The modified Dunn procedure is an open realignment of the capital femoral epiphysis performed through a surgical dislocation approach.18
 Like the original Dunn osteotomy, the modified Dunn corrects a slipped capital femoral epiphysis (SCFE) at the site of maximal deformity (ie, the physis).4
Like the original Dunn osteotomy, the modified Dunn corrects a slipped capital femoral epiphysis (SCFE) at the site of maximal deformity (ie, the physis).4
 The procedure can be performed on both unstable SCFEs and stable SCFEs.
The procedure can be performed on both unstable SCFEs and stable SCFEs.
ANATOMY
 The main source of perfusion to the femoral epiphysis is the medial femoral circumflex artery (MFCA) (FIG 1).
The main source of perfusion to the femoral epiphysis is the medial femoral circumflex artery (MFCA) (FIG 1).
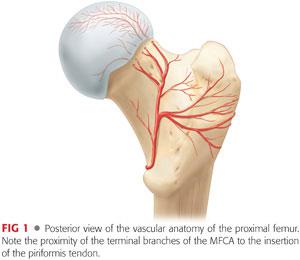
 The deep branch of the MFCA runs posterior to the obturator externus and perforates the hip capsule just distal to the piriformis and proximal to the superior gemellus.5
The deep branch of the MFCA runs posterior to the obturator externus and perforates the hip capsule just distal to the piriformis and proximal to the superior gemellus.5
 It ends in two to four retinacular branches that enter the superior aspect of the femoral head.
It ends in two to four retinacular branches that enter the superior aspect of the femoral head.
 The intact external rotator muscles, especially the obturator externus, protect the MFCA during surgical dislocation of the hip.
The intact external rotator muscles, especially the obturator externus, protect the MFCA during surgical dislocation of the hip.
 To safely reduce a SCFE, which typically displaces posterior and inferior to the neck, tension must be minimized on the retinacular vessels.18
To safely reduce a SCFE, which typically displaces posterior and inferior to the neck, tension must be minimized on the retinacular vessels.18
PATHOGENESIS
 The posteroinferior translation of the epiphysis relative to the femoral neck alters the resting position of the femoral head within the acetabulum.
The posteroinferior translation of the epiphysis relative to the femoral neck alters the resting position of the femoral head within the acetabulum.
 This altered position combined with the residual offset of the metaphysis can dramatically affect the motion of the hip joint.
This altered position combined with the residual offset of the metaphysis can dramatically affect the motion of the hip joint.
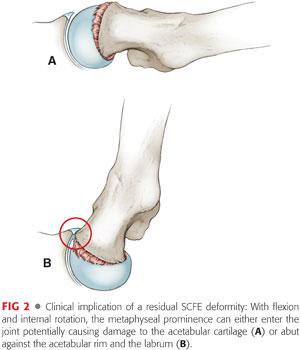
 With flexion and internal rotation, the metaphyseal prominence can either enter the joint potentially causing damage to the acetabular cartilage or abut against the acetabular rim and the labrum13 (FIG 2).
With flexion and internal rotation, the metaphyseal prominence can either enter the joint potentially causing damage to the acetabular cartilage or abut against the acetabular rim and the labrum13 (FIG 2).
 In situ fixation, while effective for preventing further slippage, does not correct the deformity associated with SCFE.
In situ fixation, while effective for preventing further slippage, does not correct the deformity associated with SCFE.
NATURAL HISTORY
 Historically, the long-term outcome after in situ screw fixation has generally been considered to be good.1,2
Historically, the long-term outcome after in situ screw fixation has generally been considered to be good.1,2
 Recent studies, however, have established an association between the residual proximal femoral metaphyseal deformity left by in situ treatment and the development of femoroacetabular impingement, lost hip motion, and premature osteoarthritis.6,9,11,17
Recent studies, however, have established an association between the residual proximal femoral metaphyseal deformity left by in situ treatment and the development of femoroacetabular impingement, lost hip motion, and premature osteoarthritis.6,9,11,17
 Increasing slip severity has been associated with poorer outcome.3
Increasing slip severity has been associated with poorer outcome.3
PATIENT HISTORY AND PHYSICAL FINDINGS
 Patients with an unstable SCFE usually present acutely with an abrupt onset of symptoms.
Patients with an unstable SCFE usually present acutely with an abrupt onset of symptoms.
Pain is intense and can be elicited even with simple logrolling of the affected extremity.
By definition, patients with unstable SCFE are unable to ambulate with or without walking aids.10
Further forced range of motion (ROM) of the affected hip is discouraged to minimize the risk of additional trauma to the femoral head blood supply.
 Patients with severe, stable SCFE can present with variable duration of symptoms.
Patients with severe, stable SCFE can present with variable duration of symptoms.
Patients complain of hip or groin pain, thigh pain, or knee pain and usually occurs without a history of trauma.
By definition, patients are able to ambulate with or without walking aids and typically present with an antalgic gait and an external foot progression angle.10
Hip flexion, abduction, and internal rotation in the flexed position will be markedly decreased in a severe SCFE as a result of femoroacetabular impingement.
Many patients will also demonstrate obligate external rotation which is forced external rotation that results from passive hip flexion.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Plain radiographs of the pelvis, including anteroposterior (AP) and frog-leg lateral views, should be obtained in any pediatric patient with hip, thigh, or knee pain and are generally diagnostic of a SCFE.
Plain radiographs of the pelvis, including anteroposterior (AP) and frog-leg lateral views, should be obtained in any pediatric patient with hip, thigh, or knee pain and are generally diagnostic of a SCFE.
The femoral epiphysis is typically displaced posterior (on the frog view) and slightly inferior (on the AP view) in relation to the femoral neck.
 A widened physis on AP or lateral views can be an early sign of SCFE.
A widened physis on AP or lateral views can be an early sign of SCFE.
 The severity of a SCFE can be described by displacement relative to the width of the metaphysis:
The severity of a SCFE can be described by displacement relative to the width of the metaphysis:
Mild: less than one-third the width
Moderate: one-third to half the width
Severe: more than half the width
 Another method of describing slip severity is measuring the difference between the epiphyseal shaft angle on each side16:
Another method of describing slip severity is measuring the difference between the epiphyseal shaft angle on each side16:
Mild: less than 30 degrees
Moderate: 30 to 50 degrees
Severe: 50 degrees or greater
 In cases with delayed presentation in which the modified Dunn is being considered, computed tomography (CT) scan can be used to more accurately assess the degree of displacement and the amount of physeal closure.
In cases with delayed presentation in which the modified Dunn is being considered, computed tomography (CT) scan can be used to more accurately assess the degree of displacement and the amount of physeal closure.
 In certain patients with unstable SCFEs who present late, bone scan or contrast magnetic resonance imaging (MRI) can be used to assess the perfusion to the epiphysis.
In certain patients with unstable SCFEs who present late, bone scan or contrast magnetic resonance imaging (MRI) can be used to assess the perfusion to the epiphysis.
DIFFERENTIAL DIAGNOSIS
 SCFE
SCFE
 Legg-Calvé-Perthes disease
Legg-Calvé-Perthes disease
 Hip labral tear
Hip labral tear
 Femoral neck fracture
Femoral neck fracture
 Septic arthritis of the hip
Septic arthritis of the hip
 Knee derangement
Knee derangement
 Greater trochanteric bursitis
Greater trochanteric bursitis
NONOPERATIVE MANAGEMENT
 Immobilization in a spica cast was the historical treatment but is no longer recommended for SCFE.
Immobilization in a spica cast was the historical treatment but is no longer recommended for SCFE.
 Once SCFE is identified in any patient with an open physis, management is surgical to avoid further slippage and the possible development of femoral head avascular necrosis (AVN).
Once SCFE is identified in any patient with an open physis, management is surgical to avoid further slippage and the possible development of femoral head avascular necrosis (AVN).
SURGICAL MANAGEMENT
 The modified Dunn procedure is indicated for unstable SCFEs and for certain stable SCFEs in which the residual deformity that would be left by in situ fixation is deemed unacceptable.
The modified Dunn procedure is indicated for unstable SCFEs and for certain stable SCFEs in which the residual deformity that would be left by in situ fixation is deemed unacceptable.
 It is most easily performed when the physis is still open.
It is most easily performed when the physis is still open.
 The modified Dunn procedure is a technically demanding procedure that requires familiarity with the surgical dislocation approach and the development of the retinacular flap.
The modified Dunn procedure is a technically demanding procedure that requires familiarity with the surgical dislocation approach and the development of the retinacular flap.
