28 Hair Restoration
Introduction
The interest in hair loss can be traced back to the early Egyptian Ebers Papyrus of 1553 BC—the oldest and most complete medical text ever found. Translations of this text include treatments of hair loss with recipes composed of oils, various animal products, and magical chants. 1 The topic also received attention from Hippocrates and Aristotle in 400 BC, both who suffered from hair loss. 2
It was not until the early 1800s that the idea of hair restoration surgery was introduced. Dom Unger is credited with first introducing hair transplantation as a treatment option for hair loss. In 1822, Unger’s doctoral student, J. Dieffenbach, wrote a thesis on the allotransplantation and autotransplantation of hair, feathers, and skin of animals. 3 Since 1893, hairbearing autografts using flaps and relatively large free grafts for the treatment of traumatic alopecia have been carried out with varying degrees of success. In 1939, Japanese physician Okuda reported successfully transplanting punch autografts of hairbearing skin in the scalp of scarring alopecia patients. Because of World War II, his techniques were not acknowledged outside of Japan for many years. In 1959, N. Orentreich, recognized as the founding father of hair transplant surgery, reported the first successful hair transplant performed in the United States for male pattern alopecia. Through his research, Orentreich coined the terms donor dominance and recipient dominance, the basis of hair transplantation surgery today. 4
In 1942, anatomist James Hamilton published research on the etiology of male pattern alopecia. 5 By comparing eunuchs and eunuchoids, he suggested that the development of male pattern baldness was dependent on the interaction of androgens, genetics, and age. He is also credited for developing a classification scale for hair loss patterns. A modified version is still used today. 2
Since this time, many advancements have brought hair restoration to where it is today. U.S. Food and Drug Administration (FDA)-approved medications are available that slow or reverse hair loss. Rotational flaps, scalp reduction procedures, and tissue expanders have been added to the armamentarium of hair restoration options. Additionally, the original 4-mm punch grafts once used in hair restoration surgery have evolved to use of follicular unit graft. Follicular unit grafts are now considered the gold standard in hair transplantation, regardless of harvesting method.
Embryology
The hair follicle develops embryologically from both the ectoderm and mesoderm. The hair matrix cells and melanocytes develop from the ectoderm. The arrector pili muscle, dermal papilla, follicular sheath, and blood vessels are of mesodermal origin.
Hair development begins around the 10th week of gestation. The primary hair bud develops from an epithelial placode that projects downward into the dermis. The epithelial placode proliferates under the control of mesenchymal condensations that later become the dermal papilla. The hair follicle grows deeper into the dermis with the deepest cells becoming the bulb and hair matrix cells. By the 18th gestational week, a differentiated sebaceous gland is formed and a hair protrudes through the surface of the skin. All hair follicles on the body are formed by the 18th gestational week. 6
At birth, there are a total of 5 million hair follicles on the body. One million of these follicles are located on head and 100,000 on scalp. The average density of hairs in Caucasians at birth is 1 follicular unit per mm2 (about 175–275 hairs/cm2) ( Table 28.1 ). The midoccipital area is the densest area of the scalp, with the midmastoid and supraauriular areas being dense as well. After the development of these follicles, no new follicles are formed with aging; rather, as the surface area increases, hair density decreases.
Total number of hair follicles (birth) | 5,000,000 |
Total number of hair follicles, head (birth) | 1,000,000 |
Total number of hair follicles, scalp (birth) | 100,000 |
Density of hairs (birth) | 1 fu/mm2 |
Anatomy
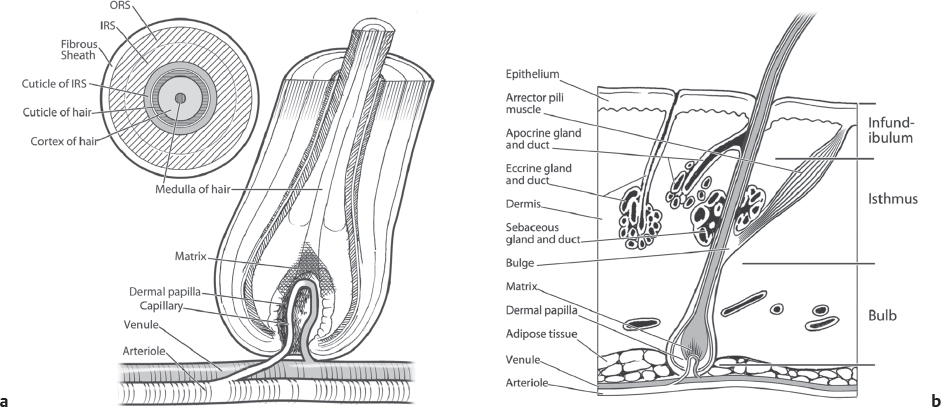
The hair follicle is divided from superficial to deep into three segments: the infundibulum, isthmus, and bulb ( Fig. 28.1 ). The infundibulum extends from the follicular ostium to the entrance of the sebaceous duct. The isthmus extends from the sebaceous duct to insertion of the arrector pili muscle. The lower segment consists of the bulb and is located between the arrector pili insertion and the base of the follicle. The bulb contains the hair matrix, dermal papilla, and the melanocytes that are responsible for hair color. The dermal papilla regulates hair growth and development and is thought to house multipotent stem cells. Under the direction of the dermal papilla, the cells of the hair matrix divide, giving rise to the growing hair shaft and internal root sheath.
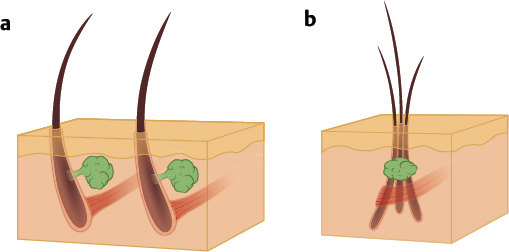
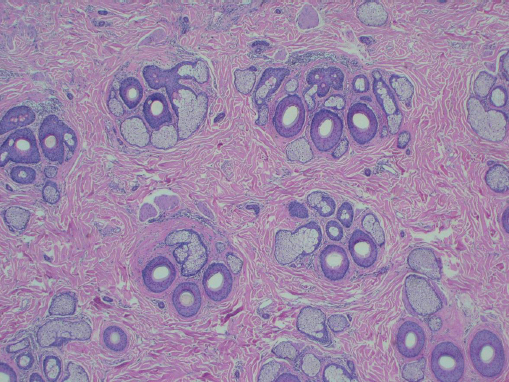
Hair follicles of the scalp naturally exist as follicular units ( Fig. 28.2 ). The follicular unit consists of one to four terminal hairs with or without several vellus hairs, a sebaceous gland and duct, arrector pili muscle, and a common vascular and neural plexus. The entire unit is surrounded by a connective tissue sheath. Using microscopy, the structure of the follicular unit and the surrounding area of non–hairbearing skin is visible ( Fig. 28.3 ).
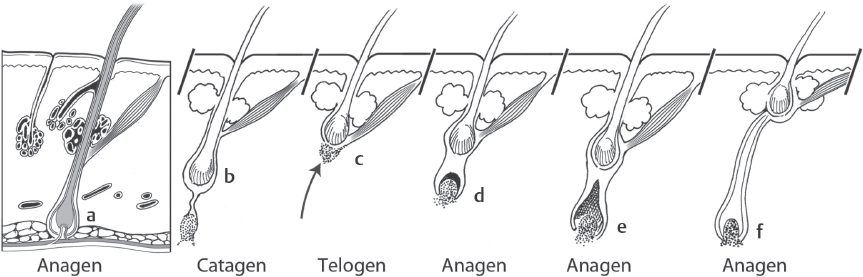
The follicular life cycle consists of three stages: anagen, catagen, and telogen. Anagen is the active growth and lasts 3 to 4 years. Approximately 90% of hairs on the scalp are in the anagen phase. The anagen phase is followed by catagen—the involution phase lasting 2 to 3 weeks. During the catagen phase, the inferior portion of follicle ascends superficially to the level of arrector pili attachment. Telogen is the resting phase lasting 3 months. Ten percent of hairs on the scalp are in the telogen phase ( Fig. 28.4 ).
Etiology
Hair loss affects at least 65 to 85% of men and 30 to 40% of women, and progresses with age 2 ( Table 28.2 ). The most common cause of thinning in both men and women is androgenic alopecia. Androgenic alopecia can present as male pattern androgenic alopecia (MPA), female pattern hair loss (FPHL), or numerous minor subtypes—including diffuse unpatterned alopecia, diffuse patterned alopecia, male pattern baldness with persistent midfrontal forelock, and senile alopecia. In general, men 18–29 years old have a 16% incidence of MPA; in their 30s, a 30% incidence of MPA; in their 40s, a 53% incidence of MPA; and in their 70s, an 80% incidence of MPA. 7 , 8 It is reported 40% of women have female pattern hair loss by age 70. 9
Men |
|
18–29 years old | 16% 8 |
30–39 years old | 30% 7 |
40–49 years old | 50% 8 |
70–79 years old | 80% 7 |
Women |
|
70 years old | 40% 9 |
Dihydrotestosterone (DHT) is the steroid that is recognized as the primary contributor to androgenic alopecia. DHT is synthesized from testosterone in hair follicles, adrenal glands, testes, and the prostate by the enzyme 5-alpha reductase, type II. During human development, DHT is necessary for embryonic sexual differentiation and virilization of the male embryo, but has no known utility in adults. In androgenic alopecia, DHT affects genetically susceptible hair follicles by binding to the androgen receptors in the hair follicle with an affinity five times higher than testosterone. 10 The result of DHT on hair development is a decrease in the percentage of hairs in the anagen phase and increase in the number in the telogen phase. 11 Additionally, coarse terminal hairs in the frontal and vertex areas undergo a miniaturization process. Follicles become finer, shorter, more lightly pigmented, and have a shorter growth period until eventually reaching the vellus stage. Hairs in the preauricular and postauricular areas change to a texture resembling beard hair. Vellus hairs in the beard, mustache, chest, and upper pubic region become terminal.
In FPHL, the pathways and processes of hair loss are not entirely known and the role of androgens remains to be defined. Microscopic evaluation in FPHL shows patterns that mimic MPA with progressive follicular miniaturization and decreased anagen phase. However, the clinical pattern of hair loss in women differs from that seen in men, which may be a result of receptor and enzymatic activity in the scalp. It is reported that 40% of women have FPHL by age 70. 38 Cytochrome P450 aromatase, an enzyme that converts testosterone to estradiol preventing the effects of DHT, is higher in women in the frontal hair follicles than in men. Additionally, women have less 5-alpha reductase types I and II and fewer androgen receptors in the frontal hair follicles compared to men. 12 These differences may be responsible for the preservation of the frontal hairline seen in FPHL. Estrogen may play a role in FPHL as well, possibly through its ability to modify androgen metabolism. This is evidenced by the phase changes seen during pregnancy, postpartum, and menopause. 13 , 14 , 15 Some women with hair loss may present more similarly to the classical male patterns of hair loss. In these cases, an evaluation for the clinical signs of hyperandrogenism including hirsutism, moderate to severe or treatment refractory acne, irregular menses, infertility, and galactorrhea is necessary. The pathways and processes of hair loss in females are not entirely known.
As opposed to hair thinning seen in androgenic alopecia, hair shedding is most commonly caused by telogen effluvium. Telogen effluvium is the massive loss, or shedding, of hair due to synchronized early entry of hair into telogen phase. It presents as a diffuse thinning of the hair on the scalp after an inciting event. Shedding lags 3 to 4 months behind an inciting event and is fully reversible. Inciting events may be emotional or physiologic stressors including eating disorders, sudden weight loss, protein deficient diet, high fever, pregnancy, chronic illness, general anesthesia, anemia, hypothyroidism, and hormonal fluctuations. Medications may also induce telogen effluvium including oral contraceptives, Accutane, vitamin A, angiotensinconverting enzyme inhibitors, and lithium.
Anagen effluvium is the pathological loss of anagen hairs associated with exposure to radiation therapy, systemic chemotherapy, or toxins. These agents affect the rapidly proliferating cells of the hair follicle causing rapid hair loss. Hair loss can occur as fast as 2 to 6 weeks after exposure and results in complete hair loss. On completion of therapy, hair returns within a month but may result in permanent alterations to the color or texture.
Alopecia areata is an autoimmune inflammatory condition that targets hair follicles. In the United States, the lifetime risk is 1.7%. 16 The pathology and triggering factors of this disorder are unknown. Clinically, patients present with sharply demarcated, round to oval, skin-colored patches of nonscarring alopecia. Patients may present with a variety of patterns of alopecia including total scalp or total body alopecia. On exam, hair follicles in the active phase have a characteristic exclamation point appearance with narrowing toward the base. There are no FDA-approved treatments for alopecia areata. The most commonly administered treatment option is intralesional steroid injections. The current topical and systemic medications have varying results. 17
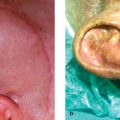
In scarring alopecia, inflammation occurs in the upper part of hair follicle leading to destruction of stem cells and the associated sebaceous gland. As a result, the hair follicle is replaced with scar tissue causing permanent hair loss. Clinically, affected areas lack hair, have smooth skin, and have no normal pore structures. In the active phase, erythematous and scaly skin is present at the periphery of the affected area. Diagnosis is achieved with a punch biopsy. Caution must be taken when proceeding with hair transplantation in patients with scarring alopecias. In active disease, grafts are unlikely to be successful and the disease can reactivate at any time resulting in graft loss. Test grafts, alopecia reduction procedures, an observation period prior to procedures, and a longer waiting period between sessions should also be considered. Scarring alopecia includes a spectrum of diseases: lichen planopilaris, central centrifugal alopecia, discoid lupus, pseudopelade (Brocq), folliculitis decalvans, tufted folliculitis, dissecting cellulitis, linear scleroderma, sarcoidosis, and frontal fibrosing alopecia.
Other less common causes of alopecia are infectious causes, trichotillomania, traction alopecia, and triangular alopecia. Infectious causes of alopecia include tinea capitis, folliculitis, and piedra, and typically exhibit a patchy, moth-eaten appearance. Patients with trichotillomania exhibit patchy areas of broken hairs of differing lengths. Like trichotillomania, traction alopecia results from mechanical stress on the hair. Tight pony tails and braided cornrow hair styles are the most common causes. Traction alopecia can result in permanent hair loss. Triangular alopecia is a rare condition primarily affecting children. It presents with an area of triangular or lancet shaped patch of vellus hairs with no terminal hairs in the frontotemporal area with the apex of the triangle pointing to the vertex.
Patient Evaluation/Consultation
A thorough patient history and physical exam are essential to determining the etiology of the patient’s hair loss and determining the best management options. Patient age must be considered prior to intervention. Although there is no minimum age limit for surgical intervention, patients younger than 22 tend to have less consistent and satisfactory results. A longterm relationship should be established with younger patients prior to intervention to observe the hair loss pattern and speed of progression while allowing the patient to mature and develop realistic expectations. Younger patients typically want to restore a more youthful hairline, which will appear abnormal beyond middle age resulting in an artificial appearance. Rather, a mature frontal hairline should be the ultimate goal. This should be addressed during the initial consultation with younger patients.
