Abstract
Bacterial infections commonly affect the skin and account for ~20% of outpatient dermatology visits. Staphylococci and streptococci cause the majority of bacterial skin conditions, which range from common infections such as impetigo and cellulitis to severe multisystem disorders such as toxic shock syndrome. Other Gram-positive and Gram-negative bacterial infections have a wide variety of localized and widespread cutaneous manifestations. In some patients, such skin findings represent signs of a systemic bacterial infection or underlying immunodeficiency. This chapter also reviews several spirochetal infections, actinomycosis, and nocardiosis.
Keywords
cutaneous microbiota, impetigo, folliculitis, furuncle, ecthyma, methicillin-resistant Staphylococcus aureus (MRSA), blistering distal dactylitis, staphylococcal scalded skin syndrome, toxic shock syndrome, scarlet fever, erysipelas, cellulitis, pseudocellulitis, botryomycosis, necrotizing fasciitis, erythrasma, pitted keratolysis, cutaneous anthrax, erysipeloid, meningococcemia, bartonellosis, cat scratch disease, bacillary angiomatosis, glanders, melioidosis, malacoplakia, tularemia, rhinoscleroma, rat-bite fever, plague, acrodermatitis chronica atrophicans, yaws, pinta, endemic syphilis, leptospirosis, actinomycosis, nocardiosis, Gram-negative bacteria, Gram-positive bacteria, spirochetes
- ▪
Roughly 20% of outpatient dermatology visits are for bacterial skin infections
- ▪
Staphylococci and streptococci cause the majority of bacterial skin conditions, which range from common infections (e.g. impetigo) to multisystem disorders (e.g. toxic shock syndrome)
- ▪
An increase in the prevalence of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) remains a concern
- ▪
Various systemic diseases and immunodeficiency states predispose patients to bacterial skin infections that can be severe and refractory to treatment
The Cutaneous Microbiota
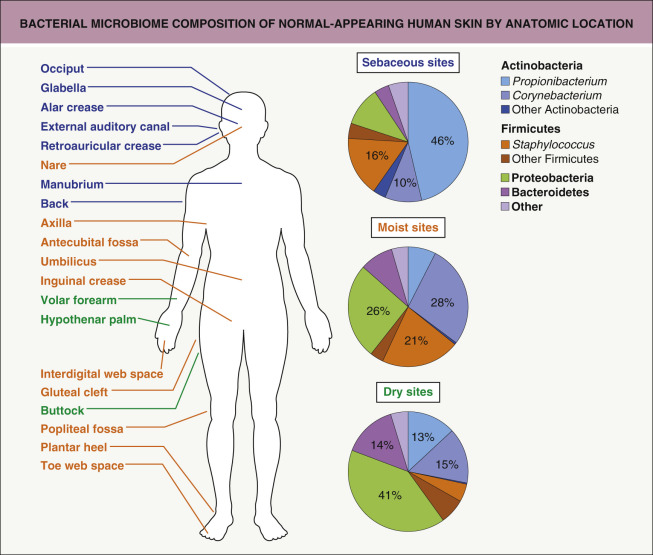
Hundreds of species of bacteria inhabit the skin as a part of the cutaneous microbiota (flora), which has been better defined utilizing DNA sequencing techniques. The normal skin micriobiota is composed of aerobic cocci, aerobic and anaerobic coryneform bacteria, Gram-negative bacteria, and yeast. Four phyla – Actinobacteria, Firmicutes, Bacteroidetes and Proteobacteria – account for the vast majority of skin bacteria ( Fig. 74.1 ) . Together these organisms help prevent skin infections by providing ecologic competition with pathogenic microorganisms and by hydrolyzing lipids of sebum to produce free fatty acids, which are toxic to many pathogenic bacteria. The ecology of particular areas of the skin is determined by the availability of moisture, presence of sebaceous lipids, and gaseous environment. Disruption of the delicate balance between host and microorganisms can result in skin disorders or infection. The nomenclature of cutaneous bacterial infections reflects the site, depth, and extent of the infection as well as the organism involved.
Gram-Positive Bacteria
Staphylococcal and Streptococcal Skin Infections
Impetigo
▪ Bullous impetigo: pemphigus neonatorum ▪ Impetigo contagiosa ▪ Non-bullous impetigo: crusted impetigo ▪ Staphylococcal impetigo ▪ Streptococcal impetigo
- ▪
Staphylococcus aureus and, to a lesser degree, group A β-hemolytic Streptococcus spp. are the major causes of impetigo
- ▪
Represents the most common bacterial skin infection in children
- ▪
Nasal carriers of S. aureus are at particular risk of developing impetigo
- ▪
Treatment decisions should consider resistance patterns of S. aureus
Introduction
Impetigo is a common, contagious, superficial skin infection that can present in non-bullous and bullous forms ( Fig. 74.2 ). The most common pathogen in both non-bullous and bullous impetigo is Staphylococcus aureus. Group A β-hemolytic Streptococcus ( Streptococcus pyogenes ) represents another important cause of non-bullous impetigo. Overall, non-bullous impetigo accounts for approximately 70% of cases. Table 74.1 compares the clinical characteristics and complications of bullous and non-bullous impetigo.
| CHARACTERISTIC FEATURES OF BULLOUS AND NON-BULLOUS IMPETIGO | ||
|---|---|---|
| Non-bullous impetigo | Bullous impetigo | |
| Epidemiology |
|
|
| Clinical lesions |
|
|
| Distribution |
|
|
| Associated findings |
|
|
| Clinical course |
|
|
| Complications |
|
|
* Associated with anti-DNase B and antistreptolysin O (ASO) antibodies.
Epidemiology
Impetigo frequently occurs in children, especially those <6 years of age, and worldwide it represents the most common bacterial skin infection in this group. Adults most commonly acquire impetigo through contact with infected children. Impetigo is extremely contagious, spreading rapidly via direct person-to-person contact or through fomites. In temperate climates, peak incidence is in the summer months. Predisposing factors include a warm ambient temperature, high humidity, poor hygiene, an atopic diathesis, skin trauma, and participation in contact sports (e.g. wrestling, football). Nasal, pharyngeal, axillary, and/or perineal colonization with S. aureus imparts an increased risk for developing impetigo and other staphylococcal infections.
Pathogenesis
Non-bullous impetigo is usually caused by S. aureus or (less often in temperate climates) Str. pyogenes . Infection typically occurs at sites of scratching (e.g. insect bites, atopic dermatitis), minor trauma (e.g. an abrasion, laceration, or burn) or other skin infections (e.g. varicella). Disruption of the skin barrier allows the bacteria to adhere, invade, and establish infection.
Bullous impetigo results from the local production of exfoliative toxins (ETA, ETB) by phage group II S. aureus at the site(s) of cutaneous infection; systemic elaboration of the same toxins is the cause of staphylococcal scalded skin syndrome (SSSS; see below). In both diseases, blister formation is mediated by exfoliative toxin binding to the desmosomal protein desmoglein 1 and cleaving its extracellular domain, thus leading to acantholysis within the epidermal granular layer. S. aureus can be cultured from fluid within the blisters of bullous impetigo, but not those of SSSS. Compared to non-bullous impetigo, the bullous form is more likely to develop on clinically intact skin, especially in intertriginous sites.
Clinical features
The clinical features of impetigo are outlined in Table 74.1 .
Pathology
In non-bullous impetigo , small neutrophilic vesiculopustules are present within the epidermis. Spongiosis frequently underlies the pustule. The upper dermis contains an intense infiltrate of neutrophils and lymphocytes. Gram-positive cocci are present within the vesiculopustules.