 Alternative techniques for treating unstable SCFEs include (1) positional reduction followed by percutaneous screw fixation (with at least two screws) and capsular decompression7 or (2) gentle manual open reduction performed via an anterolateral approach followed by Kirschner wire (K-wire) or screw fixation.12
Alternative techniques for treating unstable SCFEs include (1) positional reduction followed by percutaneous screw fixation (with at least two screws) and capsular decompression7 or (2) gentle manual open reduction performed via an anterolateral approach followed by Kirschner wire (K-wire) or screw fixation.12
 Alternative techniques for treating severe stable SCFEs include in situ fixation followed by osteoplasty of the femoral head–neck junction and/or proximal femoral osteotomy.
Alternative techniques for treating severe stable SCFEs include in situ fixation followed by osteoplasty of the femoral head–neck junction and/or proximal femoral osteotomy.
Preoperative Planning
 All imaging studies are reviewed.
All imaging studies are reviewed.
 Both AP and frog views should be reviewed for the presence of posteroinferior callus. This finding implies some chronicity to the SCFE and its removal is necessary to achieve an anatomic reduction.
Both AP and frog views should be reviewed for the presence of posteroinferior callus. This finding implies some chronicity to the SCFE and its removal is necessary to achieve an anatomic reduction.
 Pure displacement (ie, angulation or translation) can be corrected via the modified Dunn, but some chronic changes such as femoral neck retroversion are more difficult to address.
Pure displacement (ie, angulation or translation) can be corrected via the modified Dunn, but some chronic changes such as femoral neck retroversion are more difficult to address.
 In chronic and severe stable SCFEs, CT scans can be used to assess the status of the physis and determine the indication for the modified Dunn procedure.
In chronic and severe stable SCFEs, CT scans can be used to assess the status of the physis and determine the indication for the modified Dunn procedure.
 The contralateral hip should be evaluated both clinically and radiographically to determine whether a SCFE is present.
The contralateral hip should be evaluated both clinically and radiographically to determine whether a SCFE is present.
 In younger patients, and those with endocrinopathies, prophylactic percutaneous screw fixation of the normal side should be considered. This can be done in the supine position before commencing with the modified Dunn procedure.
In younger patients, and those with endocrinopathies, prophylactic percutaneous screw fixation of the normal side should be considered. This can be done in the supine position before commencing with the modified Dunn procedure.
 Timing of surgery for an unstable SCFE is somewhat controversial. For cases that present overnight, the author prefers performing the modified Dunn procedure the next morning with an appropriately skilled surgical team.
Timing of surgery for an unstable SCFE is somewhat controversial. For cases that present overnight, the author prefers performing the modified Dunn procedure the next morning with an appropriately skilled surgical team.
Positioning
 As with a standard surgical dislocation of the hip (see Chap. 13), the patient is placed in full lateral position, secured on a pegboard. A flat-top cushion (with cutout for the down leg) supports the operative extremity during the procedure (FIG 3).
As with a standard surgical dislocation of the hip (see Chap. 13), the patient is placed in full lateral position, secured on a pegboard. A flat-top cushion (with cutout for the down leg) supports the operative extremity during the procedure (FIG 3).
 A hip drape with a sterile side bag is used, which will capture the leg during the dislocation maneuver.
A hip drape with a sterile side bag is used, which will capture the leg during the dislocation maneuver.
Approach
 As with other surgical dislocations of the hip, the Kocher-Langenbeck or Gibson approach may be used.
As with other surgical dislocations of the hip, the Kocher-Langenbeck or Gibson approach may be used.
 The Kocher-Langenbeck incision is followed by splitting of the gluteus maximus muscle. In obese patients, this approach does facilitate anterior exposure.
The Kocher-Langenbeck incision is followed by splitting of the gluteus maximus muscle. In obese patients, this approach does facilitate anterior exposure.
 The abductors and gluteus maximus muscles can be spared by performing a Gibson approach, which proceeds between the gluteus medius and maximus. This results in less hip extensor dysfunction but may make anterior exposure more difficult especially in obese patients.
The abductors and gluteus maximus muscles can be spared by performing a Gibson approach, which proceeds between the gluteus medius and maximus. This results in less hip extensor dysfunction but may make anterior exposure more difficult especially in obese patients.
TECHNIQUES
 Exposure
Exposure
For a detailed description of the surgical dislocation approach please refer to Chapter 13. The following technique for the modified Dunn procedure begins after the hip capsule has been fully exposed.
 Hip Arthrotomy, Provisional Fixation, and Dislocation
Hip Arthrotomy, Provisional Fixation, and Dislocation
A Z-shaped capsulotomy is performed, with the longitudinal arm of the Z in line with the anterior neck of the femur (TECH FIG 1A).
The inferior limb extends along the intertrochanteric line toward but proximal to the lesser trochanter. A cuff of capsular tissue should be left for later repair.
Superior limb extends along the acetabular rim posteriorly toward the piriformis tendon. Care should be taken not to injure the labrum (TECH FIG 1B).
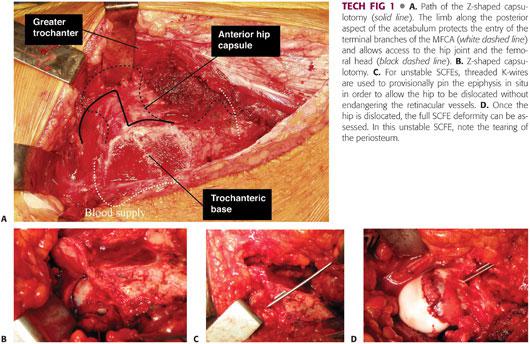
It is helpful to tag the corners of the capsulotomy to help distinguish this layer from the retinacular flap which will be developed later in the procedure.
At this point, the hip should be gently manipulated to assess the intraoperative stability of the slip and the acuity of the injury.
Unstable slips characteristically have torn periosteum anteriorly with the epiphysis clearly disengaged from the metaphysis.
Stable slips move as a single unit.
For unstable SCFEs, one to two threaded K-wires are used to provisionally pin the epiphysis in situ to allow the hip to be dislocated without endangering the retinacular vessels (TECH FIG 1C).
The wires are cut with approximately 2 cm out of bone to keep the fixation out of the way but still allow later removal.
A bone hook is placed around the neck.
The limb is then gently flexed, adducted, and externally rotated, and the bone hook is used to subluxate the hip. Curved scissors are inserted into the joint, and the ligamentum teres is transected to allow full dislocation of the hip.
The full SCFE deformity can now be assessed. It is important to evaluate the acetabulum as well for chondral or labral pathology (TECH FIG 1D).
 Retinacular Flap
Retinacular Flap
The retinacular flap is best developed with the hip located.
The periosteum is split longitudinally on the femur distal to the trochanteric osteotomy site, and a periosteal elevator is used to start the periosteal flap posteriorly (TECH FIG 2A).
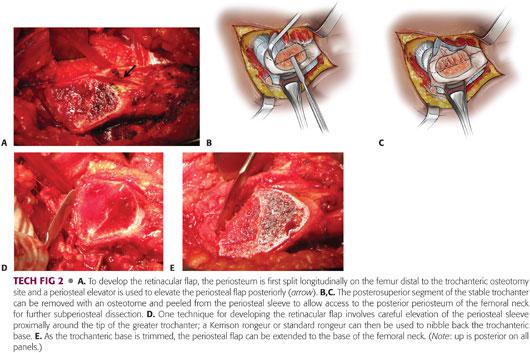
Similarly, the periosteum on the anterior neck can be split in line with the capsulotomy and elevated.
To reduce tension on the retinacular flap and allow the epiphysis to be safely mobilized, the posterosuperior third of the trochanteric base needs to be removed.
The apophyseal cartilage in skeletally immature patients provides a useful landmark for the extent of trochanteric resection.
The original technique uses an osteotome to perform the osteotomy (at the site of apophyseal cartilage) followed by careful peeling of the freed trochanteric fragment from the periosteal flap18 (TECH FIG 2B,C).
Alternatively, the subperiosteal flap can be carefully extended proximally around the tip of the greater trochanter and a Kerrison rongeur or standard rongeur can be used to “nibble” back the trochanteric base (TECH FIG 2D).
As the trochanteric base is trimmed, the periosteal flap can be extended to the base of the femoral neck (TECH FIG 2E).
 Mobilization of the Epiphysis
Mobilization of the Epiphysis
The hip is dislocated to complete the retinacular flap.
The periosteum over the anterior neck is split and elevated over the superior neck and the piriformis fossa to connect to the previously created periosteal sleeve (TECH FIG 3A).
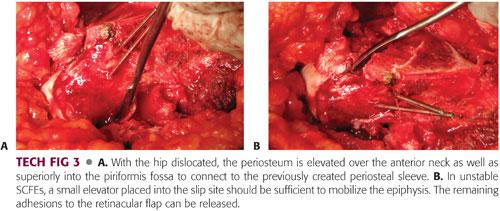
A sponge can be placed inside the acetabulum to prevent the femoral head from falling posteriorly as it is mobilized.
While manually stabilizing the epiphysis, the provisional K-wires are removed.
In unstable SCFEs, a small elevator placed into the slip site should be sufficient to mobilize the epiphysis. The remaining adhesions to the retinacular flap can be addressed (TECH FIG 3B).
In stable SCFEs, the physis should be localized and an elevator or osteotome used to separate the epiphysis from the metaphysis.
Once the epiphysis has been freed, any residual physis should be curetted to facilitate bone healing.
 Reducing the Epiphysis
Reducing the Epiphysis
The fully exposed metaphysis/neck should be inspected for signs of chronic callus. This is typically located posterior and inferior. Removing this with an osteotome and rongeur helps facilitate epiphyseal reduction (TECH FIG 4A).
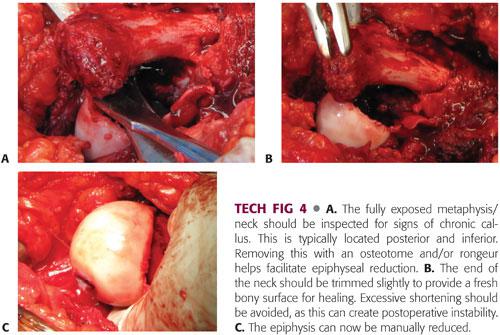
The end of the neck should be trimmed slightly to provide a fresh bony surface for healing. Excessive shortening should be avoided as this can create postoperative instability (TECH FIG 4B).
The epiphysis can now be manually reduced. There should be minimal tension on the retinacular flap during this maneuver. If excessive tension persists, the retinacular flap should be extended and the epiphysis and metaphysis should be revisited to be sure that all callus has been removed (TECH FIG 4C).
 Epiphyseal Fixation
Epiphyseal Fixation
Initial fixation is achieved by inserting a threaded K-wire from proximal to distal through the fovea and exiting out the anterolateral femur (TECH FIG 5A).
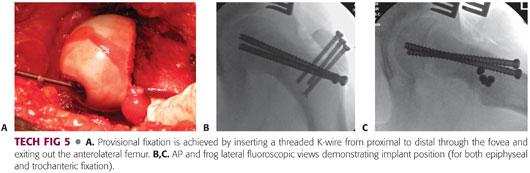
The hip is carefully relocated to allow assessment of reduction and placement of definitive fixation.
To confirm adequate epiphyseal reduction, the C-arm can be brought underneath the table and rotated to provide an AP view of the hip. The limb can be manually frogged to achieve a lateral view of the proximal femur.
If the reduction is acceptable, definitive fixation for the epiphysis should be placed distal or anterior to the trochanteric base to facilitate later removal if necessary.
The author prefers using two 6.5-mm cannulated screws to minimize the risk of implant failure; however, two or three solid 4.5-mm screws or multiple threaded K-wires can also be used.14
Implant position can be checked using the C-arm (TECH FIG 5B,C).
The initial threaded K-wire can now be removed if desired.
 Monitoring Perfusion
Monitoring Perfusion
Perfusion of the epiphysis can be monitored periodically throughout the case (eg, during development of the retinacular flap, after epiphyseal reduction, etc.) by placing a small 1.5-mm drill hole into the anterior head away from the weight-bearing surface. Fresh bleeding should be visualized from the head.
A more objective means of perfusion monitoring involves placement of an intracranial pressure catheter into the small drill hole. A discrete waveform should be visualized (TECH FIG 6).
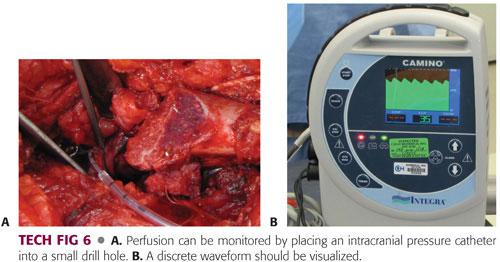
If perfusion is lost after epiphyseal reduction, the tension on the retinacular flap should be checked. If necessary, the flap should be extended, the neck slightly shortened, and the epiphysis rereduced in a tension-free manner.
 Deep Closure
Deep Closure
A few tacking sutures can be placed loosely in the anterior periosteum; but in general, the retinacular flap should not be repaired to avoid causing unnecessary tension.
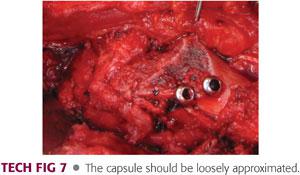
The capsule is loosely approximated with interrupted heavy (no. 1) absorbable sutures. Again, it is important to avoid creating unnecessary tension on the retinacular flap (TECH FIG 7).
 Trochanteric Osteotomy Fixation
Trochanteric Osteotomy Fixation
The trochanteric wafer is reduced to the same or slightly distal position and held in place using a ball spike probe.
Three guidewires for a 4.5-mm cannulated screw system are inserted across the osteotomy site aiming slightly distal (toward the lesser trochanter) (TECH FIG 8).
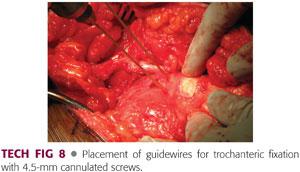
The reduction of the osteotomy and the wires can be confirmed with the C-arm.
The wires are then measured and overdrilled. Fully threaded 4.5-mm cannulated screws are used to facilitate later removal if necessary.
Alternatively, solid 3.5- or 4.5-mm screws can be used.
 Closure
Closure
The vastus lateralis is repaired using a running absorbable suture (eg, 2-0 Vicryl).
The fascia lata is closed in a watertight fashion using interrupted heavy absorbable suture (eg, no. 1 Vicryl), followed by layered closure of the dermis (2-0 Vicryl) and the subcuticular layer (4-0 Monocryl).
PEARLS AND PITFALLS | |
Difficulty distinguishing capsule from retinacular flap during closure |
|
Excessive tension on retinacular flap during mobilization of the epiphysis |
|
Excessive tension on retinacular flap during reduction of the epiphysis |
|
Implant failure |
|
Tension on the retinacular flap during closure |
|
AVN |
|
POSTOPERATIVE CARE
 Patients are made toe-touch weight bearing with crutches. Weight bearing is generally advanced around 8 weeks based on early radiographic signs of healing at the physis.
Patients are made toe-touch weight bearing with crutches. Weight bearing is generally advanced around 8 weeks based on early radiographic signs of healing at the physis.
 Physical therapy is then initiated for gait training and abductor strengthening.
Physical therapy is then initiated for gait training and abductor strengthening.
 If radiographic healing progresses as expected, patients are generally cleared for sports around 6 months after surgery.
If radiographic healing progresses as expected, patients are generally cleared for sports around 6 months after surgery.
 After the immediate postoperative period, patients are followed every 2 to 3 months for at least a year with AP and frog lateral radiographs to monitor for the development of osteonecrosis.
After the immediate postoperative period, patients are followed every 2 to 3 months for at least a year with AP and frog lateral radiographs to monitor for the development of osteonecrosis.
OUTCOMES
 The initial series describing the modified Dunn procedure consisted of 40 patients followed for a minimum of 1 year and 3 years from two institutions.18 The authors reported excellent functional outcomes, improved ROM, near-anatomic slip angles, and no cases of AVN.
The initial series describing the modified Dunn procedure consisted of 40 patients followed for a minimum of 1 year and 3 years from two institutions.18 The authors reported excellent functional outcomes, improved ROM, near-anatomic slip angles, and no cases of AVN.
 Two additional follow-up studies have also reported excellent functional scores and anatomic radiographic results following the modified Dunn procedure in both stable and unstable SCFEs.
Two additional follow-up studies have also reported excellent functional scores and anatomic radiographic results following the modified Dunn procedure in both stable and unstable SCFEs.
One series of 23 patients reported excellent clinical and radiographic outcomes in 21 patients. The poor outcomes occurred in 2 patients who developed osteonecrosis and osteoarthritis.15
Another series of 30 SCFEs reported near-anatomic reduction in all cases. Twenty-eight patients had excellent clinical outcomes with one patient developing AVN. Failure of implants requiring revision surgery occurred in four hips.8
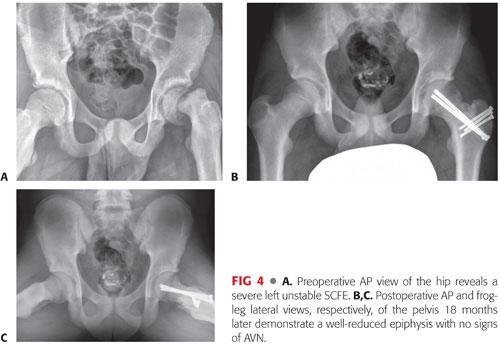
 A multicenter study of 27 unstable SCFEs treated with the modified Dunn procedure demonstrated higher rates of AVN (26%) and failed epiphyseal fixation (15%) than previous reports.14 Patients that did not develop osteonecrosis had excellent clinical and radiographic results (FIG 4).
A multicenter study of 27 unstable SCFEs treated with the modified Dunn procedure demonstrated higher rates of AVN (26%) and failed epiphyseal fixation (15%) than previous reports.14 Patients that did not develop osteonecrosis had excellent clinical and radiographic results (FIG 4).
COMPLICATIONS
 Chondrolysis
Chondrolysis
 AVN (more common with unstable SCFEs)
AVN (more common with unstable SCFEs)
 Nonunion
Nonunion
 Failed epiphyseal fixation
Failed epiphyseal fixation
REFERENCES
1. Boyer DW, Mickelson MR, Ponseti IV. Slipped capital femoral epiphysis. Long-term follow-up study of one hundred and twenty-one patients. J Bone Joint Surg Am 1981;63(1):85–95.
2. Carney BT, Weinstein SL, Noble J. Long-term follow-up of slipped capital femoral epiphysis. J Bone Joint Surg Am 1991;73(5):667–674.
3. Castañeda P, Ponce C, Villareal G, et al. The natural history of osteoarthritis after a slipped capital femoral epiphysis/the pistol grip deformity. J Pediatr Orthop 2013;33(suppl 1):S76–S82.
4. Dunn DM. The treatment of adolescent slipping of the upper femoral epiphysis. J Bone Joint Surg Br 1964;46:621–629.
5. Gautier E, Ganz K, Krugel N, et al. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br 2000;82(5):679–683.
6. Goodman DA, Feighan JE, Smith AD, et al. Subclinical slipped capital femoral epiphysis. Relationship to osteoarthrosis of the hip. J Bone Joint Surg Am 1997;79(10):1489–1497.
7. Gordon JE, Abrahams MS, Dobbs MB, et al. Early reduction, arthrotomy, and cannulated screw fixation in unstable slipped capital femoral epiphysis treatment. J Pediatr Orthop 2002;22(3):352–358.
8. Huber H, Dora C, Ramseier LE, et al. Adolescent slipped capital femoral epiphysis treated by a modified Dunn osteotomy with surgical hip dislocation. J Bone Joint Surg Br 2011;93(6):833–838.
9. Leunig M, Casillas MM, Hamlet M, et al. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Orthop Scand 2000;71(4):370–375.
10. Loder RT, Richards BS, Shapiro PS, et al. Acute slipped capital femoral epiphysis: the importance of physeal stability. J Bone Joint Surg Am 1993;75(8):1134–1140.
11. Mamisch TC, Kim YJ, Richolt JA, et al. Femoral morphology due to impingement influences the range of motion in slipped capital femoral epiphysis. Clin Orthop Relat Res 2009;467(3):692–698.
12. Parsch K, Weller S, Parsch D. Open reduction and smooth Kirschner wire fixation for unstable slipped capital femoral epiphysis. J Pediatr Orthop 2009;29(1):1–8.
13. Rab GT. The geometry of slipped capital femoral epiphysis: implications for movement, impingement, and corrective osteotomy. J Pediatr Orthop 1999;19(4):41–424.
14. Sankar WN, Vanderhave KL, Matheney T, et al. The modified Dunn procedure for unstable slipped capital femoral epiphysis: a multicenter perspective. J Bone Joint Surg Am 2013;95(7):585–591.
15. Slongo T, Kakaty D, Krause F, et al. Treatment of slipped capital femoral epiphysis with a modified Dunn procedure. J Bone Joint Surg Am 2010;92(18):2898–2908.
16. Southwick WO. Osteotomy through the lesser trochanter for slipped capital femoral epiphysis. J Bone Joint Surg Am 1967;49(5):807–835.
17. Tannast M, Goricki D, Beck M, et al. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res 2008;466(2):273–280.
18. Ziebarth K, Zilkens C, Spencer S, et al. Capital realignment for moderate and severe SCFE using a modified Dunn procedure. Clin Orthop Relat Res 2009;467(3):704–716.
< div class='tao-gold-member'>