Information about the onset of hair loss, speed of progression, and prior management approaches should be obtained. Prior management approaches may include topical concealing sprays, medical intervention, and prior surgical procedures. The family history should include both the maternal and paternal family history. The patient’s motivations and goals must be addressed. The patient should be educated in order to have realistic expectations of hairline placement, density, and the need for multiple procedures. The limitations of transplanting the entire vertex, midscalp, and frontal hairline in a lifetime should be addressed.
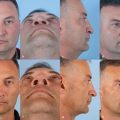
On physical exam, the pattern of hair loss should be documented. In males, the Hamilton classification modified by Norwood is used to classify the pattern and severity of hair loss 18 ( Fig. 28.5 ). Males with androgenic alopecia usually present with hair recession in the frontotemporal areas and hair loss in the vertex area. With age, these areas enlarge and coalesce. However, patients seldom fit exactly into a single category and must be classified based on the closest fitting. In the Hamilton-Norwood classification scheme, males are classified as:
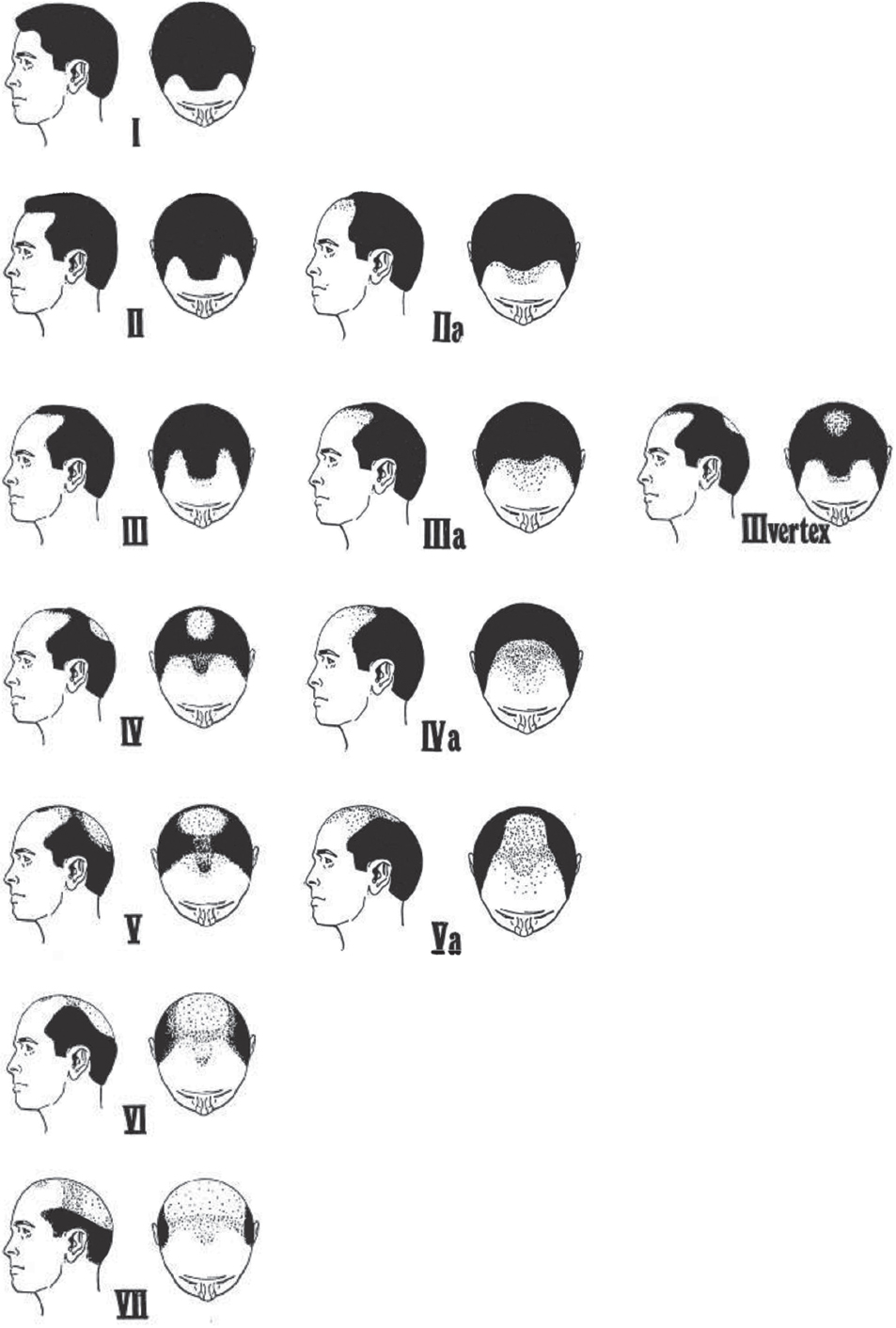
Type 1: Adolescent or juvenile hairline resting on upper brow crease with no or very minimal recession along the anterior border of the hairline in the frontotemporal region.
Type 2: Triangular areas of recession in frontotemporal hairline that extend no farther posteriorly than 2 cm anterior to a line drawn in a coronal plane between the external auditory meatus.
Type 3: Deep frontotemporal recession that is bare or sparsely covered with hair. The recession extends farther posteriorly than a line drawn 2 cm anterior to the coronal plane at the external auditory meatus.
Type 3 Vertex: Early hair loss primarily in the vertex. Patients can also have some frontal recession, but it does not exceed that seen in type 3.
Type 4: Hair loss in frontotemporal and vertex regions with a band of moderately dense hair across the top separating these two areas.
Type 5: Continued hair loss in frontotemporal and vertex regions with narrower and sparser bridge of hair separating the two areas.
Type 6: Complete loss of the bridge separating the frontotemporal and vertex areas resulting in a single large bald area. The lateral hair of scalp remains relatively high.
Type 7: Narrow and fine horseshoe band of hair laterally and on the occiput.
Norwood Class A patterns occur in approximately 3 to 12% of individuals studied and describe a front to back progression of the hairline without the usual peninsula of hair in midfrontal region or vertex balding. 18 , 19
Type 2A: Frontal recession no farther than 2 cm from the midcoronal line
Type 3A: Frontal recession approaching or reaching the midcoronal line
Type 4A: Extends beyond midcoronal line
Type 5A: Does not reach vertex, beyond this, cannot distinguish between usual type 5 and 6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree