Dermal fillers remain popular for facial rejuvenation but with its increasing use, the potential for more complications including blindness is present. This article focuses on the mechanism of filler-associated blindness, possible treatments, and future directions. Unfortunately, to date there is no proven treatment to reverse filler-induced blindness or visual compromise. It is essential for all injectors to discuss the potential ocular risks including blindness with their patients and obtain informed consent before filler injection.
Key points
- •
Blindness and orbital ischemia can occur with dermal filler injections. The mechanism of action is thought to be inadvertent intraarterial injection of filler product causing ischemia.
- •
Injectors should be aware of the risk of blindness and visual compromise/orbital ischemia and discuss this small but potential risk with patients before filler injection and obtain informed consent.
- •
There is no proven treatment to reverse filler-induced blindness to date.
- •
Some injectors suggest the use of retrobulbar hyaluronidase for filler-induced blindness; however, it has its own risks and is unproved to date.
Overview
Fillers are commonly thought of as a straightforward and safe treatment and continue to be a popular nonsurgical facial rejuvenation treatment option. According to the American Society for Aesthetic Plastic Surgery, an estimated 870,000 filler injection procedures were performed in the United States in 2018. Dermal fillers come from a variety of materials including hyaluronic acid, calcium hydroxyapatite, and poly- l -lactic acid (PLLA), with more than 30 dermal fillers approved by the Food and Drug Administration. Hyaluronic acid gel fillers are the most popular dermal filler due to safety and side-effect profile, reversibility, variety of formulations, and applications. However, hyaluronic acid gel fillers still have associated complications. Although most of these complications are mild and reversible, rare but reported complications include tissue ischemia, skin necrosis, and permanent blindness. To reduce the likelihood of these complications, implementing “Global Filler Safety” is essential, which includes understanding the facial anatomy, proper patient and filler product selection, and proper placement of filler in a safe anatomic location. As with any other procedure, an informed consent should include a thorough discussion with the patient about the risks and potential complications.
Although the incidence of filler-induced blindness is unknown, increasingly more cases of visual compromise are expected to be seen with the growing use of fillers. Beleznay and colleagues found 146 total reported cases of sudden vision loss secondary to filler injections, with 98 cases found between 1906 and 2015 and another 48 cases between 2015 and 2018. The actual cases of filler-induced blindness are likely underreported, as we do not have a good way to capture all cases.
Injectors, staff, and patients should be aware of this known complication. Even with the appropriate understanding and implementation of filler injections, blindness can happen. Signs and symptoms associated with visual compromise include immediate loss of vision, double vision, pain, ophthalmoplegia, droopy eyelids (ptosis), headache, soft tissue ischemia, and stroke symptoms due to cerebral infarcts. , The highest risk areas or “danger zones” for visual compromise from filler injection are the glabella, nasal area, nasolabial fold, and temple. , ,
This article focuses on the mechanism of filler-associated blindness, possible treatments, and future directions.
Mechanism of visual compromise due to dermal fillers
The primary mechanism of filler-induced blindness is due to direct intraarterial filler injection, leading to embolization of filler material via vascular pathways to the ophthalmic artery.
Filler injected at pressures that overcome arterial pressure and friction forces due to viscous flow can lead to ophthalmic artery embolization. With enough pressure, the filler material moves initially in a retrograde path, then after reduction of injection pressure, filler material moves in an anterograde direction with blood flow, resulting in a shower of emboli anteriorly, including into the ophthalmic artery circulation.
The main branches of the ophthalmic artery include the supratrochlear, supraorbital, dorsal nasal, lacrimal, ethmoidal, palpebral, muscular, posterior ciliary, and central retinal arteries. The facial artery may anastomose with the ophthalmic artery via the angular artery. In a cadaver study, the supratrochlear, supraorbital, and dorsal nasal artery directly connected to the ophthalmic artery in all cadavers, and the angular artery anastomosed to the ophthalmic artery in 54% ( Fig. 1 ) —this corresponds with the common “danger zones” for visual compromise or blindness. Although these arteries may present more direct routes to the ophthalmic artery, there are many other anastomoses from the arteries of the face with the ophthalmic artery, so nearly any area of the face is at some risk of vision-threatening complications.
