67 Congenital Auricular Deformities
Introduction
The malformations of the ear have an almost infinite variability of morphologic changes. Therefore, the correction or reconstruction or, more precisely, construction of an auricle requires an enormous methodologic range of different surgical techniques. In particular, in malformation surgery, we are often confronted both with functional disorders and aesthetic impairments. The rehabilitation of affected children must take into account both aspects and combine each conceptually. This applies in particular for severe ear malformations with their externally visible disfigurement and conductive deafness. For these malformations, the plastic head and neck surgeon must also ensure hearing rehabilitation or perform the necessary steps him- or herself.
Embryology
The auricle begins its development between 21 and 22 days postconception with an ectodermal thickening in the ear region, in which a dimple develops around the 28th day postconception. 1 By day 38 postconception, six mesenchymal hillocks evolve, arranged around the first branchial cleft, three each on the first (mandibular) and second branchial arch (hyoid arch). The first cranial, later ventral hillocks of the mandibular arch form the anterior part of the external ear (tragus and crus). The caudal and dorsal, later three hillocks of the hyoid arch form the posterior auricular shares. The concha and the external auditory canal develop from the first branchial cleft.
In addition to an increasing formation and enlargement, the ear moves from an initially anterocaudal into a dorsocranial position.
During embryonic and fetal development, the relative amount of the mesenchyme from the mandibular arch decreases significantly. It is estimated that about 85% of mature pinna structure is formed solely by the hyoid arch. 1
Disturbances of this complex development and differentiation process at different temporal and structural levels lead to the almost infinite variety of form variants and dysplasias of variable severity.
Anatomy of the External Ear
The external ear includes the pinna and the external auditory canal. The pinna has a characteristic relief facing frontward and a less distinguished posterior relief. With the exception of the cartilage-free lobule, the static basis of the auricle is formed by an elastic cartilage, which has a thickness of 1 to 3 mm. 1 , 2 , 3
The anterior skin is fixed to the perichondrium without subcutaneous tissue in between, being almost immobile. It is 0.8 to 1.2 mm thick. In contrast, the posterior side has a subcutaneous layer of 1.2 to 3 mm, which leads to a certain mobility. 3 The blood supply of the auricle is variable and dominated by branches of the superficial temporal and posterior auricular vessels. Innervation of the auricle is due to branches of N. auricularis magnus, N. auriculotemporalis, and N. occipitalis minor. The auricular muscles are relatively unimportant and small. Two muscles might be detected intraoperatively: M. auricularis posterior and M. auricularis superior. In addition, the auricle is stabilized by one posterior and two anterior ligaments. 1
The lymphatic fluid of the auricle is drained into the superficial and deep neck nodes, into the parotid gland, and toward the submandibular nodes and, in addition, the postauricular and mastoid lymph nodes.
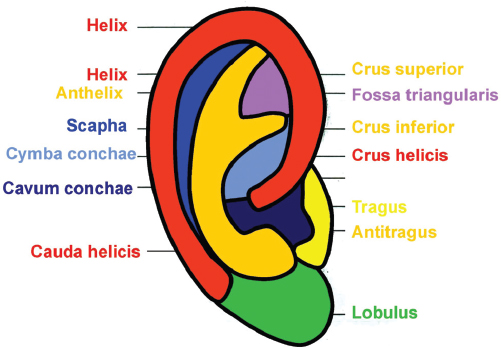
Aesthetic Units of the Auricle
The aesthetic units of the auricle are shown in Fig. 67.1 . In all reconstructive procedures, they should be respected. Incisions should be placed along their borders and not through them as much as possible.
Anthropometry of the Auricle
The size and position of the auricle as well as its relief are of utmost importance for corrective and reconstructive auricular surgery. The knowledge of this information in relation to age, sex, and body stature is necessary for the individual surgical planning. 3
The length of the auricle depends on body stature as well as age. On average, the auricle reaches 85% of its final length by the age of 6 and 90% at age 9. Later during life, the length of the auricle increases only slowly, mainly due to changes of the soft tissue of the lobule, which is more or less a lobule chalasis instead of real growth.
The width of the auricle also depends on body stature and age, but reaches 95% of its final value by the age of 6.
In contrast, the auricular projection (i.e., its width seen from a strictly anterior perspective) is almost constant throughout life. On average, the ear projection is 20 ± 4 mm. Its normal range is between 12 and 28 mm. These data are important for the indication and planning of otoplasty.
Classification and Surgery of Auricular Deformities
History
The history of the treatment of external ear malformations is closely linked to the reconstruction of traumatic or oncologic defects. Celsus was probably the first who described the reconstruction of partial auricular defects. 1 Known, however, are the statements of Susruta (about fourth century AD), who described a technique for reconstruction of the lobule from skin of the cheek. In medieval Europe (15th century), the Sicilian Branca family was well known for their techniques to reconstruct noses and ears with flaps of skin from the upper arm. The famous Italian surgeon Gaspare Tagliacozzi (1545–1599) also described techniques for the partial replacement of an ear. However, he deserves major credit for having his methods for the first time also illustrated pictorially.
Well into the 19th century, reconstructions for complete replacement of an ear were considered impossible; they were frowned upon. Also, one of the forefathers of German plastic surgery, the famous Berlin surgeon Dieffenbach, described only techniques for reconstruction of partial auricular defects. At the same time, he resolutely rejected methods for complete auricular reconstruction.
From about the mid-20th century, there are detailed reports of total auricular reconstruction. First and foremost, the pioneering work of Tanzer, Converse, Brent, Nagata, and Weerda must be mentioned here. 1 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 According to increasing deformity, severity, and reconstructive effort, the auricular dysplasias can be divided into three levels of severity according to recommendations of Weerda. 1
First-Degree Dysplasia
These dysplasias are only minor malformations. All structures of the auricle are present. Surgical correction means realignment of the given tissue without the necessity for grafts.
Protruding Ears
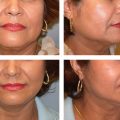
This is characterized by a flat to complete absence of the antihelix, including the crura. Usually there is also a pseudo-concha hyperplasia and an enlarged helical-mastoid distance of more than 20 mm. Despite the relatively mild malformation, since the first description of an otoplasty by Ely in 1881 to today, approximately 100 additional possibilities for the correction of prominent ears have been described. 1 Which of the correction options one chooses in each individual case depends on the individual’s underlying pathology, knowledge from the surgeon’s “surgical school,” and the experiences and preferences of the attending surgeon.
In very thin and soft cartilage, we use the Mustarde technique. 17 Starting from a posterior skin incision, approximately 1 cm below and parallel to the helix, in this case, first the entire cartilaginous auricular surface is exposed while sparing the perichondrium. With thin needles, the scapha and the junction of the concha and antihelix are marked. With multiple mattress sutures—we use Ethibond 4-0 (Ethicon) for this—the ear is then folded at the previous needle marks. If necessary, a conchal setback and a lobule plasty can also be performed (see below).
For thicker cartilage, we use the Converse technique ( Fig. 67.2 ). 18 , 19 Access, preparation of the cartilaginous auricular surface, and markings are equivalent to the above outlined technique of Mustarde. The posterior surface of the ear cartilage is then incised where the markings were made, but under strict preservation of ventral perichondrium. The incisions are at the base of the newly defined antihelix. Shaping a harmonious running antihelix is completed with several mattress sutures. In addition, if necessary, a conchal setback and lobule plasty might be performed (see below).

In cases of very rigid and solid cartilage, but also in revisions, we apply a modified Chongchet or Crikelair technique. 20 , 21 Unlike the techniques described above, here the ear cartilage is severed in the region scapha and then prepared anteriorly. By anterior scoring the cartilage (not deeper than twothirds of cartilage thickness) in the running direction of the marked antihelix, a harmonious shape is formed. If the effect is not be sufficient, the Mustarde technique can be introduced with some supportive mattress sutures appropriately. The chondrocutaneous flap is moved back and the cartilage reattached in the area of the severed scapha with some slowly absorbable sutures. Conchal setback and lobule plasty can be performed when needed.
This technique follows the same principle as the Stenström technique with the difference that in the latter the skin over the antihelix is only tunneled and the anterior surface of the cartilage is scored with small special instruments (e.g., after Drommer, Fa Robomed, Kolbingen). 22
A conchal setback is indicated when the helix-mastoid distance is more than 20 mm after one of the featured antihelix plasties. 23 , 24 Its operational technical basis consists of the resection of retroauricular fat, muscle, and connective tissue, and the fixation of the concha to the periosteum of the mastoid plane with stitches. Usually we place one to three slowly absorbable sutures (e.g., Polysorb 4-0 [Covidien]).
A real hyperplasia of the conchal cavum (usually pseudo-hyperplasia) should be corrected by a sickle- or crescent-shaped cartilage excision from the concha via the usual retroauricular access. 25
The lobule plasty stands at the end of nearly all otoplasties for protuding ears. Only in a few cases can this be dispensed with. In addition to reverse Y-shaped skin excisions of the posterior lobule-concha area and scoring of the cauda helixis, we prefer first and foremost a very simple yet highly effective technique for changing the position of the lobule. 26 , 27 Here, the lobule is mobilized subcutaneously, starting from the retroauricular incision. The predefined highest point of the lobule is taken by a subcutaneous suture and anchored to the cavum conchae in the anterior region. With the so defined mattress suture, the earlobe can be very finely moved toward the head. We use a slowly absorbable 5-0 suture (PDS [Ethicon]) for this.
For all protruding ears, the retroauricular wound closure is usually performed in a continuous technique. As suture material, we use, in the case of the often still small and partially uncooperative children, 5-0 quickly absorbable sutures (Vicryl Rapid [Ethicon]). A bandage (Ototect [Spiggle and Theiss]) remains for about 1 week. At night we recommend wearing a headband for an additional 6 weeks.
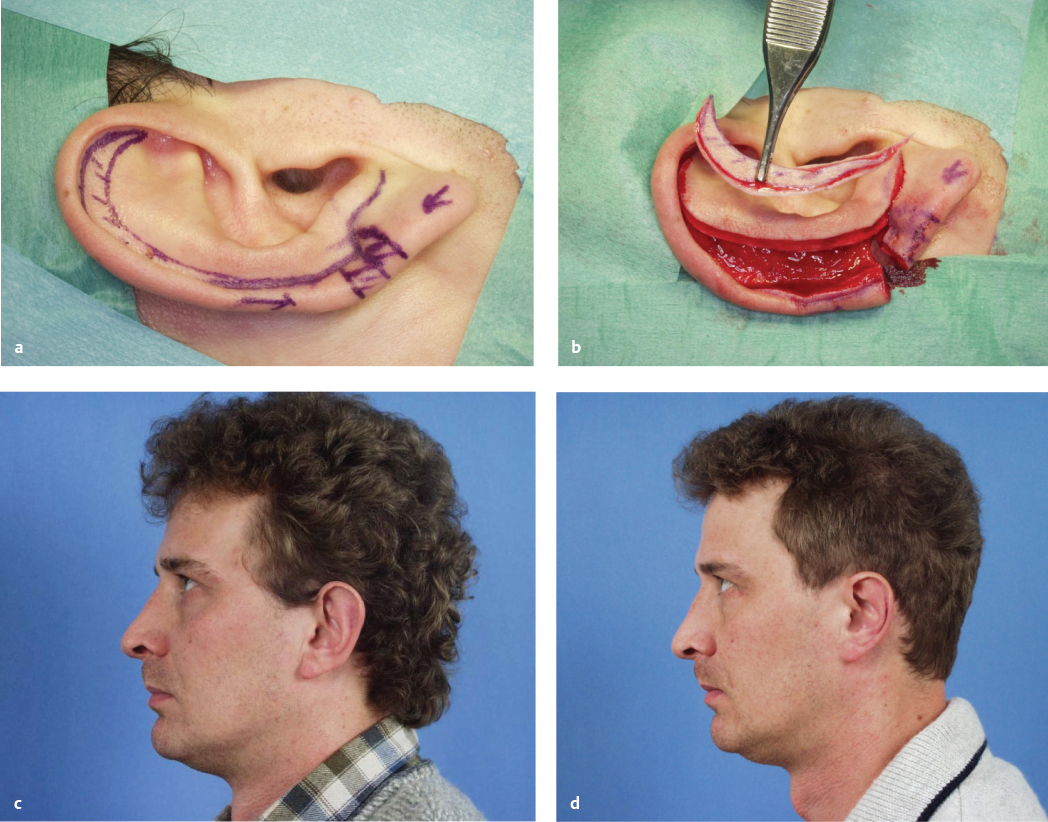
Macrotia
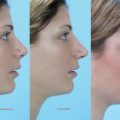
Macrotia denotes an auricle that is too large with regard to the patient’s body stature. Often it is combined with abnormal protrusion. Since its predominant feature is the hypertrophic scapha, the method of choice is the modified Gersuny technique ( Fig. 67.3 ).
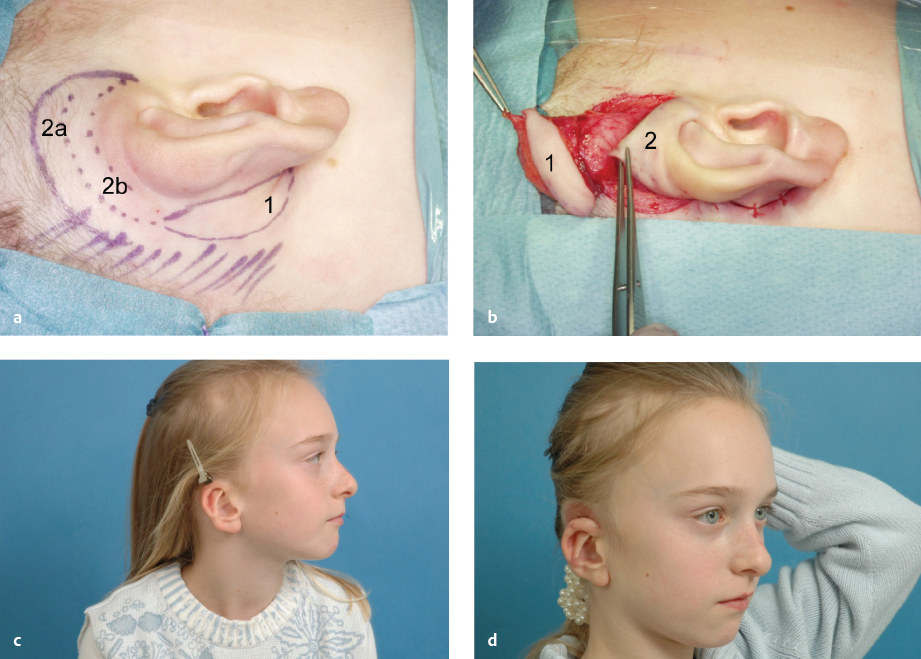
Cryptotia
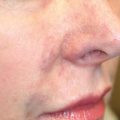
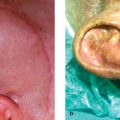
Cryptotia ( Fig. 67.4 ) is relatively rare in Europe, although more common in Japan. It is characterized by the upper helical rim being present, but hidden under the scalp. The goal of its surgical correction is to increase projection of the upper part of the auricle and to construct the upper sulcus. This technique is similar to the second step of total auricular reconstruction. The incision is done 1 cm above the hidden but palpable helical rim and the skin mobilized above the level of the hair follicles. Then the hidden upper part of the auricular cartilage is mobilized leaving it covered with connective tissue for its nutritive blood supply. The retroauricular sulcus is covered with the prepared thin skin. An interesting alternative to using a fullthickness skin graft is the application of a cranially pedicled island flap transposed into the new sulcus ( Fig. 67.4 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree