertise. The defect in sweating can manifest in overheating.
Investigations Recommended
Biopsy is generally not useful
Genetic testing, if available
Bacterial and fungal cultures as needed to consider secondary infection
Table 5.5
First line therapy
Careful monitoring in the first week of life with a humidified environment and emollients |
Emollients |
Topical tazarotene or keratolytic agents (including N-acetylcysteine with urea) |
Topical or systemic antimicrobials as needed for bacterial or fungal infection |
Antihistamines if helpful for the pruritus |
- 1.
Ichthyosis in the newborn [8]. This article reviews the potential complications and management of ichthyosis, particularly collodion babies.
- 2.
Collodion baby: an update with a focus on practical management [57]. This review summarizes the clinical characteristics, complications, outcomes, and differential diagnosis of the collodion baby, and suggests practical management.
- 3.
Care of the newborn with ichthyosis [32]. This review summarizes the phenotypic presentations of ichthyosis in the neonatal period and discusses management.
- 4.
Nine children with ichthyosis (2–17 years of age), of whom three had ARCI, applied tazarotene 0.05 % or 0.1 % cream or gel to up to 90 % of the body surface for 1 month to 2 years. In the majority, blood levels of tazarotene were undetectable, and in others, very low [58].
- 5.
Topical tazarotene can be particularly helpful for decreasing ectropion and is a successful alternative to surgical intervention for many patients [38].
- 6.
Ten percent N-acetylcysteine in 5 % urea is effective and safe as a topically applied cream; the odor of sulfur has recently been minimized by the addition of rosemary water [19].
Table 5.6
Second line therapy
Topical vitamin D3 analogs |
Liarozole 5 % cream (not available in the US)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|