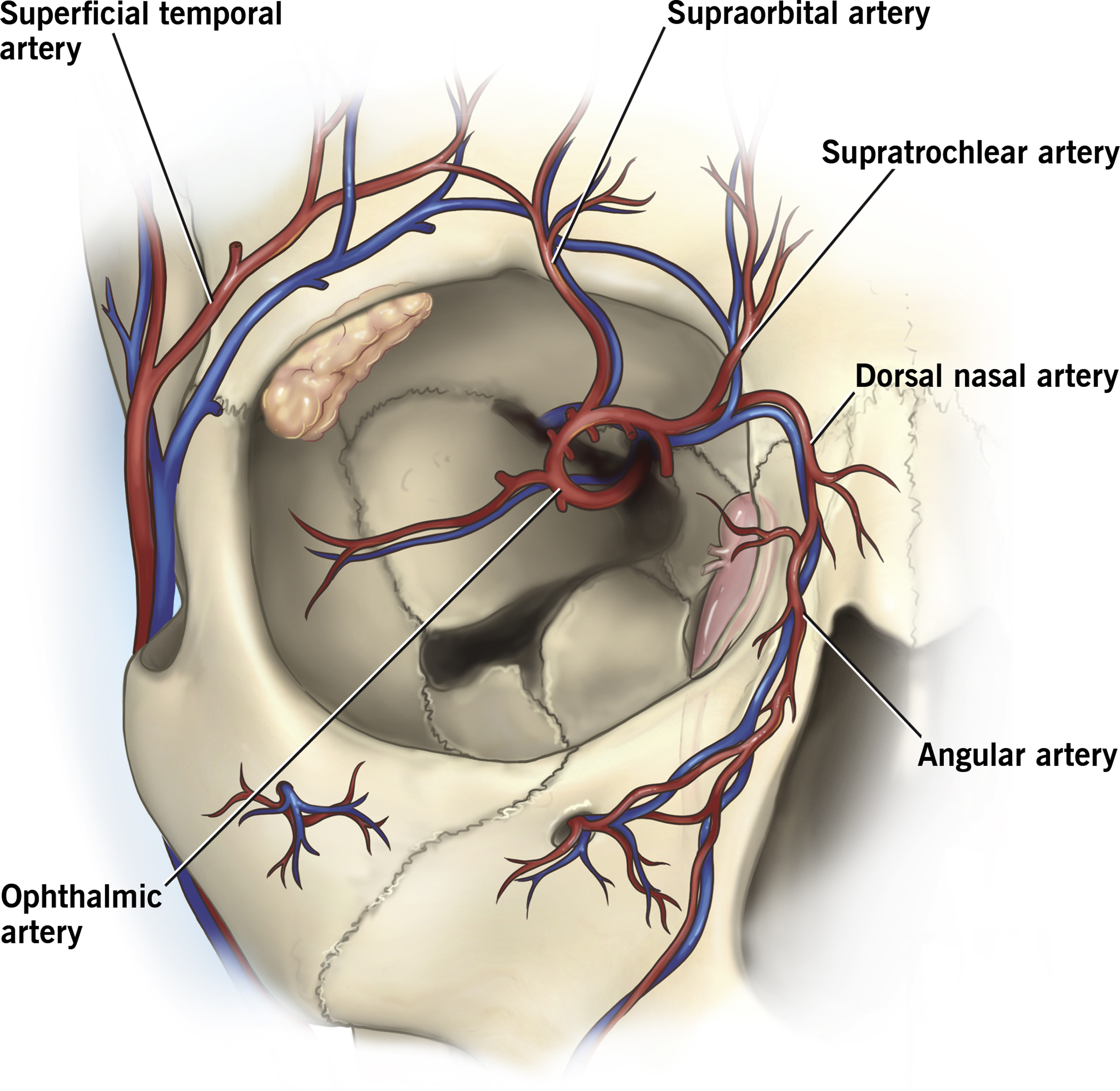
The degree of visual compromise depends on the level of obstruction within the ophthalmic artery and is likely related to the filler particle size. The approximate diameter of the ophthalmic artery is 2 mm and the central retina artery is 160 μm. , If the filler product is larger than the vessel, ischemia downstream will result.
All major types of filler materials can result in visual compromise, and particle size likely has an impact on arterial occlusion. Autologous fillers, such as fat, vary in particle size. Hyaluronic acid particle sizes are 400 μm for Restylane (Galderma, Ft. Worth, TX) and 750 to 1000 μm for Perlane (Galderma, Ft. Worth, TX). Juvederm (Allergan, Irvine, CA) products are not passed through sizing screens during processing and are instead “cohesive molecules” of cross-linked hyaluronic acid. Radiesse (BioForm Medical, San Mateo, CA) is a CaHA with particle size between 25 and 45 μm. Sculptra (Dermik Laboratories, Berwyn, PA) is a PLLA filler with particles ranging from 1 to 63 μm. Artefill (Suneva Medical, San Diego, CA) is a polymethyl-methacrylate microsphere (PMMA) filler with particles of 30 to 50 μm. In the Beleznay and colleagues 2015 review of filler-induced vision loss cases from 1906 to 2015, the most common filler causing vision loss was autologous fat (47.9%), followed by hyaluronic acid (23.5%). In their follow-up study in 2019, of cases between 2015 and 2019, hyaluronic acid was now the most common filler type (81.3%) associated with vision loss, followed by CaHA, and only one case of autologous fat injection, which likely represents the increased popularity of hyaluronic acid filler use but could also be related to the size of the filler material, with PLLA and PMMA smaller than the diameter of the central retinal artery (160 μm).
In filler-associated vision loss, the most common type of obstruction is ophthalmic artery occlusion, followed by central retinal artery occlusion (CRAO) and branch retinal artery occlusion. Ophthalmic artery occlusion causes not only CRAO but also occlusion of the downstream vessels (ciliary arteries) and can result in ptosis, ophthalmoplegia, and anterior segment ischemia ( Table 1 ). Ophthalmoplegia and anterior segment ischemia may improve after ophthalmic artery occlusion; however, CRAO will not likely improve.
| Artery | Signs/Symptoms if Occluded |
|---|---|
| Ophthalmic artery |
|
| Central retinal artery |
|
| Branch retinal artery |
|
| Muscular arteries |
|
| Dorsal nasal artery |
|
| Supraorbital and supratrochlear arteries |
|
| Ciliary arteries |
|
| Lacrimal artery |
|
Of note, in cases of filler-induced blindness, filler embolization likely involves many arterial areas, beyond the ophthalmic artery. In the cadaver head perfusion model by Cho and colleagues, after injection of the supratrochlear artery, filler was also seen in the dorsal nasal and supraorbital artery in addition to the ophthalmic artery, which is consistent with findings of skin necrosis in patients with filler-related vision loss. Filler may embolize not only to the ophthalmic artery but also to other arteries such as the supratrochlear, supraorbital, dorsal nasal, and angular arteries.
Prevention of filler-induced blindness
Although there is no method or technique that will reliably prevent vision loss, risk-modifying strategies may reduce the likelihood of filler-induced vision loss.
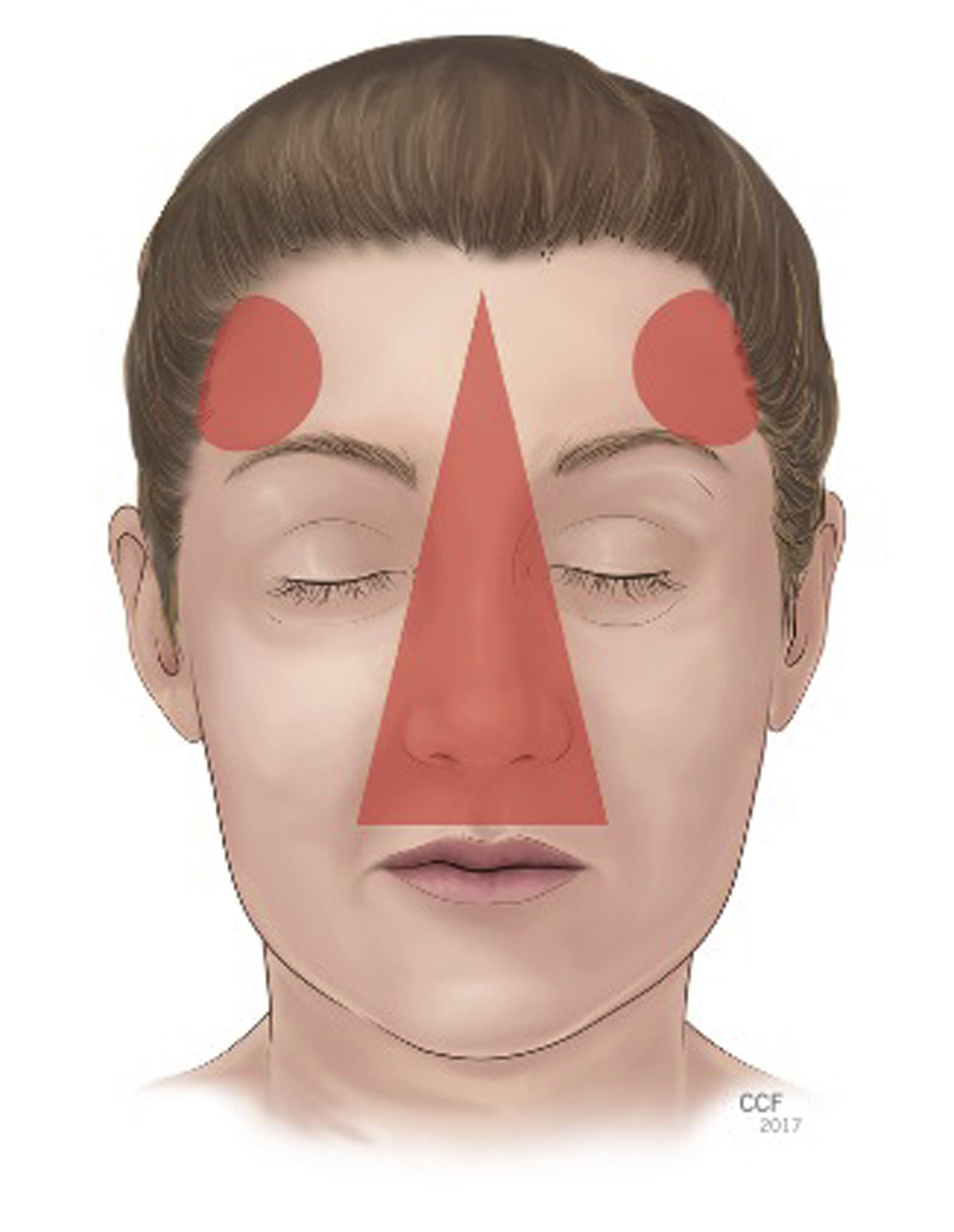
Before injecting dermal fillers, it is important to have a baseline level of understanding of the relevant facial vascular anatomy and proper placement of the filler material. Although any area of the face has a potential risk of ophthalmic artery embolization, the common “danger zones” for vascular compromise include the glabella, nasal area, nasolabial folds, forehead, and temple ( Fig. 2 ). , ,
The possibility of loss of vision associated with fillers should be discussed with patients before injection and before informed consent is obtained. Signs of filler-induced visual compromise include vision loss, sharp pain, ophthalmoplegia, ptosis, central nervous system (CNS) complications, and nausea/vomiting. Pain may be variably present and can be masked by lidocaine, which comes in most of the filler products or by previously administered topical anesthetic agents or nerve blocks. If signs of filler-induced blindness are noticed, the filler injection should be immediately stopped, and practitioners should be prepared to manage complications.
Filler injectors should avoid high pressures and volumes on injection. In a study using a cadaver head perfusion model, the average injection pressure needed to embolize the ophthalmic artery by cannulating and injecting hyaluronic acid filler in the supratrochlear artery was 166.7 mm Hg. The investigators noted that during injection “it is easy for the injector to exert the pressure well above 200 mm Hg within 2 to 3 seconds” and concluded that this provides support to the clinical recommendation to avoid high injection pressures during filler injections.
In addition, while injecting fillers, small aliquots should be used per pass. One cadaver study showed the entire volume of the supratrochlear artery from glabella to orbital apex is 0.085 mL. Therefore, small volumes, for example, less than 0.1 mL, should be used per pass.
Some have proposed cannulas over needles for filler injections; however, filler-induced blindness can result from injections using either needles or cannulas. In a review of 48 cases of blindness after filler injections from 2015 to 2018, in only 33% (16 of 48) were needle or cannula use documented, with 10 cases with needles and 6 with cannulas, with cannula diameters ranging from 27G to 23G. The force needed to penetrate an arterial wall increases with both larger diameter cannulas and needles. For 27G needles and cannulas, the force needed to penetrate an artery is similar. For larger diameters (22G and 25G), cannulas require greater force to penetrate arterial walls than needles. No clinical studies have shown cannulas are safer than needles or that they reduce the risk of arterial perforation.
Reflux of the filler syringe is a commonly recommended technique to evaluate for intraarterial placement of the needle or cannula. In a study of 17 filler products (mostly hyaluronic acid, 1 PLLA, and 2 CaHA products), the syringe and needles provided in the filler package only showed a positive reflux in 53% of fillers, although larger bore needles did lead to positive aspirations in all filler materials. In a similar study of aspiration using a variety of needle types and soft tissue fillers, it was found that 112 of 340 aspiration tests (33%) had positive aspiration results within 1 second of aspiration. After 10 seconds of aspiration, 128/140 aspiration tests (38%) showed false-negative aspiration results. Unlike aspiration tests when injecting fluids, filler materials have different rheological properties that make aspiration a less reliable test to confirm intraarterial placement of the needle or cannula.
Recommended injection techniques should be used, such as injecting small volumes per pass of the needle, continuously moving the tip of the needle or cannula during injection and using a low injection pressure, small aliquots of filler material, and possibly aspiration before injection; however, this has high false-negative results. Other recommendations to prevent intraarterial embolization of filler products include using local vasoconstrictors (eg, epinephrine) to constrict the blood vessels and limit entry, limiting the total volume of filler used, and occlusive pressure in the area of the supraorbital notch ( Box 1 ). ,
- 1.
Understand periorbital anatomy.
- 2.
Inject small volumes per pass and less than 0.1 mL in any one area.
- 3.
Keep moving the tip of the injection needle/cannula.
- 4.
Attempt aspiration before injection; however, sometimes because of the length of the needle or nature of the filler product, it may not produce a flashback.
- 5.
Use low injection pressure, do not force injection, especially in areas of previous scarring, injection, or surgery.
- 6.
Consider the use of blunt cannulas, but remember they do not eliminate risk.
- 7.
Smaller needles and cannulas may be more likely to penetrate vascular walls.
- 8.
Always know where the tip of the needle or cannula is in 3-dimensional space, including depth (can use nondominant hand to protect globe and feel tip of cannula before injection)
- 9.
Consider local anesthesia with epinephrine to vasoconstrict blood vessels; however, blanching may delay the recognition of ischemic complications.
Treatments for filler-induced blindness
Unfortunately, to date, there are no proven treatments to reverse filler-induced blindness. Vision recovery is time sensitive, and it is thought that the retinal circulation needs to be restored within 90 minutes of onset to prevent permanent vision loss. If visual compromise or blindness develops after injection of fillers, the patient should be referred immediately to an ophthalmologist, preferably a retina specialist. Before initiating treatment, it is important to confirm the degree of vision loss (check visual acuity or at least determine light perception or no light perception) and evaluate the pupillary reaction for a relative afferent pupillary defect. It is important to evaluate for other signs of ischemia in the skin, orbit, and CNS. Proptosis (bulging of eye), extraocular motility, ptosis (droopy eyelid), and redness of the eye can be also present with filler-induced visual compromise.
Even with early recognition and prompt referral and evaluation, visual compromise associated with filler injection is often irreversible due to the terminal effects on the retina. The other signs such as ptosis and ophthalmoplegia will recover over time due to the rich blood supply and ability of muscles to regain function.
Treatment options for filler-induced blindness resulting in blindness and CRAO include traditional treatments for CRAO to try and dislodge emboli: lowering intraocular pressure (IOP) with ocular massage, IOP-lowering eye drops, systemic medications such as acetazolamide and mannitol, and possibly anterior chamber paracentesis. Although none of these treatments have been shown to reverse CRAO, most are relatively low risk and can be attempted in filler-induced blindness.
Hyaluronidase
High-dose hyaluronidase is the only proven treatment of the reversal of soft tissue ischemia associated with inadvertent intraarterial injection of hyaluronic acid gel fillers. Hyaluronidase has been suggested as a treatment option for filler-related blindness, but this remains unproved. Hyaluronidase is an enzyme that depolymerizes hyaluronic acid by degrading glycoside bonds and may be used to reverse the effects of hyaluronic acid filler material. Hyaluronidase is derived from a variety of formulations: purified bovine testicular hyaluronidase (Amphadase, Hydase), purified ovine testicular hyaluronidase (Vitrase), and recombinant human DNA (Hylenex). Hyaluronidase products are standardized so that 1 International Unit (IU) is equivalent in hydrolysis of hyaluronic acid, regardless of the source of formulation. The effectiveness of hyaluronidase depends on many factors, including the amount of filler needed to be reversed, surface area of hyaluronic acid for interaction with hyaluronidase, temperature, and pH.
Hyaluronidase may take several hours to hydrolyze hyaluronic acid filler. Using 150 IU of hyaluronidase can hydrolyze 0.1 cc of hyaluronic acid in approximately 4 hours. By depolymerizing hyaluronic acid filler materials, hyaluronidase may reverse ischemic complications. Different hyaluronic acid fillers respond at different rates, given different formulations, concentrations, and cross-linking properties. In one study, hyaluronidase in the form of Vitrase and Hylenex were used in vitro: Restylane showed the most degradation, followed by Juvederm, then Belotero (most resistant to degradation). In general, more hyaluronidase is required for larger volumes of filler, and higher concentrations of hyaluronidase dissolves filler faster.
Hyaluronidase for filler-associated blindness potentially can be administered as an intraarterial, intravitreal, intravenous (IV), or retrobulbar injection. Although there are reported cases of improvement in vision with retrobulbar and intraarterial hyaluronidase injections, this has not been proved and is not consistently effective.
Intraarterial hyaluronidase given near the ophthalmic artery, via the supratrochlear, supraorbital arteries, or with interventional radiology, could potentially dissolve hyaluronic acid filler causing filler-induced blindness. , Given the small time window for efficacy of hyaluronidase, intraarterial injections with interventional radiology are highly unlikely to be coordinated within a short time window, as this requires transfer to a hospital, administration of anesthesia, and establishing femoral artery access. Although supraorbital and supratrochlear arteries are close to the skin surface, this route relies on hyaluronidase being injected into the artery and following the path of least resistance, which may not be toward the filler material embolus. Two case reports , have shown that supraorbital and/or supratrochlear injections helped symptoms, although visual acuity and pressures were not checked. In addition, direct cutdown and cannulation of the supraorbital and/or supratrochlear artery has been proposed; however this requires surgical exposure, knowledge of the anatomy, and surgical expertise.
Intravitreal hyaluronidase potentially could provide a more direct access of hyaluronidase to the retinal and choroidal vessels, as it could diffuse across these vessels. Intravitreal hyaluronidase has been reported in the use of vitreous hemorrhage in diabetic retinopathy, showing no serious safety issues. In addition, there are risks associated with retinal injections such as cataract formation or acceleration, raised IOP, retinal tears or detachments, and endophthalmitis. The authors, in their unpublished rabbit study data (Catherine J. Hwang, unpublished data, 2016), have not found reversal with intravitreal injections alone and needs to be administered by a skilled eye specialist.
IV hyaluronidase may be promising, as it could be administered in the office or emergency room setting by peripheral vein. In a rabbit model, Chiang and colleagues showed improvement in ophthalmic and retinal artery perfusion with hyaluronidase and urokinase when given IV within 30 minutes, although large doses were required (5000 IU/kg). Urokinase is thought to help by dissolving any arterial thrombosis in the area of occlusion. IV hyaluronidase may be a promising route, as it could be administered quickly, has been shown to be safe in large doses, and can be administered by peripheral venous access. , However, this large dose of IV hyaluronidase in a 50 kg human (250,000 IU) may be cost prohibitive.
Retrobulbar hyaluronidase is another route of administration for filler-induced vision loss. Retrobulbar hyaluronidase theoretically works given its ability diffuse through arterial walls into intraarterial hyaluronic acid filler material. Hyaluronidase, however, has not been found to penetrate the optic nerve sheath in in-vitro studies of human optic nerves. In one rabbit study, retrobulbar hyaluronidase administered at 30 minutes using 1000 IU of hyaluronidase failed to reverse or restore filler-associated vision loss. In a separate rabbit study, there seemed to be an improvement with 3000 IU retrobulbar hyaluronidase injection 5 to 10 minutes after filler retinal artery occlusion. However, in this study there were no pretreatment electroretinograms to verify abnormal function before retrobulbar hyaluronidase, so it is unknown if the eyes were truly occluded pretreatment and the hyaluronidase was given remarkably rapidly. In the literature, the volume of retrobulbar hyaluronidase used for filler-induced blindness ranges from 120 IU up to 6000 IU. Although there have been a few cases reported with successful use of retrobulbar hyaluronidase, there was no vision checked before treatment, questioning the reported visual improvement after treatment.
Given there are no proved treatments of filler-induced blindness, retrobulbar hyaluronidase is a frequently attempted treatment option but does have risks including retrobulbar hemorrhage and inadvertent globe perforation. In addition, less than 7 cc of hyaluronidase must be used, as higher volumes risks increased orbital pressure and optic disc edema. Retrobulbar hyaluronidase, if administered, should be given by a trained professional, and the patient should understand that the treatment may not reverse the visual loss, can have some other risks, but could help with orbital pain.
Future directions and studies
An ideal filler would be without complications, fully reversible, and could age with the individual. Unfortunately, an ideal treatment of visual compromise and blindness remains to be determined. Further research efforts are needed to find a treatment or possible prevention protocol. The ideal filler would be one that does not lead to complications, particularly the devastating complication of vision loss, and would have a reliable treatment if a complication were to occur.
Further studies may evaluate various routes of hyaluronidase administration (intraarterial, IV, intravitreal, and retrobulbar) by themselves or in combination as well as possible other adjunctive medications such as thrombolytics, corticosteroids, and vasodilators. The authors look forward to the day when they have a proved treatment of filler-induced blindness.
Summary
Dermal fillers remain popular treatments for facial rejuvenation. Although relatively safe and effective, complications can occur, including visual compromise and blindness. Before any filler injection, careful planning and informed consent including vision loss and blindness should be discussed with the patient. In addition, injectors, staff, and patients should all be aware of and educated of potential complications, be able to identify filler complications, and promptly initiate treatment. For filler-induced vision loss, there are no proved treatments to date. Various rescue methods can be attempted such as those used for CRAO (ocular massage, lowering IOP, rebreathing into a bag) and potentially hyaluronidase. Hyaluronidase can be administered in various routes to attempt to reverse filler-induced blindness but unfortunately none have been proved to be effective. Regardless, hyaluronidase should always be available at all times when injecting hyaluronic acid fillers, as it is the only proved treatment to reverse filler-induced skin ischemia.
Disclosure
The authors have nothing to disclose.
Unrestricted grant award from Research to Prevent Blindness to the Department of Ophthalmology at Cole Eye Institute (RPB1508DM).
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree