Acellular dermal matrices (ADMs) have been used for postmastectomy breast reconstruction, primary and secondary breast augmentation, and reduction mammaplasty. In postmastectomy breast reconstruction, ADMs can be used to either create an implant pocket in single-stage reconstruction or to create the inferolateral portion of the tissue expander pocket in two-stage reconstruction. Specific deformities after cosmetic breast augmentation such as contour irregularities and implant malposition can be addressed with ADMs. The use of ADMs is a safe alternative for the correction of breast deformities after reconstructive and aesthetic breast surgery.
- •
Acellular dermal matrices (ADMs) are useful in primary prosthetic breast reconstruction as well as in the treatment of secondary deformities.
- •
A periareolar incision gives excellent access to the breast in secondary revision.
- •
When implanting ADMs, it is important to use a single, thick layer of the product.
- •
Patient selection is an important factor; in the postmastectomy setting, ADM-assisted reconstruction is appropriate in patients who have an adequate skin envelope.
- •
ADMs may alleviate the occurrence of complications by reducing the inflammatory changes that cause capsular contracture and capsule formation.
- •
One drawback to the use of ADMs is their cost.
| Reconstructive Breast Surgery | Aesthetic Breast Surgery | Reduction Mammaplasty | |
|---|---|---|---|
| Surface Irregularities | Implant Malposition | ||
|
|
|
|
| Breast Reconstruction | Aesthetic Breast Surgery | Reduction Mammaplasty | |
|---|---|---|---|
| Surface Irregularities | Implant Malposition | ||
|
|
|
|
Overview of ADM s in breast surgery
ADMs became available in 1994 and the most commonly used ADMs in breast surgery are AlloDerm ® (LifeCell, Branchburg, NJ, USA), Strattice™ (LifeCell Corporation, Branchburg, NJ, USA), DermaMatrix ® (MTF/Synthes CMF, West Chester, PA, USA) and FlexHD ® (Ethicon, New Brunswick, NJ, USA).
AlloDerm ® regenerative tissue matrix is produced by removing the epidermis and cells from human cadaveric skin. Strattice™ reconstructive tissue matrix (LifeCell Corp., Branchburg, NJ, USA) is derived from porcine dermis denuded of cells and sterilized using electron beam irradiation. DermaMatrix ® (MTF/Synthes CMF, West Chester, PA, USA) is human skin in which both the epidermis and dermis are removed from the subcutaneous layer of tissue in a process using sodium chloride solution, rendering it sterile and preserving the original dermal collagen matrix. FlexHD ® acellular hydrated dermis is derived from donated human allograft skin.
ADM Biomechanical Differences
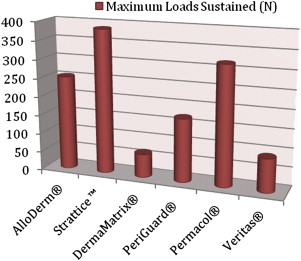
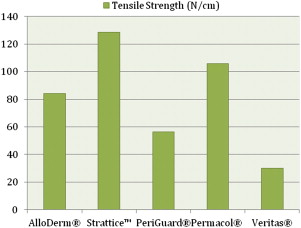
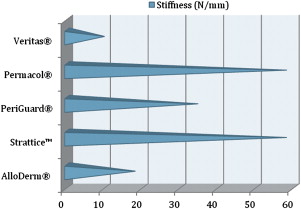
A few biomechanical differences that are of clinical relevance exist between the various ADMs. AlloDerm ® has been reported to have increased elasticity compared with DermaMatrix ® and Strattice™. This quality is relevant in situations in which increased elasticity is preferred, such as in addressing capsular contracture, or when the goal is for the ADM to conform to the inferolateral curvature of the breast. When the ADM is needed to provide support, such as in repositioning a displaced implant, a less elastic ADM like Strattice™ is preferred. Figs. 1–3 provide a comparison of the different biomechanical properties of various types of biologic meshes.
ADM Size
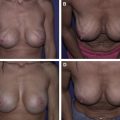
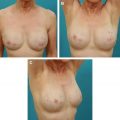
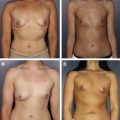
When deciding on the size of ADM to use, the patient is assessed preoperatively, and any surface irregularities or implant malposition are marked with the patient standing, sitting, lying down, and flexing the pectoralis major muscles. These markings aid in choosing the size of the ADM to be used. Intraoperatively, the sheet of ADM is fashioned to the appropriate size of defect at the time of placement. With respect to the sizes of ADM to use, a 4-cm × 16-cm piece of AlloDerm ® is typically used; the thickness of ADM varies from 1.3 to 1.8 mm, and the thickness used depends on the indication. Spear and colleagues devised a guideline for selection of AlloDerm ® size based on the arc length of the inframammary fold and the lateral mammary fold; for arc lengths of 18 cm or less, Spear and colleagues advocate a thick piece of 4 × 12 cm. For arc length greater than 18 cm, they advocate a thick piece of 4 × 16 cm.
ADM Preparation
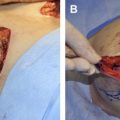
In terms of preparation of the ADM, the matrix is hydrated in saline as instructed by the manufacturer. Different hydration times are required depending on the product; AlloDerm ® requires at least 30 minutes of rehydration before its application but DermaMatrix ® can be rehydrated in 3 minutes. It is important to examine the ADMs for dermal elements, such as hair, and to remove them if present. Sterile technique must be used when handling ADMs. The dermal matrix is handled by only 1 surgeon, after either changing or cleansing the gloves. The product is taken from the saline bath where it is soaking and placed directly in the wound so that it does not contact the operative field or the patient’s skin.
ADM Placement
The ADM has a distinct polarity, and this must be identified intraoperatively. The dermal side has a smooth, shiny appearance that seems to absorb blood that it contacts. This side should be placed in contact with the underside of the mastectomy flap because it has been shown to be more likely to revascularize. In addition, the dermal side is potentially more seroma forming, and is thus kept away from the implant. The basement membrane side is dull and rough in appearance, and seems to repel blood that it contacts. This side is placed down so that it contacts the implant. It is important to avoid layering the ADM material because it is an avascular foreign body and this can increase the risk of infection and seroma formation. For postoperative care, soft compression and a surgical bra postoperatively may be helpful in minimizing dead space. Patients should remain on antibiotics to cover gram-positive skin flora for a 7-day period.
Uses of ADM s in breast reconstruction
Between one-half and two-thirds of women undergoing postmastectomy breast reconstruction choose alloplastic reconstruction, which makes prosthesis-based reconstruction the most common method of reconstruction in these patients. After mastectomy, an implant or a tissue expander is placed underneath the pectoralis major muscle, and the muscle covers the superior and medial poles of the prosthesis. The exposed inferior and lateral poles can be covered with subcutaneous tissue or by elevating serratus anterior or pectoralis minor muscles ; however, incomplete or inadequate coverage of the prosthesis can result in a higher risk of visible rippling, implant visibility or exposure, and contour irregularities. Some patients have a deficiency of the soft tissue envelope because of either an atrophic pectoralis major, its native insertion site on the chest wall, or because of intraoperative trauma or resection during the course of the mastectomy.
ADMs are a solution to this problem because they can be used either to create the implant pocket in single-stage reconstruction or to maintain the inferolateral portion of the tissue expander pocket in 2-stage reconstruction ( Fig. 4 ). After the mastectomy has been performed, a subpectoral pocket is developed. The boundaries of this pocket are the lateral border of the pectoralis major muscle to the second rib superiorly, the sternum medially, and the level of the contralateral inframammary fold inferiorly. The inferior attachment of the pectoralis major muscle is dissected from the chest wall.
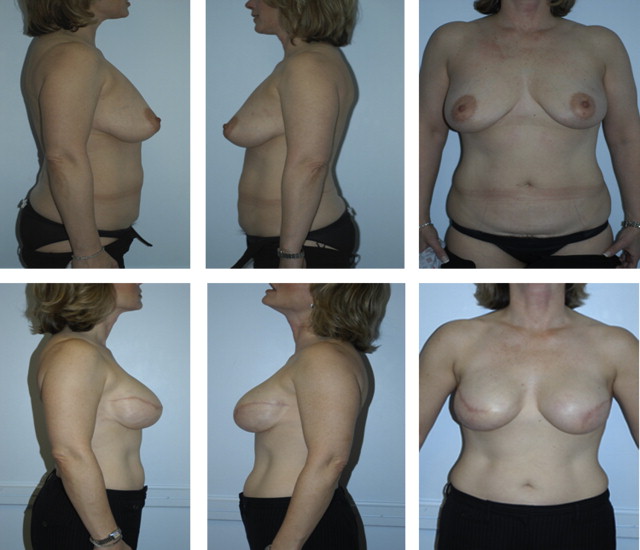
Various techniques have been described to attach the ADM to local tissue. See Box 1 for some technique tips for using ADMs in breast reconstruction.
- •
An ADM sling or hammock can be used to anchor the lower pole of the pectoralis major muscle by attaching it to the lower pole of the released pectoralis major superiorly, the serratus anterior flap laterally, and the chest wall inferomedially.
- •
After irrigating the pocket with bacitracin in sterile saline, the tissue expander or implant is then placed in the subpectoral pocket, covering the superior and medial poles of the prosthesis.
- •
The ADM is placed with the dermal side facing the mastectomy skin flaps and first secured by suturing it to the chest wall.
- •
The free edge of the pectoralis is then sutured to the ADM with a running suture.
- •
Two closed-suction drains are placed below the mastectomy skin flaps; 1 drain is placed in the axilla because this area tends to drain moderate amounts in most cases and the second drain runs along the inframammary fold.
- •
Mastectomy skin flaps are trimmed to yield bleeding margins for closure in 2 layers and dressed.
- •
Tension-free closure of the mastectomy flaps is crucial.
- •
Ensuring that the flaps are as thick as possible is also important.
- •
It is preferable to orient the incision on top of the muscle as opposed to directly over the ADM.
- •
Because textured implants can cause noticeable rippling, these implants are to be avoided.
Benefits of using ADMs in postmastectomy reconstruction include
- •
Providing soft tissue coverage over the prosthesis, especially when the skin flaps are deficient
- •
Allowing for control of the inframammary fold, permitting the creation of a larger implant pocket
- •
Decreasing the rate of capsular contracture.
These factors can potentially improve aesthetic outcomes.
ADMs can also augment the perfusion of a vulnerable skin envelope by offloading the mechanical stress caused by the implant weight on the skin envelope and the skin closure.
Using ADMs can reduce the time needed for the entire reconstructive process by reducing the time or need for tissue expansion; in turn, this concept can expedite the start of adjuvant chemotherapy and radiation if needed. By limiting the amount of dissection during implant or expander placement, using ADMs may decrease postoperative pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree