Abstract
Local anesthetics have one of two chemical structures – esters or amides. Allergic reactions are far more common with ester anesthetics. In dermatology, lidocaine, an amide, is by far the most commonly employed local anesthetic. When used within recommended dosage ranges, local anesthetics are extremely safe. Vasovagal reactions are the most common side effect of injections of local anesthesia. Epinephrine is added to the local anesthetic because as a vasoconstrictor, it lengthens the duration of anesthesia and reduces intraoperative bleeding. Mastering the art of painless anesthesia is important for patient comfort.
Nerve blocks are very effective for anesthesia of the central face, digits, and palmoplantar surfaces. Ring block anesthesia is especially useful on the scalp. Tumescent anesthesia achieves an almost bloodless field and can be used to safely anesthetize large areas, especially for liposuction. For anxious patients or for long procedures, oral anxiolytics and narcotics can improve patient tolerance for the procedure.
Keywords
amide anesthetic, anesthesia, benzocaine, epinephrine, ester anesthetic, hyaluronidase, lidocaine, local anesthesia, nerve block, tumescent anesthesia, ring block
- ▪
Local anesthetics are classified into two groups – amides and esters, depending upon the linkage in their intermediate chain
- ▪
“Anesthetic allergies” vary from palpitations secondary to epinephrine and vasovagal reactions, to urticaria and anaphylaxis from preservatives or the local anesthetic (especially esters)
- ▪
Additives to the local anesthetic include epinephrine, sodium bicarbonate and hyaluronidase
- ▪
Nerve blocks are particularly helpful when performing procedures on the face, digits and palmoplantar surfaces
Introduction
Effective anesthesia is an essential component of dermatologic surgery, and almost all dermatologic surgical procedures can be performed under local anesthesia. Local anesthetics have been in use since the 1880s with the introduction of cocaine hydrochloride, which was extracted from the leaves of the South American bush Erythroxylon coca . This was followed by procaine in 1904, tetracaine in 1930, and the first amide local anesthetic, lidocaine, was introduced in 1943 . The introduction of lidocaine was a major breakthrough because lidocaine is far less likely to cause an allergic reaction than the ester-type anesthetics that preceded it.
Local anesthesia has several advantages over general anesthesia, including reduced morbidity (especially in poor-risk patients), reduced cost, reduced procedure time, and faster recovery. The main disadvantages are some limitation on the extent of a procedure and the possibility of greater patient discomfort from the injections. Knowledge of the physiology, dosage, side effects, and proper “painless” local anesthesia technique are essential for performing dermatologic surgery and to keep patients safe and satisfied.
Discussion
Physiology and Structure
Local anesthetics act by blocking sodium channels in the axon cell membrane, and this prevents sodium from entering the nerve cell. The nerve cell is not depolarized and consequently the action potential is blocked. The cationic form of the anesthetic appears to bind to the inner pore of voltage-gated sodium channels, possibly leading to both narrowing of the pore lumen (steric block) and creation of an electrostatic barrier to permeation . Smaller unmyelinated C-type nerve fibers that conduct pain sensation are blocked more quickly and easily than intermediate fibers that also carry sensations of heat and cold. The myelinated A-type fibers that carry pressure sensation and motor fibers are blocked last. Clinically, this is evident when an area seems fully anesthetized for scalpel surgery, but the patient still feels the pressure of the surgeon’s fingers at the surgical site.
All local anesthetics consist of three parts ( Fig. 143.1 ):
- •
a secondary or tertiary amine end
- •
an aromatic end
- •
an intermediate connecting chain that contains an ester or amide.
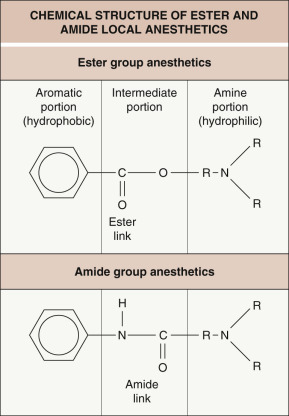
The aromatic portion is hydrophobic and lipophilic. This is essential to allow the anesthetic to diffuse through nerve cell membranes. The amine portion is hydrophilic and is responsible for the anesthetic’s water solubility, which is important for preparing, storing, and administering the anesthetic .
Local anesthetics are weak organic bases, which, to be water-soluble and injectable, require the addition of a hydrochloride salt. In aqueous solution, the salt equilibrates between the ionized and non-ionized form. The ionized form is water-soluble, allowing injection into and diffusion through tissue. However, it is the non-ionized, lipid-soluble base that can diffuse into the nerve cell membrane. Within the sodium channel’s inner pore, it is the ionized cation that is responsible for blocking nerve conduction (see above) .
The dissociation constant (pKa) of each anesthetic determines the proportion of the anesthetic base and its cation at a given pH. The pKa of all local anesthetics is higher than physiologic pH. For most local anesthetics at a pH of 7.4, 80% or more is in the cationic ionized form. Alkalinization of the anesthetic solution, as is done with the addition of sodium bicarbonate, will speed its onset of action as more of the anesthetic will be in the non-ionized form. However, if the pH is raised too much, the anesthetic may precipitate out of solution. Anesthetic sensitivity to pH also helps explain why infected tissue is difficult to effectively anesthetize . The inflammatory response surrounding the infection acidifies the site (reduces the pH), which reduces the proportion of the anesthetic in the non-ionized, lipid-soluble form.
Pharmacology
Local anesthetics are classified into two groups, depending on the linkage in the intermediate chain ( Table 143.1 ). Amide anesthetics have an amide linkage and ester anesthetics have an ester linkage (see Fig. 143.1 ). They differ in how they are metabolized and in the risk of sensitization. Ester anesthetics are hydrolyzed by plasma pseudocholinesterase, and the metabolites are excreted by the kidneys. Patients with a deficiency of functional pseudocholinesterase, who are often diagnosed after prolonged paralysis following administration of standard doses of succinylcholine, are at an increased risk of ester anesthetic toxicity. The metabolite para-aminobenzoic acid (PABA) is responsible for the allergic reactions seen with ester anesthetics. Amide anesthetics are metabolized by the hepatic microsomal cytochrome P450 enzyme system, and the metabolites are excreted by the kidneys. Patients with severe liver disease may be at increased risk of amide anesthetic toxicity.
| LOCAL ANESTHETICS FOR INFILTRATIVE AND NERVE BLOCK ANESTHESIA | |||||
|---|---|---|---|---|---|
| Generic name | Trade name ® | Onset (min) | Duration plain (h) | Maximum dose plain (mg for 70 kg person) | Maximum dose with epinephrine (mg for 70 kg person) |
| Amides | |||||
| Articaine | Septocaine | 2–4 | 0.5–2 | 350 | 500 |
| Bupivacaine hydrochloride | Marcaine | 5–8 | 2–4 | 175 | 225 |
| Etidocaine | Duranest | 3–5 | 3–5 | 300 | 400 |
| Levobupivacaine hydrochloride | Chirocaine | 2–10 | 2–4 | 150 | Not available |
| Lidocaine | Xylocaine | Rapid | 0.5–2 | 350 | 500 (3500 dilute) |
| Mepivacaine | Carbocaine | 3–20 | 0.5–2 | 300 | 500 |
| Prilocaine hydrochloride | Citanest | 5–6 | 0.5–2 | 400 | 600 |
| Ropivacaine * | Naropin | 1–15 | 2–6 | 200 | Not available |
| Esters | |||||
| Chloroprocaine hydrochloride | Nesacaine | 5–6 | 0.5–2 | 800 | 1000 |
| Procaine | Novocaine | 5 | 1–1.5 | 500 | 600 |
| Tetracaine | Pontocaine | 7 | 2–3 | 100 | Not available |
* Addition of epinephrine has no effect on onset or duration of action of ropivacaine.
Local anesthetics differ in the speed of onset, duration of action and potency, depending on each compound’s intrinsic chemical characteristics ( Table 143.1 ). A low pKa leads to rapid onset of anesthesia, as more of the anesthetic will be in the non-ionized form. Greater lipid solubility is associated with higher anesthetic potency, as the compound penetrates the nerve cell membrane more easily. Duration of action is determined by the strength of anesthetic binding to the sodium channel pore.
For pregnant women, the local anesthetic of choice is lidocaine. It is classified as pregnancy category B, which means that, in animal studies, no teratogenic effects have been documented. Studies in pregnant women who received lidocaine during the first trimester of pregnancy have shown no increase in anatomic abnormalities in the newborns. However, it is recommended that lidocaine, as with all other pharmacologic agents, be used cautiously during the first 4 months of pregnancy, when maximum organogenesis takes place. Lidocaine crosses the placenta into the fetus. Lidocaine can be safely used in nursing mothers with the realization that some of the anesthetic may be excreted in the mother’s milk.
Lidocaine can be safely used in children, but the maximum recommended dosage should be adjusted downward based on the child’s weight and age. Care must be taken in premature infants due to cardiovascular effects. Parabens, used as preservatives, are bound to albumin. In a jaundiced newborn, they could displace bilirubin from the albumin, worsening the hyperbilirubinemia . For this reason, only paraben-free anesthetics should be used in newborns.
Additions to Local Anesthetics
Epinephrine
All local anesthetics, except for cocaine hydrochloride, relax vascular smooth muscle, which results in vasodilation. This causes increased bleeding at the operative site and reduced duration of anesthetic action as the anesthetic is rapidly removed from the surgical site via the dilated blood vessels. The addition of epinephrine (adrenaline) has the beneficial effect of constricting blood vessels, which prolongs the duration of anesthesia 100% to 200% by slowing removal of the anesthetic from the surgical site. Also, there is reduced intraoperative bleeding due to the vasoconstriction. The addition of epinephrine provides more effective anesthesia by decreasing the volume of anesthetic needed. The reduced absorption rate decreases anesthetic toxicity and allows larger doses to be used safely. The vasoconstrictive effect of epinephrine, manifested by skin blanching, takes about 15 minutes to fully develop. While they usually coincide, blanching does not always denote the anesthetized area.
Epinephrine is premixed with local anesthetics at a concentration of 1 : 100 000 or 1 : 200 000. However, concentrations as low as 1 : 1 000 000 achieve effective vasoconstriction, while concentrations >1 : 100 000 are associated with a greater risk of side effects. The concentration used in a given patient should be individualized. Patients who have relative contraindications to epinephrine should receive lower concentrations, while highly vascular areas such as the scalp should receive higher concentrations. The maximum dosage used at a concentration of 1 : 100 000 is determined by the anesthetic with which the epinephrine is premixed ( Table 143.1 ).
Epinephrine is a strong β- and α-agonist and, as such, it must be used cautiously in patients with altered β- and α-receptors. Absolute contraindications to the use of epinephrine include hyperthyroidism and pheochromocytoma. Patients taking β-blockers, monoamine oxidase inhibitors, tricyclic antidepressants, and phenothiazines are more sensitive to epinephrine. Therefore, epinephrine should be used with caution, with the dose and concentration reduced accordingly. In patients taking β-blockers, severe hypertension developing after injection of epinephrine-containing anesthetics has been reported . This is probably due to unopposed α-adrenergic activity with its associated vasoconstriction. Fortunately, this reaction seems to be quite rare , and mainly occurs when higher doses are used. Patients with severe hypertension or with severe cardiovascular disease (especially coronary artery disease) may have their underlying disease exacerbated if large amounts of epinephrine are administered with the local anesthetic. High doses of epinephrine can induce labor. However, the low doses of epinephrine used in cutaneous surgery can be safely used during pregnancy. Epinephrine use in the periorbital area in patients with narrow angle glaucoma should be avoided, as it may aggravate the patient’s glaucoma.
The use of epinephrine on digits has been controversial. Historically, epinephrine was not used on digits for fear of causing vasoconstriction that might result in digital necrosis. More recent studies have not demonstrated any increased risk from epinephrine in digital anesthesia. It appears that most cases of digital necrosis occurred due to vessel compression from too much anesthetic volume being injected (tamponade), constricting circumferential dressings, tourniquets, postoperative hot soaks (possibly due to heat-induced edema), infection, use of vasoconstrictive anesthetics such as cocaine hydrochloride, or non-standard mixing of lidocaine with epinephrine . There is no evidence of digital necrosis due solely to commercially available lidocaine with epinephrine . However, epinephrine is clearly contraindicated for digital anesthesia in patients with peripheral artery disease. I have found that combining lidocaine with dilute epinephrine (1 : 500 000) and small volumes provides safe digital anesthesia. Ring blocks of the digit should be avoided.
Self-limited systemic side effects of epinephrine include palpitations, anxiety, fear, diaphoresis, headache, tremor, weakness, tachycardia, and elevated blood pressure. These signs and symptoms can be seen on occasion even with normal doses used for skin surgery, but they usually resolve within a few minutes ( Table 143.2 ). They are more commonly seen when injecting highly vascular areas, especially the face and scalp. Skin necrosis from vasoconstriction is an extremely rare complication of epinephrine injection and would be an issue only in patients with severe vascular compromise at the injection site.
| DIFFERENTIAL DIAGNOSIS OF LOCAL ANESTHETIC SYSTEMIC REACTIONS | ||||
|---|---|---|---|---|
| Diagnosis | Pulse rate | Blood pressure | Signs and symptoms | Emergency management |
| Vasovagal reaction | Low | Low | Excess parasympathetic tone; diaphoresis, hyperventilation, nausea | Trendelenburg, cold compress, reassurance |
| Epinephrine reaction | High | High | Excess α- and β-adrenergic receptor stimulation; palpitations | Reassurance (usually resolves within minutes), phentolamine, propranolol |
| Anaphylactic reaction | High | Low | Peripheral vasodilation with reactive tachycardia; stridor, bronchospasm, urticaria, angioedema | Epinephrine 1 : 1000 0.3 ml subcutaneous, antihistamines, corticosteroids, fluids, oxygen, airway maintenance |
| Lidocaine overdose | ||||
| 1–6 mcg/ml | Normal | Normal | Circumoral and digital paresthesias, restlessness, drowsiness, euphoria, lightheadedness | Observation |
| 6–9 mcg/ml | Normal | Normal | Nausea, vomiting, muscle twitching, tremors, blurred vision, tinnitus, dysgeusia (e.g. metallic taste), confusion, excitement, psychosis | Diazepam, airway maintenance |
| 9–12 mcg/ml | Low | Low | Seizures, cardiopulmonary depression, arrhythmia | Respiratory support |
| >12 mcg/ml | None | None | Coma, cardiopulmonary arrest | Cardiopulmonary resuscitation and life support |
Serious side effects of epinephrine injection include cardiac arrhythmias (e.g. ventricular tachycardia, ventricular fibrillation), cardiac arrest, and cerebral hemorrhage. None of these should be expected to occur at the doses used for skin surgery. However, it is prudent to limit the total dose injected in patients with severe cardiac disease.
Epinephrine is stable only in an acidic environment. Therefore, when epinephrine is premixed with local anesthetics, the pH is lowered into the 3.5 to 5.5 range with the addition of acidic preservatives such as sodium metabisulfite to stabilize the epinephrine. This acidic solution not only is more painful at the time of injection , but also slows the onset of anesthetic action, as less anesthetic is in the non-ionized form. By preparing the mixture fresh daily and using it by the end of the day, the mixture has a higher pH, which is less painful. Adding 0.5 ml epinephrine (1 : 1000) to 50 ml plain lidocaine will give a final concentration of 1 : 100 000 epinephrine. Alternatively, the lidocaine with epinephrine can be neutralized with sodium bicarbonate (see below).
Sodium bicarbonate
Injecting the standard mixture of lidocaine with epinephrine at a pH of 3.5 to 5.5 is quite painful, with significant stinging due to the acidic pH. The pH can be neutralized with the addition of sodium bicarbonate. Adding one part of 8.4% sodium bicarbonate to ten parts lidocaine with epinephrine will bring the pH into a more physiologic 7 to 8 range. This mixture significantly reduces the pain of anesthetic injection . Both components can be drawn up into the syringe, immediately before injection. Alternatively, 5 ml sodium bicarbonate (8.4%) can be added to a 50 ml bottle of lidocaine with epinephrine. As the epinephrine activity is lost at a rate of 25% per week in an alkaline or neutral environment, the mixture should be labeled with the date prepared, kept refrigerated, and used within about 1 week.
Hyaluronidase
Hyaluronidase (derived from bovine testicular hyaluronidase) depolymerizes hyaluronic acid, which breaks up ground substance, allowing anesthetics to diffuse further away from the injection point. In addition, there is less distortion of the injected structures . The greater diffusion of anesthetic may decrease its duration of action. Hyaluronidase is most useful for periorbital surgery and to increase the rate of successful nerve blocks. It is prepared by adding 150 units (U) to 30 ml of local anesthetic. Allergic reactions to hyaluronidase are rare, but they can occur. Some surgeons recommend an intradermal skin test before using hyaluronidase. In addition, the preparation may contain thimerosal preservative, which can cause contact dermatitis.
Anesthetic Mixtures
In an attempt to take advantage of different anesthetic properties, some surgeons combine two local anesthetics in one syringe. For example, a combination of lidocaine, for its rapid onset of action, with bupivacaine hydrochloride, for its longer duration of action, is often used. However, a study looking at such combinations has found that these mixtures do not live up to their promise. The mixture seems to take on the properties of one of the components to the exclusion of the other . Therefore, most surgeons will inject the rapid-onset anesthetic first and the longer-acting anesthetic later, to minimize the pain of injection and maximize the duration of action.
Side Effects
Vasovagal reactions
By far the most common side effect of local anesthetic injection is a vasovagal reaction, in which the vagus nerve discharges due to patient anxiety, resulting in an increase in parasympathetic tone ( Table 143.2 ). Vasovagal reactions are manifested by dizziness, diaphoresis, syncope, bradycardia, and hypotension. Placing the patient in the Trendelenburg position will rapidly relieve the patient’s symptoms. A cold towel on the forehead can also be helpful. Oxygen, fluids or epinephrine are usually not necessary. To avoid significant vasovagal reactions, all patients should have local anesthetic infiltrated in the recumbent position.
Allergic reactions
The most important side effect of local anesthetics is the development of allergic reactions. The allergy is usually a type I IgE-mediated reaction manifested by urticaria, angioedema, bronchospasm, and, on rare occasions, anaphylaxis with associated hypotension and tachycardia ( Table 143.2 ). Most true local anesthetic allergies have been reported with ester anesthetics; amide anesthetics are implicated only very rarely. Preservatives added to multidose vials, especially methylparaben and sodium metabisulfite, have frequently been shown to be the cause of “local anesthetic” allergy .
There is some allergy cross-reactivity amongst the various ester anesthetics and amongst the amide anesthetics, but there is no cross-reactivity between the ester and amide anesthetic classes. It is PABA (the metabolite of ester anesthetics) that is thought to be responsible for the ester anesthetic allergic reactions. Ester anesthetics can also cause type IV delayed hypersensitivity reactions and cross-react with several contact sensitizers, including PABA, para-amino salicylic acid, and para-phenylenediamine (PPD).
Patients who present with a history of “local anesthetic” allergy need to be questioned in detail about the “allergy” with a review of relevant medical records whenever possible. Often, the “allergy” is a result of a vasovagal reaction or epinephrine sensitivity. If the reaction seems to represent a true allergic reaction and the offending anesthetic is known (usually an ester), using an anesthetic from the other class (usually an amide) in a preservative-free solution is a reasonable option. Skin testing, by an allergist, may be warranted if the offending agent is not certain . The testing should include pinprick followed by intradermal tests of an ester anesthetic, an amide anesthetic, methylparaben, and sodium metabisulfite. Alternatively, for small procedures, adequate anesthesia can be obtained with intradermal injection of 1% diphenhydramine solution. Epinephrine may be added to counteract vasodilation caused by diphenhydramine, to enhance the anesthetic effect, and to reduce systemic antihistamine symptoms . Intradermal normal saline with benzyl alcohol preservative will achieve very brief anesthesia through pressure effects on cutaneous nerve endings and the anesthetic properties of the benzyl alcohol preservative. It can be used for very short procedures such as a shave biopsy .
Limited allergic reactions can be managed with oral antihistamines and prednisone. However, patients developing bronchospasm, angioedema, or hemodynamic compromise require immediate emergency management including subcutaneous epinephrine, bronchodilators, parenteral antihistamines, corticosteroids, intravenous fluids, and oxygen ( Table 143.2 ).
Local side effects
Bruising and edema are frequently seen after local anesthetic infiltration, especially in the periorbital area. Periorbital edema will often develop after surgery on the forehead and frontal scalp. Transient motor nerve paralysis is sometimes seen. This may be delayed for some time after the sensory nerves have become anesthetized, because of the large myelinated nerve fibers involved. The paralysis may persist for several hours after the sensory nerves have returned to normal. Informing the patient when a motor nerve has been affected by the anesthetic will eliminate distressed patient telephone calls later in the day. Prolonged sensory nerve paresthesia may develop if a sensory nerve is injured due to intraneural injection. This is most commonly seen after nerve blocks. It can be minimized by avoiding intraneural injections and by using small-gauge needles for injection.
Overdosage
If the local anesthetic dosage administered is kept within the recommended ranges, clinical symptoms of local anesthetic overdose are unlikely to be encountered. Maximum recommended dosages of lidocaine are 5 mg/kg plain (i.e. with no epinephrine), 7 mg/kg if with epinephrine and at standard (1–2%) concentrations, and 35–50 mg/kg for tumescent lidocaine anesthesia with dilute concentrations of lidocaine (0.05–0.1%) as well as epinephrine concentrations ( Table 143.1 ) .
The symptoms of local anesthetic overdose are directly related to the serum blood level, with worsening CNS and cardiovascular signs and symptoms as the level increases ( Table 143.2 ). CNS symptoms start with circumoral and digital numbness and tingling, followed by lightheadedness, tinnitus, visual disturbances, slurred speech, muscle twitching, and, finally, seizures and coma. Cardiovascular and pulmonary symptoms develop at significantly higher doses than do early CNS symptoms and include hypotension, arrhythmias, respiratory depression, and cardiac arrest.
When lidocaine is administered, there are over 150 potential drug–drug interactions. Most are minor or only relevant when large doses of lidocaine are used. Patients receiving medications that inhibit the liver’s cytochrome P450 system (e.g. erythromycin, ketoconazole, itraconazole; see Ch. 131 ), and therefore inhibit lidocaine metabolism, can exhibit signs of lidocaine overdose at lower doses. With large doses of lidocaine, patients taking class I and III antiarrhythmic drugs (e.g. amiodarone) are at increased risk of cardiac arrhythmias and those taking CNS depressants (e.g. opiates) are at increased risk of CNS toxicity.
Bupivacaine hydrochloride has a greater risk of cardiac toxicity than lidocaine. Prilocaine hydrochloride metabolizes to ortho-toluidine, which is an oxidizing agent capable of converting hemoglobin to methemoglobin. This can become significant with large doses of more than 500 mg. Benzocaine has also been implicated in causing methemoglobinemia when used on mucosal surfaces . The treatment of methemoglobinemia is methylene blue infusion.
Topical Anesthetics
Skin
Historically, the only anesthetic used for topical anesthesia of keratinized skin was benzocaine, which is an ester anesthetic. Anesthesia was generally effective only when applied to traumatized skin. Little, if any, anesthesia was achieved in intact normal skin. Of greater concern was the high rate of allergic contact dermatitis seen with benzocaine.
There are now several topical anesthetics that achieve moderate superficial anesthesia of intact skin ( Table 143.3 ). EMLA ® is available in a cream formulation or as a disc. It is a eutectic mixture of 2.5% lidocaine and 2.5% prilocaine hydrochloride. EMLA ® is able to achieve superficial anesthesia, with the degree of anesthesia related to the amount and duration of application before surgery. The cream is applied as a thick layer under occlusion at least 1 hour before the procedure while the disc itself provides occlusion. It is especially useful for reducing the pain of non-ablative laser procedures and to reduce the pain of local anesthetic or other injections. Some dermatologists who perform CO 2 laser ablative resurfacing solely under EMLA ® anesthesia prepare the skin with vigorous degreasing followed by two applications of EMLA ® 1 hour apart under occlusion. Additional EMLA ® is applied to the skin after the first pass with the laser has removed the epidermis.