In bullous impetigo , there is cleavage of the upper epidermis, typically within the granular layer. Acantholysis mimicking pemphigus foliaceus may be observed. Relatively few inflammatory cells are present within the blister cavity, and a neutrophilic infiltrate is often found in the upper dermis. Gram-positive cocci may be evident.
Diagnosis and differential diagnosis
The diagnosis of impetigo is usually made clinically; exudate from beneath the crust or fluid from intact bullae can be sent for culture to confirm the diagnosis and determine susceptibility to antibiotics. Leukocytosis is seen in approximately half of patients with impetigo and regional lymphadenopathy is common. The differential diagnosis of non-bullous and bullous impetigo is presented in Table 74.2 .
| DIFFERENTIAL DIAGNOSIS OF NON-BULLOUS AND BULLOUS IMPETIGO | ||
|---|---|---|
| Most common | Less common | |
| Non-bullous impetigo |
|
|
| Bullous impetigo |
|
|
Treatment
For healthy patients with a few superficial lesions and no systemic symptoms, topical mupirocin, retapamulin, or fusidic acid (not available in the US) are often equally (if not more) effective than oral antibiotics. However, S. aureus can develop resistance to each of these agents . Treatment should also include cleansing the affected area and removing crusts, which can be facilitated by wet dressings.
The extent of skin involvement, the presence of complications (e.g. cellulitis, lymphangitis, bacteremia), comorbid conditions (e.g. atopic dermatitis, varicella), the patient’s immune status, and local drug-resistance patterns (e.g. the prevalence of community-associated methicillin-resistant S. aureus [CA-MRSA]) should be considered when deciding whether topical, oral, or intravenous therapy is the most appropriate treatment ( Table 74.3 ). The risk of developing post-streptococcal glomerulonephritis following streptococcal impetigo is not affected by treatment and is greater with certain subtypes of Str. pyogenes (see Table 74.1 ) . In contrast to pharyngitis, a link between streptococcal pyoderma and acute rheumatic fever has not been established. Methods for “decolonizing” the nares and skin of patients with recurrent staphylococcal impetigo are discussed in the folliculitis section below.
| EMPIRIC TREATMENT OF CUTANEOUS STAPHYLOCOCCAL AND STREPTOCOCCAL INFECTIONS IN ADULTS | |
|---|---|
| Organism/situation | Suggested antibiotics |
| Streptococcal infection |
|
Suspected MSSA infection
|
|
Suspected MRSA infection (see Table 74.4 )
| |
| Other oral options | |
| Intravenous options for severe disease | |
| Penicillin-allergic patients |
|
| Additional special considerations |
|
* Does not provide coverage of group A streptococci; if coverage of the latter is desired, a β-lactam should also be prescribed.
† Intravenous form also available.
‡ Not currently available in the US.
** e.g. 0.5 cup of household bleach (6–8.25% sodium hypochlorite) in a full 40-gallon bathtub, or 0.5–1 teaspoon per gallon in a spray bottle.
Bacterial Folliculitis
Introduction
Bacterial folliculitis is a superficial or deep infection of the hair follicle. A furuncle develops when the entire follicle and surrounding tissue are involved (see below).
Epidemiology and pathogenesis
S. aureus is the most common infectious cause of folliculitis (see Ch. 38 ). Gram-negative folliculitis occasionally develops in acne vulgaris patients treated with prolonged courses of oral antibiotics. In addition, pseudomonal folliculitis can result from the use of improperly chlorinated hot tubs and whirlpools . Predisposing factors for staphylococcal folliculitis include occlusion, maceration, and hyperhydration of the skin; shaving, plucking, or waxing hair; use of topical corticosteroids; hot and humid weather; atopic dermatitis; and diabetes mellitus.
Clinical features
Staphylococcal folliculitis most frequently involves the face (especially the beard area), scalp, chest, back, axillae, and buttocks (see Ch. 38 ). The appearance depends on the depth of follicular involvement. Superficial folliculitis (impetigo of Bockhart) presents with small (1–4 mm) pustules or crusted papules on an erythematous base. The lesions are frequently clustered and heal without scarring. Sycosis barbae, a type of deep folliculitis, appears as large erythematous papules, often with a central pustule and sometimes coalescing to form plaques studded with pustules and crusts. Bacterial folliculitis may be pruritic or (especially with deeper involvement) tender .
Diagnosis and differential diagnosis
The diagnosis of bacterial folliculitis is usually made based on clinical inspection. Gram staining and bacterial cultures can help to identify the causative organisms, which is especially useful in severe, recurrent or treatment-resistant cases. The differential diagnosis includes other forms of folliculitis (see Ch. 38 ) as well as acne vulgaris, rosacea, chloracne, pseudofolliculitis barbae, and keratosis pilaris.
Treatment
Superficial staphylococcal folliculitis can be treated with antibacterial washes that contain chlorhexidine or sodium hypochlorite. Topical antibiotics such as mupirocin or clindamycin may also be used for 7–10 days to treat localized lesions. When staphylococcal folliculitis is widespread or recurrent, oral β-lactam antibiotics (e.g. a β-lactamase-resistant penicillin or first-generation cephalosporin), tetracyclines, or (depending on local resistance patterns) macrolides can be prescribed (see Table 74.3 ). Although pseudomonal folliculitis is often self-limited, ciprofloxacin is an option for more severe cases.
In patients with recurrent staphylococcal folliculitis and their close contacts, application of mupirocin 2% ointment twice daily to the nares for 5–10 days can be used to eradicate nasal carriage of S. aureus ; methods to decolonize the skin (e.g. axillae, perineum/groin, submammary area) include topical mupirocin, washes containing chlorhexidine or triclosan (OTC product banned in the US), and dilute sodium hypochlorite baths (e.g. 0.5 cup household bleach [6–8.25% sodium hypochlorite] in a full standard bathtub). Elimination of bacterial contamination of potential fomites such as keyboards, toys, and sports equipment (e.g. shoulder pads, wrestling mats) should also be considered, e.g. by using ethanol- or sodium hypochlorite-based disinfectants.
Abscesses, Furuncles, and Carbuncles
Introduction
Abscesses and furuncles (boils) are collections of pus that are “walled-off” from the surrounding tissues. Whereas an abscess can occur anywhere in or on the body, a furuncle, by definition, involves a hair follicle. A contiguous collection of furuncles is termed a carbuncle.
Epidemiology and pathogenesis
Furuncles most often occur in adolescents and young adults. S. aureus is usually the causative organism, although anaerobic bacteria are occasionally cultured from recurrent furuncles in the anogenital region. Predisposing factors include chronic S. aureus carriage, close personal contact with affected individuals (e.g. households, athletic activities), diabetes mellitus, obesity, poor hygiene, immunodeficiency syndromes (e.g. chronic granulomatous disease, hyperimmunoglobulin E syndrome), and hereditary sensory and autonomic neuropathy due to defects in nerve growth factor β or its high-affinity receptor (see Table 6.10 ) .
Clinical features
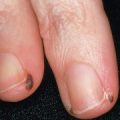
Abscesses are localized collections of pus that are usually inflamed. They can arise in any cutaneous site. Furuncles are acute, inflammatory abscesses of individual hair follicles and the surrounding tissue, and, as such, occur only in hair-bearing skin. The most common locations are the face, neck, axillae, buttocks, thighs, and perineum. Sites prone to friction or minor trauma, such as the area under a belt, are also susceptible. Furuncles usually begin as firm, tender, red nodules that progressively enlarge, becoming painful and fluctuant ( Fig. 74.3 ); rupture results in decreased pain. Systemic symptoms are usually absent, although regional lymphadenopathy may develop. Furunculosis (multiple or recurrent furuncles) can be associated with chronic S. aureus carriage.
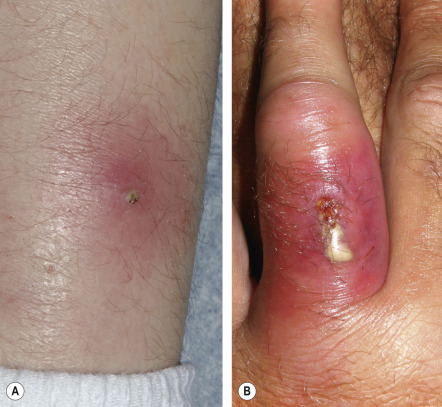
Carbuncles are collections of furuncles that extend deep into the subcutaneous tissue. The surface usually displays multiple draining sinus tracts and occasionally ulcerates. They usually occur in areas with thicker skin (e.g. nape of neck, back, thigh). Systemic symptoms are often present. Carbuncles are slow to heal and lead to scarring.
Pathology
Biopsy specimens reveal a dense neutrophilic infiltrate in the subcutaneous tissue. Furuncles are characterized by an acute, suppurative reaction involving the follicle below the infundibulum, as well as perifollicular necrosis with fibrinoid debris.
Diagnosis and differential diagnosis
Diagnosis is based primarily on clinical appearance. Gram stains and cultures from the lesion support the diagnosis. Extensive furuncles and carbuncles can be associated with leukocytosis. A ruptured epidermoid or pilar cyst, hidradenitis suppurativa, and cystic acne should be considered in the differential diagnosis.
Treatment
For simple furuncles, warm compresses may promote maturation, drainage, and resolution. Larger or deeper fluctuant lesions typically require incision and drainage. Systemic antibiotic therapy is recommended (in conjunction with incision and drainage if possible) in the following situations: (1) furuncles around the nose, in the external auditory canal, or in other locations where drainage is difficult (e.g. elsewhere on the face, hands, genitalia); (2) severe or extensive disease (e.g. multiple sites); (3) lesions with surrounding cellulitis/phlebitis or associated with signs/symptoms of systemic illness; (4) lesions not responding to local care; and (5) patients with concerning comorbidities or immunosuppression . Given the high proportion of furuncles that are caused by MRSA, empiric coverage with antibiotics such as doxycycline, trimethoprim–sulfamethoxazole (TMP-SMX), or (depending on local resistance patterns) clindamycin should be considered (see Table 74.3 and below). Recent large controlled studies found that administration of a systemic antibiotic (TMP-SMX or clindamycin) following incision and drainage of a solitary, uncomplicated cutaneous abscess increases the cure rate (~80–85%, vs ~70% with placebo) and decreases the likelihood of recurrence . However, this benefit must be weighed against the potential for antibiotic side effects. Patients with recurrent furunculosis may benefit from eradication of S. aureus from the nares, axillae, and perineum (see Bacterial folliculitis above).
Methicillin-Resistant Staphylococcus aureus
Introduction
Methicillin resistance was first noted in isolates of S. aureus in 1961. Although initially associated with nosocomial infection (healthcare-associated [HA]-MRSA), in recent years community-associated (CA)-MRSA has become a growing problem among young, otherwise healthy individuals . Table 74.4 compares CA- and HA-MRSA. Although there is considerable regional variation, overall, MRSA represents the most common cause of purulent skin and soft tissue infections presenting to emergency departments in the US . MRSA also represents one of the ESKAPE pathogens that have emerged due to widespread overuse of antibiotics ( Table 74.5 ); these organisms have become increasingly problematic sources of nosocomial infections, especially among burn victims.
| COMPARISON OF COMMUNITY-ASSOCIATED (CA) AND HEALTHCARE-ASSOCIATED (HA) METHICILLIN-RESISTANT STAPHYLOCOCCAL AUREUS (MRSA) | ||
|---|---|---|
| CA-MRSA | HA-MRSA | |
| Epidemiology |
|
|
| Risk factors |
|
|
| Typical sites/types of infection |
|
|
| Molecular characteristics |
|
|
| Antibiotic susceptibility |
|
|
* Increased risk of staphylococcal infections, but not generally in the proportion due to MRSA.
| THE ESKAPE PATHOGENS |
|---|
| Enterococcus faecium |
| Staphylococcus aureus |
| Klebsiella pneumoniae |
| Acinetobacter baumannii |
| Pseudomonas aeruginosa |
| Enterobacter species |
Clinical features
Furunculosis is the most frequent manifestation of CA-MRSA infection (see Fig. 74.3 ). These lesions can evolve into large abscesses, cellulitis, or necrotic plaques which may be misdiagnosed as spider bites. Less often, impetigo, SSSS, and folliculitis are seen, and life-threatening complications such as septic shock, toxic shock syndrome, and necrotizing fasciitis occasionally develop.
Pathogenesis
The cause of methicillin resistance is the production of an altered penicillin-binding protein 2a (PBP2a) that has decreased affinity for β-lactam antibiotics, which interfere with bacterial cell wall synthesis by binding to PBPs. PBP2a is the protein product of the mecA gene, which is located within a specific mobile genetic element called the staphylococcal cassette chromosome mec (SCC mec ). Acquisition of different SCC mec elements by S. aureus led to the emergence of HA-MRSA (usually SCC mec types I–III) and CA-MRSA (usually SCC mec types IV and V) strains; methicillin-resistant coagulase-negative staphylococci are thought to represent a reservoir of SCC mec for CA-MRSA. Many CA-MRSA strains also encode virulence factors such as Panton–Valentine leukocidin (PVL), a pore-forming cytotoxin that can cause destruction of leukocytes and tissue necrosis.
As a reflection of their distinct SCC mec elements, CA-MRSA and HA-MRSA have different patterns of antibiotic resistance. CA-MRSA is typically susceptible to multiple non-β-lactam antibiotics, whereas HA-MRSA is usually resistant to several antimicrobial classes, including aminoglycosides, macrolides, and clindamycin. However, some isolates of the USA300 clone, which currently causes most CA-MRSA skin infections in the US, have developed resistance to macrolides, clindamycin, tetracycline (less often doxycycline), quinolones, and mupirocin .
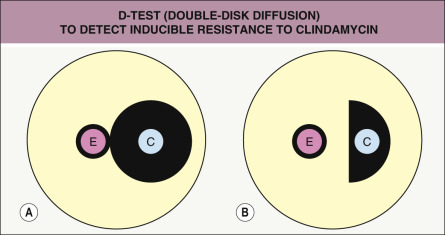
Resistance to macrolides (e.g. erythromycin) in staphylococci may result from: (1) active drug efflux via a pump encoded by the msrA / msrB gene (frequent in MRSA isolates); (2) synthesis of macrolide-inactivating enzymes; or (3) modification of the bacterial ribosome by the erythromycin ribosomal methylase encoded by erm genes, which produces cross-resistance to clindamycin . Erythromycin stimulates expression of erm , which leads to clinically relevant resistance. If initial sensitivity testing shows resistance to erythromycin and susceptibility to clindamycin, resistance to clindamycin will still develop if erm is present, since bacterial variants with constitutive expression of this gene commonly arise and are selected for during clindamycin therapy. This “inducible resistance” can be assessed with the D-test (double-disk diffusion) ( Fig. 74.4 ).
Diagnosis and treatment
When S. aureus infection is suspected, culture and susceptibility testing should be performed, whenever feasible, to guide antibiotic selection. Prior to obtaining these results, empiric antibiotic therapy can be chosen based on the prevalence of MRSA and resistance patterns in a given community as well as the severity of the infection (see Table 74.3 ). Vancomycin should be considered for patients with severe infections, especially in geographic regions where CA-MRSA is prevalent and in individuals with a history of MRSA colonization or injection drug use . PCR-based assays (e.g. BD Max TM StaphSR) have been developed for rapid detection of MRSA from nasal or potentially skin swab samples, but they do not provide other drug susceptibility information.
Blistering Distal Dactylitis
Blistering distal dactylitis is a localized infection of the volar fat pad of a finger or, less often, a toe, with occasional involvement of the nail fold or more proximal portion of the digit. Darkening of the skin is often observed for several days to a week before blister formation. The infection most commonly occurs in children aged 2–16 years.
Group A Streptococcus spp. or S. aureus are usually the causative organisms. Inoculation may follow local skin trauma or autoinoculation from nose picking . The differential diagnosis includes herpetic whitlow, a thermal or chemical burn, acute paronychia, bullous impetigo, and frictional bullae.
Drainage and a 10-day course of an oral antistaphylococcal antibiotic are recommended. Although topical therapy with mupirocin has been described, systemic therapy can prevent development of new sites of infection as well as local extension.
Ecthyma
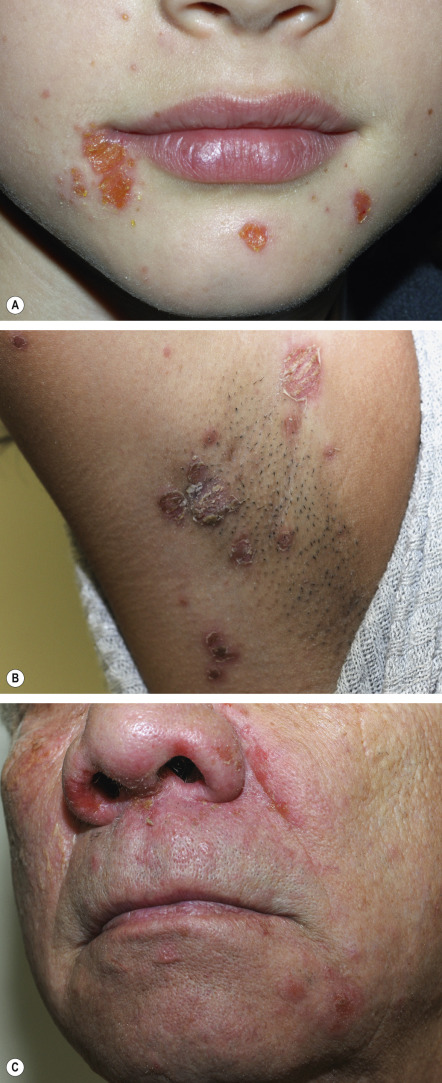
Ecthyma is a deep form of non-bullous impetigo characterized by extension into the dermis to produce a shallow ulcer that heals with scarring ( Fig. 74.5 ). It can be caused by a primary infection with Str. pyogenes or streptococcal superinfection of a pre-existing ulceration or excoriated insect bite. Outbreaks of ecthyma have occurred among infantry units where skin trauma, poor hygiene, and crowded living conditions facilitate disease spread . Table 74.6 summarizes the clinical aspects and treatment of ecthyma .
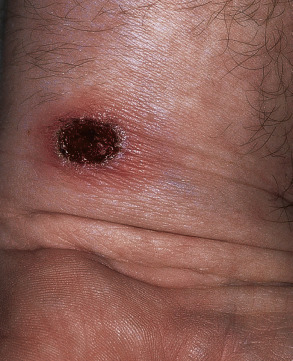
| CLINICAL FEATURES OF ECTHYMA | |
|---|---|
| Clinical findings |
|
| Risk factors |
|
| Complications |
|
| Diagnosis |
|
| Differential diagnosis |
|
Staphylococcal Scalded Skin Syndrome
▪ Ritter’s disease ▪ Pemphigus neonatorum
Introduction
Staphylococcal scalded skin syndrome (SSSS) is caused by hematogenous dissemination of the same exfoliative toxins that lead to bullous impetigo when produced locally. Cleavage of the epidermis through the granular layer results in the formation of tender, flaccid bullae .
Epidemiology
SSSS is primarily a disease of infants and young children, who have decreased renal toxin clearance (especially neonates) and/or a lack of toxin-neutralizing antibodies. Occasionally, adults with chronic renal insufficiency or immune suppression are affected. Outbreaks occurring in neonatal nurseries are usually secondary to asymptomatic carriage of a toxigenic strain of S. aureus by healthcare workers or parents. A male predominance exists, with a 2 : 1 male : female ratio in sporadic cases and 4 : 1 in epidemics .
Pathogenesis
Most cases of SSSS are caused by phage group II strains (e.g. types 55, 71) of S. aureus , which can be methicillin-sensitive or resistant and produce exfoliative (also known as epidermolytic) toxins (ETs). ETA (chromosomally encoded) and ETB (plasmid encoded) are serine proteases that bind and cleave desmoglein 1 (Dsg1). This causes splitting of the desmosomes, which leads to disruption of the epidermal granular layer and bulla formation. Desmoglein 1 is targeted by autoantibodies in pemphigus foliaceus, which has identical histologic features to SSSS.
In contrast to bullous impetigo, where the effects of the ETs are limited to the site(s) of infection, in SSSS the toxin diffuses from a focus of infection and (in the absence of specific antitoxin antibody) spreads hematogenously to produce widespread effects. In children, the infectious focus is usually in the nasopharynx or conjunctivae, whereas staphylococcal pneumonia or bacteremia may be present in adults .
Clinical features
There is often a prodrome of malaise, fever, irritability, and tenderness of the skin. The patient may have purulent rhinorrhea or conjunctivitis as a manifestation of the underlying staphylococcal infection. Erythema typically first appears on the head (accompanied by variable facial edema) and in intertriginous sites, often with generalization within 48 hours. The skin subsequently develops a wrinkled appearance owing to the formation of flaccid, sterile bullae within the superficial epidermis ( Fig. 74.6 ). The Nikolsky sign is positive. Classically, the flexural areas are the first to exfoliate, leaving behind moist skin and thin, varnish-like crusting. Patients also demonstrate characteristic periorificial (e.g. perioral, periocular) crusting and radial fissuring (see Fig. 34.17 , Fig. 81.16 ). Intraoral lesions do not occur.
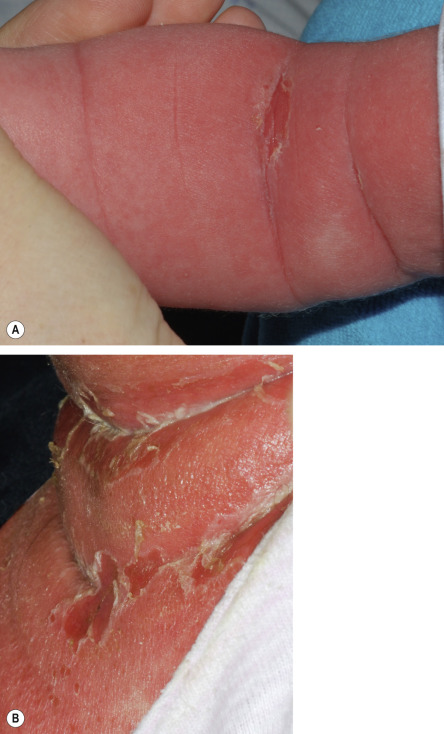
Scaling and desquamation continue for the next 3–5 days, followed by re-epithelialization without scarring. With proper treatment, SSSS resolves in 1–2 weeks, usually without sequelae. The mortality rate is ≤4% for children but may approach 60% in adults .
Pathology
Histologic examination shows a sharply demarcated zone of cleavage at or below the stratum granulosum. There are usually no inflammatory cells in the bullae. The upper dermis also lacks an inflammatory infiltrate, and no organisms are seen on Gram stain of biopsy specimens.
Diagnosis and differential diagnosis
SSSS is generally a clinical diagnosis. Although cultures taken from intact bullae are negative, S. aureus may be cultured from the conjunctiva, nasopharynx, perianal area, or pyogenic foci on the skin. Blood cultures are almost always negative in children but may be positive in adults. The leukocyte count can be elevated or normal. Examination of frozen sections is occasionally helpful in confirming the level of the split in the bullae. Slide latex agglutination, double immunodiffusion, or enzyme-linked immunosorbent assay (ELISA) tests can identify the toxins responsible for SSSS.
The differential diagnosis may include a drug reaction, a viral exanthem, sunburn, Kawasaki disease, extensive bullous impetigo, toxic shock syndrome, graft-versus-host disease (GVHD), and pemphigus foliaceus. Differentiation from toxic epidermal necrolysis (TEN) is outlined in Table 74.7 and Fig. 81.16 .
| COMPARISON OF TOXIC EPIDERMAL NECROLYSIS AND STAPHYLOCOCCAL SCALDED SKIN SYNDROME | ||
|---|---|---|
| TEN | SSSS | |
| Cause | Usually drug-induced | Infection with toxin-producing S. aureus |
| Age | Primarily adults | Primarily infants and young children |
| Histology | Dermal–epidermal separation with epidermal necrosis; dermis has a variable inflammatory infiltrate | Granular layer split in epidermis; dermis lacks inflammatory infiltrate |
| Distribution of rash | Areas of sparing often present | Generalized with flexural accentuation |
| Mucous membranes | Involved, erosions | Uninvolved |
| Nikolsky sign | In some areas, difficult to elicit | Present in seemingly uninvolved skin |
| Face | Vermilion lip erosions and crusting | Perioral and periocular crusting and radial fissuring with mild facial swelling |
| Treatment | IVIg, cyclosporine, corticosteroids (short course), and TNF inhibitors Supportive care (similar to a burn victim) | Antibiotics (β-lactamase-resistant ± clindamycin) and supportive care |
Treatment
Patients with severe, generalized forms of SSSS require hospitalization and parenteral antibiotics. Oral treatment with a β-lactamase-resistant antibiotic (e.g. dicloxacillin, cephalexin) for a minimum of 1 week is usually sufficient for milder disease, although SSSS is occasionally caused by MRSA. Clindamycin administration may help to reduce bacterial toxin production, but up to 50% of strains implicated in SSSS are clindamycin resistant, so monotherapy with this agent is not recommended . Identification and decolonization of S. aureus carriers (see Folliculitis section above) are important, especially in hospital-acquired cases .
Toxic Shock Syndrome
Introduction
Toxic shock syndrome (TSS) is an acute multisystem disease caused by a S. aureus exotoxin ( Table 74.8 ).
| CASE DEFINITIONS FOR THE TOXIC SHOCK SYNDROMES | |
|---|---|
| Toxic shock syndrome | |
| |
|
|
| |
| Streptococcal toxic shock syndrome | |
| |
or
and
and | |
| |
† Defined as a probable case, excluding any other possible etiology.
Epidemiology and pathogenesis
In the early 1980s, there was an outbreak of TSS in young women using highly absorbent tampons (“menstrual” TSS). The incidence of menstrual TSS then declined substantially following changes in tampon manufacturing and use, and by the early 2000s it accounted for approximately half of TSS cases . TSS can also occur in patients who have undergone surgical procedures. In addition, it may develop in association with cutaneous pyodermas, postpartum infections, abscesses, burns, and infections associated with nasal packing or insulin pump infusion sites. Non-menstrual TSS affects both sexes equally.
TSS is due to infection or colonization with strains of S. aureus that produce toxic shock syndrome toxin-1 (TSST-1). This toxin is thought to act as a “superantigen” that binds to major histocompatibility complex (MHC) class II molecules of antigen-presenting cells (APCs) and the Vβ region of T-cell receptors in a non-antigen-specific manner, which leads to massive release of cytokines and chemokines as well as clonal T-cell expansion (see section on Streptococcal TSS) . Recent investigations implicate invariant natural killer T cells, mucosa-associated invariant T (MAIT) cells, and interleukin-17A-producing effector memory T cells in this hyperinflammatory “cytokine storm” response to bacterial superantigens . Of note, the neonatal TSS-like exanthematous disease (see Ch. 10 ) occurs during the first week of life due to colonization with TSST-1-producing S. aureus (usually methicillin-resistant) and has a relatively mild course due to the immature, relatively anergic state of T cells in newborns.
Clinical features
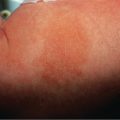
TSS is characterized by the sudden onset of high fever with myalgias, vomiting, diarrhea, headache, and pharyngitis. Rapid progression to hypotensive shock can occur, but the clinical spectrum ranges from relatively mild to fulminant fatal disease. The dermatologic manifestations are more extensive and predictable in staphylococcal TSS than in streptococcal TSS ( Table 74.9 ). Patients usually develop diffuse erythema or a scarlatiniform exanthem that starts on the trunk and spreads to the extremities ( Fig. 74.7 ). Additional findings include erythema and edema of the palms, soles, and oral mucosa as well as a strawberry tongue, hyperemia of the conjunctivae, and (with disease progression) generalized non-pitting edema. Desquamation of the hands and feet occurs 1–3 weeks after the onset of symptoms.
| THE TOXIC SHOCK SYNDROMES | ||
|---|---|---|
| Staphylococcal | Streptococcal | |
| Typical patient | Young (15–35 years) and healthy | Young (20–50 years) and healthy |
| Diffuse macular erythroderma | Very common | Less common |
| Vesicles and bullae | Rare | Uncommon (5%) |
| Localized extremity pain | Rare | Common |
| Soft tissue infection | Rare | Common |
| Hypotension | 100% | 100% |
| Renal impairment | Common | Common |
| Predisposing factors | Surgical packing, surgical meshes, abscesses, contraceptive sponge, tampon | Lacerations, bites, bruises, varicella |
| Positive blood cultures | <15% | >50% |
| Mortality | <3% | 30–60% |
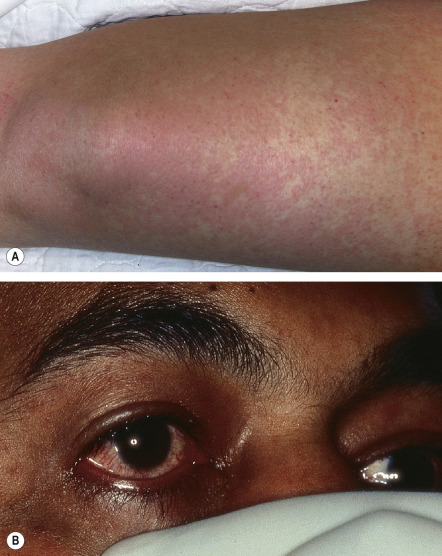
After recovery, Beau’s lines and nail shedding can be seen. In severe cases, telogen effluvium may occur. With proper treatment, the majority of patients completely recover. However, complications include decreased renal function, prolonged weakness and fatigue, protracted myalgias, vocal cord paralysis, upper extremity paresthesias, carpal tunnel syndrome, arthralgias, amenorrhea, and gangrene .
Pathology
Biopsy specimens of the eruption show an infiltrate of neutrophils and lymphocytes in the superficial dermis. Papillary dermal edema as well as epidermal spongiosis and exocytosis may be noted. Hair follicles and eccrine sweat glands display similar findings.
Diagnosis and differential diagnosis
A high index of suspicion is required in order to make a timely diagnosis. Table 74.8 outlines the case definition of TSS. The possibility of streptococcal TSS (see Table 74.9 and below) should also be considered. TSS can have clinical overlap with Kawasaki disease, scarlet fever, SSSS, early TEN, Rocky Mountain spotted fever, and leptospirosis.
Treatment
Severe cases of TSS require intensive monitoring and supportive therapy. Hypotension can be treated with intravenous fluids and vasopressor agents. Foreign bodies (e.g. surgical mesh, nasal packing, tampons) should be removed and abscesses drained. Beta-lactamase-resistant antibiotics are used to eradicate the toxin-producing staphylococci; MRSA is responsible for only a small proportion of TSS cases . Some advocate using antibiotics that suppress protein (and thereby toxin) production, such as clindamycin, and administration of intravenous immunoglobulin (IVIg) may help to neutralize the toxin . In severe cases of shock unresponsive to antibiotics, low-dose corticosteroids have been used .
Scarlet Fever
▪ Scarlatina
Introduction
Primarily a disease of children, scarlet fever was often fatal in the pre-antibiotic era. The erythematous exanthem and enanthem are due to toxins produced by group A β-hemolytic streptococci.
Epidemiology and pathogenesis
Scarlet fever is caused by streptococcal pyrogenic exotoxins (SPEs) types A, B, and C (also referred to as erythrogenic toxins), which are produced by group A streptococci and lead to immune activation. The majority of cases occur between 1 and 10 years of age; by the age of 10 years, 80% of the population has developed anti-SPE antibodies that prevent development of the eruption. Scarlet fever usually follows tonsillitis or pharyngitis and is most common during the late fall, winter, and spring in temperate climates. However, it occasionally develops as a complication of wound (“surgical scarlet fever”), post-burn, pelvic, or puerperal infections .
Clinical features
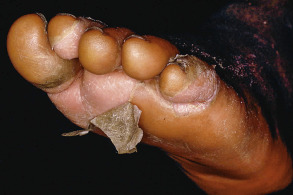
Scarlet fever is typically preceded by the sudden onset of a sore throat, headache, malaise, chills, anorexia, nausea, and high fevers. Patients, especially young children, may experience vomiting, abdominal pain, and seizures. The eruption begins 12–48 hours later as blanchable erythema on the neck, chest, and axillae. There is subsequent generalization (usually within 12 hours) and development of tiny superimposed papules with a sandpaper-like texture (“sunburn with goose pimples”). Pastia’s lines (linear petechial streaks) are seen in the axillary, antecubital, and inguinal areas. The cheeks are flushed with circumoral pallor. The throat is red and edematous, developing an exudate after 3–4 days; palatal petechiae and tender cervical adenopathy are often evident. The tongue is initially white with bright red papillae, but later becomes beefy red (“strawberry tongue”). Desquamation occurs after 7–10 days, most prominently on the hands and feet ( Fig. 74.8 ), and can last for 2–6 weeks. Possible complications of scarlet fever and the underlying streptococcal infection include otitis, mastoiditis, sinusitis, pneumonia, myocarditis, meningitis, arthritis, hepatitis, acute glomerulonephritis, and rheumatic fever .
Pathology
Biopsy specimens reveal engorged capillaries and dilated lymphatics, most prominent around hair follicles. Dermal edema, perivascular neutrophilic infiltrates and small areas of hemorrhage represent additional findings. Spongiosis and parakeratosis are seen during the desquamative stage.
Diagnosis and differential diagnosis
Clinical diagnosis is usually not difficult. There is almost always an elevated leukocyte count with a left shift. Eosinophilia of 10–20% is often seen after 2–3 weeks of convalescence. Hemolytic anemia can occur, and mild albuminuria and hematuria may be present early in the disease. Nasal and/or throat cultures grow group A streptococci. Detection of antistreptolysin O (ASO) and anti-DNase B antibodies can also be useful in confirming the streptococcal infection.
The differential diagnosis of scarlet fever may include a drug eruption, a viral exanthem, TSS, early SSSS, Kawasaki disease, and recurrent toxin-mediated perineal erythema. Infection with Arcanobacterium haemolyticum , a Gram-positive rod, can result in pharyngitis and a scarlatiniform exanthem in adolescents and young adults .
Treatment
As with other group A streptococcal infections, penicillin (or amoxicillin) is the drug of choice; a 10–14-day course is usually sufficient. A clinical response can be expected within 24–48 hours. Antibiotic treatment as long as 10 days after the onset of symptoms will prevent the development of rheumatic fever. In penicillin-allergic patients, a first-generation cephalosporin (if no history of an immediate-type reaction), clindamycin, or a macrolide can be used; of note, some strains of group A streptococci are resistant to macrolides.
Streptococcal Toxic Shock Syndrome
▪ Streptococcal toxic shock-like syndrome ▪ Toxic streptococcal syndrome
Introduction
Streptococcal TSS is a rapidly progressive, life-threatening illness caused by infection with toxin-producing group A and rarely group G streptococci .
Epidemiology and pathogenesis
Streptococcal TSS mainly affects healthy individuals between 20 and 50 years of age but can also occur in children (mean age, 4–5 years) . A disruption of the cutaneous barrier usually serves as the portal of bacterial entry. In contrast to the occult infections that predominate in staphylococcal TSS, many cases are associated with invasive soft tissue infections (e.g. necrotizing fasciitis) with virulent (e.g. M types 1 and 3) strains of group A streptococci, and the majority of patients are bacteremic . Toxins that have been implicated include SPEs A, B, and C as well as the more potent streptococcal mitogenic exotoxin Z (SMEZ) . Streptolysin O may also act synergistically with SPE-A.
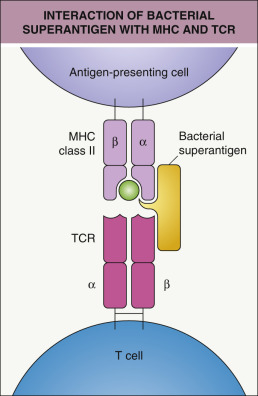
Similar to staphylococcal TSS, the clinical manifestations of streptococcal TSS result from massive cytokine release due to superantigen activity of bacterial exotoxins . Superantigens bind directly (without intracellular processing) to MHC class II molecules on APCs (outside the antigen-presenting groove) and the Vβ region of the T-cell receptor ( Fig. 74.9 ), thereby stimulating T cells in a relatively nonspecific manner. A given superantigen–T-cell interaction may lead to the activation of 5–30% of the entire circulating T-cell population, compared to ~0.01% for conventional antigens. This leads to production of huge amounts of cytokines, especially tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and IL-6; there is also upregulation of Toll-like receptors (TLRs) 2 and 4, which augments deleterious effects of endotoxin from Gram-negative bacteria (e.g. those colonizing the gut) as well as streptococci, resulting in further elaboration of proinflammatory mediators. As a consequence, patients develop clinical manifestations such as fever, erythematous eruptions, vomiting, hypotension, and tissue injury in multiple organ systems . The use of nonsteroidal anti-inflammatory drugs (NSAIDs), which lessen fever and other signs of infection, might delay the diagnosis and treatment of a streptococcal soft tissue infection, allowing TSS to occur .
Clinical features
Streptococcal TSS is defined as a group A streptococcal infection with an early onset of shock and organ failure (see Table 74.8 ). The most common initial symptom is severe local pain in an extremity. Although 50% of patients display signs of a soft tissue infection (e.g. swelling, tenderness, erythema), some present with only pain and no obvious physical findings. The development of a violaceous hue, bullae, or necrosis points to a deeper infection, such as necrotizing fasciitis or myositis, and portends a worse outcome.
The disease may begin insidiously with nonspecific flu-like symptoms, such as fever, chills, myalgias, and diarrhea. CNS symptoms (e.g. confusion, altered consciousness) are commonly seen. A generalized blanching macular erythema is observed much less often than in staphylococcal TSS, but blistering is more likely to occur. Desquamation on the hands and feet eventually appears in 20% of patients. Shock and multi-organ failure usually develop 48–72 hours after the onset of symptoms. Complications of streptococcal TSS can include renal failure, disseminated intravascular coagulation, and acute respiratory distress syndrome. The mortality rate for streptococcal TSS ranges from 30% to 60%.
Pathology
Biopsy specimens from skin lesions may show spongiosis, necrotic keratinocytes, subepidermal blister formation, and a neutrophilic and/or lymphocytic perivascular infiltrate in the dermis .
Diagnosis and differential diagnosis
Published criteria require isolation of group A streptococci from a sterile site for a “definite” diagnosis, combined with hypotension and clinical or laboratory abnormalities in two or more organ systems (see Table 74.8 ). Serum creatinine often rises early in the course, and creatine phosphokinase is elevated in the setting of necrotizing fasciitis or myonecrosis. The white blood cell count may be increased or normal with a substantial left shift . Although staphylococcal and streptococcal TSSs share some common features, major differences exist (see Table 74.9 ).
Treatment
Most cases require intensive supportive therapy. Hypotension should be treated with aggressive intravenous fluid and vasopressors. Clindamycin inhibits the production of bacterial toxins and is a component of first-line antimicrobial treatment (together with penicillin); linezolid also blocks toxin production, and administration of IVIg to neutralize toxins may be of benefit . Early surgical intervention (e.g. drainage, debridement, fasciotomy, amputation) is crucial for necrotizing soft tissue infections and can be life-saving .
Erysipelas
▪ St Anthony’s fire
Introduction
Erysipelas is a superficial variant of cellulitis caused primarily by group A streptococci that affects the dermis with prominent lymphatic involvement; in contrast, classic cellulitis is centered in the deep dermis and subcutaneous tissues .
Epidemiology and pathogenesis
Erysipelas is usually a disease of the very young, the aged, the debilitated, and those with lymphedema or chronic cutaneous ulcers. Women outnumber men, but boys are more commonly affected than girls in the pediatric age group. Erysipelas is usually caused by group A streptococci; groups G, B, C, and D streptococci are occasionally implicated. S. aureus , Pneumococcus spp., Klebsiella pneumoniae , Yersinia enterocolitica , and Haemophilus influenzae type b can also cause an erysipelas-like infection .
Clinical features
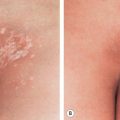
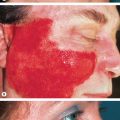
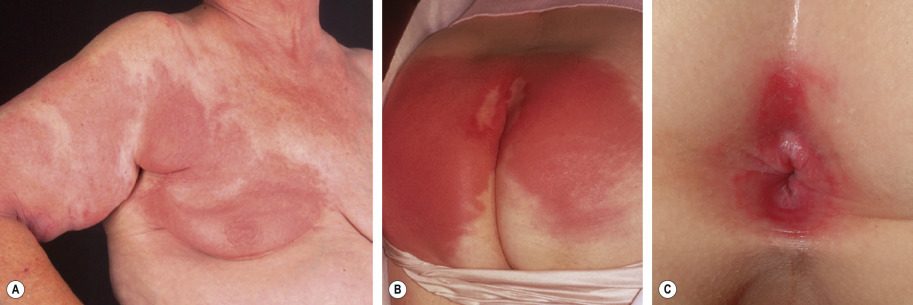
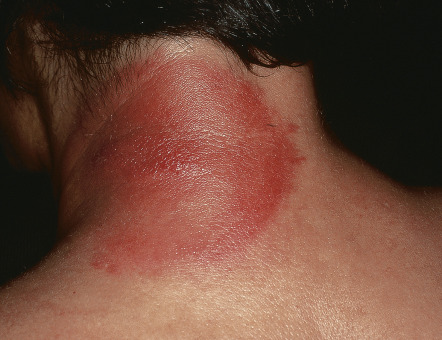
Although erysipelas classically affects the face, the lower extremity is the most common location. After an incubation period of 2 to 5 days, there is an abrupt onset of fever, chills, malaise, and nausea. A few hours to a day later, a sharply marginated erythematous plaque with a ridge-like border develops and progressively enlarges. It is clearly demarcated from the surrounding skin ( Fig. 74.10A,B ), hot, tense, and indurated with non-pitting edema. The affected area is painful to palpation and may burn. Regional lymphadenopathy is usually present, with or without lymphangitis. Pustules, vesicles, bullae, and small areas of hemorrhagic necrosis may also form. Complications of erysipelas are uncommon and usually occur in patients with underlying disease . When the infection resolves, desquamation and postinflammatory pigmentary changes may ensue.
Pathology
Biopsy specimens reveal diffuse edema and a neutrophilic infiltrate in the dermis. Dilation of the lymphatics, foci of suppurative necrosis, and dermal–epidermal separation are often seen. There is no primary necrotizing vasculitis, thrombosis, or leukocytoclasis.
Diagnosis and differential diagnosis
Diagnosis is based primarily on clinical findings. Laboratory evaluation shows an elevated leukocyte count with a left shift. Blood cultures are positive in only ~5% of cases. Although cultures from pustules or bullae may be helpful, the sensitivity of cultures of skin biopsy specimens is low, especially in immunocompetent hosts. Anti-DNase B and ASO titers are useful indicators of the streptococcal etiology. Direct immunofluorescence and latex agglutination tests can be used to detect streptococci within skin specimens.
The differential diagnosis of erysipelas includes other forms of cellulitis and soft tissue infections (e.g. erysipeloid, necrotizing fasciitis) as well as inflammatory causes of “pseudocellulitis” ( Table 74.10 ).
| CAUSES OF “PSEUDOCELLULITIS” | |
|---|---|
Infections and bites
Neutrophilic dermatoses
Drug reactions
| Other inflammatory disorders
Metabolic disorders
Malignancy
|
* Occasionally develops prior to onset of leukemia or due to infection.
Treatment
A 10- to 14-day course of penicillin is the treatment of choice for erysipelas caused by streptococci. Although macrolides (e.g. erythromycin) may be used in penicillin-allergic patients, some strains of Str. pyogenes are macrolide-resistant. Erysipelas may recur in patients with abnormal local circulation (e.g. lymphedema), and penicillin prophylaxis is occasionally required . The development of an effective vaccine against Streptococcus spp. could dramatically change the epidemiology of the infections caused by this microorganism .
Streptococcal Intertrigo
Intertrigo caused by group A streptococci is an under-recognized entity that usually affects infants and young children . Infants are particularly vulnerable due to irritation and friction in moist, deep skin folds of the neck, axillae, antecubital and popliteal fossae, and inguinal region. Sharply demarcated, intensely erythematous patches or thin plaques are observed in an intertriginous site, often accompanied by a foul odor. In contrast to intertriginous candidiasis, satellite lesions are uncommon. Affected children occasionally exhibit irritability, low-grade fevers, and Str. pyogenes bacteremia. In genetically predisposed children, cutaneous streptococcal infection may trigger psoriasis . Bacterial culture can confirm the diagnosis, which should be considered when simple intertrigo fails to respond to barrier creams and other measures to reduce friction and minimize moisture. A 10-day course of oral penicillin or amoxicillin is usually effective .
Perianal and Vulvovaginal (Perineal) Streptococcal Infection
▪ Perianal – perianal streptococcal disease, perianal cellulitis, perianal streptococcal dermatitis ▪ Vulvovaginal – perivaginal streptococcal disease, streptococcal vulvovaginitis
Other causes of perineal erythema or pruritus include contact dermatitis (irritant or allergic), S. aureus infection , candidiasis, seborrheic dermatitis, pinworm infestation, inflammatory bowel disease, lichen sclerosus, child abuse, and the early phase of Kawasaki disease. Perineal streptococcal infection can be diagnosed with skin culture or group A streptococcal rapid testing, although the latter is less specific. In a randomized controlled study, a 7-day course of cefuroxime was found to be more effective than a 10-day course of penicillin in the treatment of perianal streptococcal disease .
Cellulitis
Introduction
Cellulitis is an infection of the deep dermis and subcutaneous tissue that manifests as areas of erythema, swelling, warmth, and tenderness.
Epidemiology and pathogenesis
Cellulitis in immunocompetent adults is most often caused by group A streptococci or S. aureus , and the latter organism is the most frequent etiology in children. Although H. influenzae was once a common cause of cellulitis in pediatric patients, it is now rare due to routine vaccination against H. influenzae type b . A mixture of Gram-positive cocci and Gram-negative aerobes and anaerobes is often implicated in cellulitis surrounding diabetic and decubitus ulcers . Bacteria typically gain access to the dermis via a break in the skin barrier in immunocompetent individuals, but a bloodborne route is common in immunocompromised patients. Lymphedema, alcoholism, diabetes mellitus, injection drug use, and peripheral vascular disease are all risk factors for cellulitis. Recurrent bouts of cellulitis may result from damage to the lymphatic system, e.g. via prior lymph node dissection, saphenous vein harvest, or cellulitis.
Clinical features
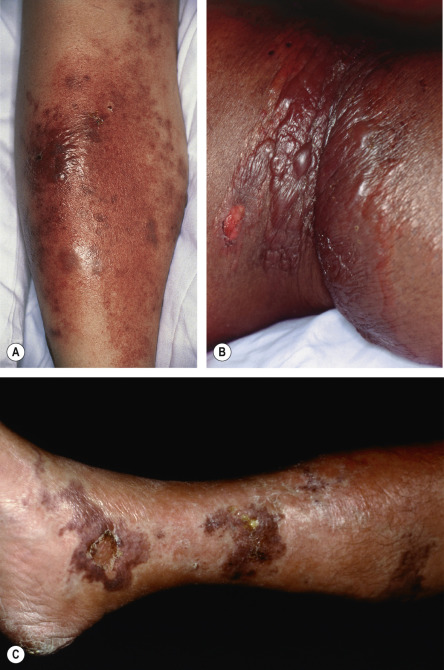
Cellulitis is often preceded by systemic symptoms such as fever, chills, and malaise. The affected area displays all four of the cardinal signs of inflammation: rubor (erythema), calor (warmth), dolor (pain), and tumor (swelling). The borders are usually ill-defined and non-palpable. In severe infections, vesicles, bullae, pustules or necrotic tissue may be present ( Fig. 74.11 ). Ascending lymphangitis and regional lymph node involvement may occur. In children, cellulitis most often affects the head and neck, whereas in adults it tends to involve the extremities. Cellulitis due to injection drug use typically affects the upper extremities, the usual sites of drug injection. Complications are uncommon but may include acute glomerulonephritis (if caused by a nephritogenic strain of streptococcus), lymphadenitis, subacute bacterial endocarditis, and recurrences related to disrupted lymphatic damage.
Pathology
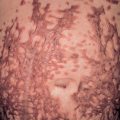
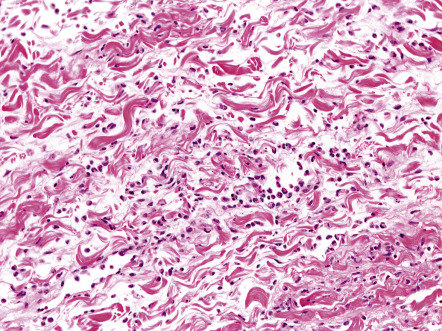
A mild or moderate inflammatory infiltrate composed of lymphocytes and neutrophils can be seen throughout the dermis, often extending into the subcutaneous fat ( Fig. 74.12 ). Additional findings include edema, which occasionally leads to subepidermal bullae, and dilation of lymphatics and small blood vessels. With special stains, the causative organism may be identified.
Diagnosis and differential diagnosis
The diagnosis of cellulitis is usually clinical ( Fig. 74.13 ). The leukocyte count is often normal or only slightly elevated. Blood cultures are almost always negative in immunocompetent hosts. An exception is H. influenzae cellulitis, where there is usually an increased leukocyte count with a left shift and positive blood cultures. Atypical organisms are more common in children and immunocompromised patients, and needle aspiration or skin biopsy may help to identify the infectious etiology. The differential diagnosis of lower extremity cellulitis includes deep vein thrombosis and inflammatory diseases such as stasis dermatitis, superficial thrombophlebitis, lipodermatosclerosis, and other forms of panniculitis. While superficial thrombophlebitis often presents with redness and tenderness, the absence of a fever and the presence of a palpable cord aid in the diagnosis. Misdiagnosis of lipodermatosclerosis as cellulitis often leads to unnecessary hospitalizations. Additional causes of “pseudocellulitis” are listed in Table 74.10 and illustrated in Figure 74.14 .
Treatment
Treatment of cellulitis is typically targeted against group A streptococci and S. aureus . For uncomplicated cases, a 10-day course of an oral antibiotic that covers these organisms (e.g. dicloxacillin, cephalexin, or clindamycin) is appropriate. Hospitalization and parenteral antibiotics may be necessary for patients who are seriously ill, have facial involvement, or fail to respond to oral therapy. If MRSA is suspected, e.g. when there is cellulitis in association with an abscess, agents such as clindamycin, TMP-SMX or doxycycline should be used (see Table 74.5 ); if group A streptococcal infection is also a possibility, the latter agents should be combined with a β-lactam. Diabetic or decubitus ulcers complicated by cellulitis require broad-spectrum coverage, such as piperacillin/tazobactam or, in penicillin-allergic patients, metronidazole plus ciprofloxacin. Adjunctive measures include immobilization and elevation of an affected extremity and the application of wet dressings to areas with bullae or exudate. If signs and symptoms do not improve after 36–48 hours of treatment, cultures and susceptibilities should be obtained and antibiotics adjusted accordingly. NSAIDs may mask the signs and symptoms of deeper necrotizing infections and should be avoided when treating cellulitis. Prophylactic low-dose penicillin has been shown to decrease the risk of recurrent lower extremity cellulitis; however the protective effects diminish upon discontinuation of therapy . The clinical features and treatment of other types of cellulitis (e.g. Erysipelothrix rhusiopathiae , Vibrio vulnificus ) are discussed below.
Pyomyositis
Pyomyositis is a primary bacterial infection of the skeletal muscles that is most commonly caused by S. aureus . Other reported etiologies include: Str. pyogenes , Str. pneumoniae, Escherichia coli , Yersinia enterocolitica , and H. influenzae as well as mycobacteria and fungi; polymicrobial infections can also occur in immunocompromised patients . Once referred to as tropical myositis, it also occurs in temperate climates. Predisposing factors include trauma, diabetes mellitus, HIV infection, injection drug use, and other forms of immunosuppression. Patients often present with a 1–2-week history of low-grade fevers, myalgias, and progressive firmness, pain, and enlargement of a deep soft tissue mass. Palpation of the affected area reveals “woody” induration. Muscle abscess formation occurs during the second stage of disease, and septicemia may follow.
MRI is the diagnostic modality of choice for the early disease; ultrasound-guided aspiration may be helpful later in the course. Treatment of staphylococcal pyomyositis includes incision and drainage as well as appropriate intravenous antibiotics until clinical improvement is noted, followed by oral therapy for a total of ≥3 weeks .
Botryomycosis