Fig. 59.1
Surgical techniques used in the venous nerve graft study (i.e. study I). Group 1 comprises an empty vein graft, Group 2 a saline filled vein graft and Group 3 a BMSC filled vein graft
Vein Muscle Graft
The results of the vein graft combined with the BMSCs encouraged us to initiate a consecutive study to eliminate limitations from the previous study and introduce a slight modification to the vein graft itself. Anatomically the vein does only have a very thin surrounding smooth muscle cuff (especially compared to an artery), and may therefore have a tendency to collapse without any internal luminal filling [9, 11–13]. This theory could prohibit or withstand nerve regeneration. Battiston et al. provided a solution to this collapsing tendency. They placed a small piece of avascularised muscle intra-luminal to provide structure and published satisfactory results [26–28].
As such we executed another experimental study comparing the autologous nerve graft to the vein-muscle graft and to the vein-muscle graft with BMSCs as a luminal additive [29]. The results did not confirm our hypothesis that the vein-muscle graft with or without the BMSCs would compete in regenerative capacity to the autologous nerve graft. The autologous nerve graft significantly outperformed the vein-muscle grafts regarding both functional and sensory regain of function. Results from the sensory testing, motor function assessments, the electrophysiological evaluation, the gastrocnemius muscle index (GMI), and histological evaluation all converge in this respect.
In this study we bridged a 15 mm sciatic nerve defect in a rodent model. Three conduits were used: (1) an autograft (2) a vein muscle graft and (3) a BMSC filled vein muscle graft. The surgical techniques are illustrated in Fig. 59.2. Similar to the above-mentioned project, functional evaluation was conducted at different time points, with a maximum follow up of 3 months. The functional results, without the compound muscle action potentials, are depicted in Figs. 59.3, 59.4 and 59.5. In addition to these tests we also performed a histological analysis, which is discussed in the already published manuscript [29].
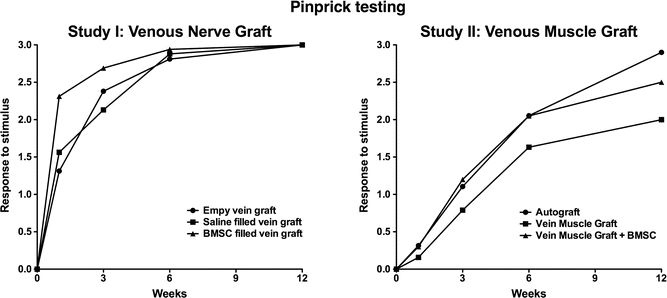
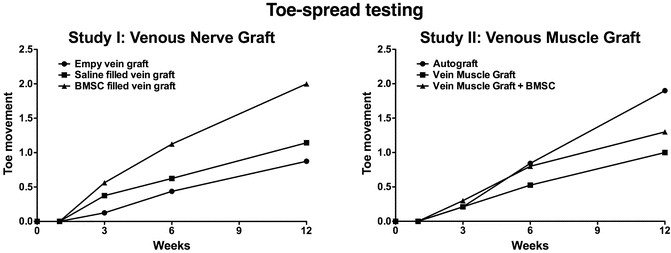
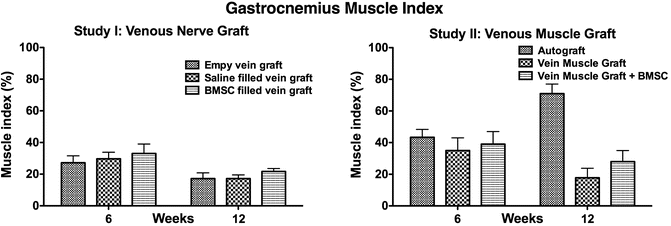

Fig. 59.2
Surgical techniques used in the venous muscle graft study (i.e. study II). Group 1 comprises an autograft, Group 2 a vein muscle graft and Group 3 a BMSC filled vein muscle graft

Fig. 59.3

Fig. 59.4

Fig. 59.5
Results of the gastrocnemius muscle index calculated in the two studies extensively discussed in this chapter. The muscle sacrificing procedure took place at 6 and 12 weeks post surgery. Study I: venous nerve graft and study II: venous muscle graft (The original data was used from the published studies [5, 29])
To illustrate the difference in regenerative capacity I would like to elucidate one specific evaluation assessment. The Gastrocnemius Muscle Index (GMI), which is a fairly simple and straightforward well-known assessment, evaluates and compares the wet muscle weight of the gastrocnemius of both the operated and non-operated leg. This ratio can inform the researcher if the motor end plates have been re-innervated after nerve trauma and because of re-innervation gain function and weight. In our study the rodents with a nerve defect reconstructed with an autograft showed an increase of the GMI comparing the rodents at 6 and 12 weeks post-surgery. The rodents operated with either the vein-muscle graft without BMSCs or the vein-muscle graft with BMSCs did not show an improvement of the GMI between 6 and 12 week. This implicates that the muscle in the autologous nerve graft treated group was already re-innervated, whereas the other two groups did not had a (sufficient) re-innervation yet [29].
Our hypothesis regarding the muscle segment was that it could prevent collapse of the vein in the early post-operative phase. As the muscle fragment is not vascularized, we expected it to become atrophic over time. This was confirmed in our H&E staining, which did not reveal any muscle tissue 6 and 12 weeks post-operatively. As previous work has already shown that any remaining muscle tissue does not obstruct the regenerating nerve [30], we can safely assume that incorporation of muscle in a vein graft will not influence nerve regeneration other than by preventing the vein from collapsing.
Regarding the effect of the luminal additive (i.e. the BMSCs), our analysis demonstrated a beneficial effect of the BMSCs, especially in early regeneration. Not only could we still find the labeled BMSCs inside the vein, but – more importantly – we also found a stronger, but non-significant, staining intensity 6 weeks after surgery of Neural Growth Factor and S-100 (a specific differentiated Schwann cell marker) in the rodents treated with vein-muscle graft with BMSCs as a luminal additive [29].
Worth mentioning in this chapter is that the final evaluation assessment of this study investigates the reinnervation of the sensory skin fibers after reconstructing a nerve defect. This data is yet to be published and is already discussed extensively in my PhD-thesis [31].
The skin sensory fibers can be divided in peptidergic fibers (containing CGRP and Substance P), which are responsible for the transmission of vital pain (temperature changes and acute pinch pain), and non-peptidergic fibers (containing P2X3) which transmit non-vital pain. Another group of fibers are the A-Delta fibers responsible for the quickest pain response transmission.
Evaluating the sensory skin fibers 3 months after autograft reconstruction a re-innervation of almost 70 % was seen. Dominance in the regeneration capacity was seen by the myelinated fibers. Regarding the evaluation of the skin fibers in the vein-muscle graft treated rodents a dominance in regeneration of the peptidergic fibers was also seen. However, no significant beneficial effect of the BMSCs was counted in the number of regenerated skin fibers of the rodent footpath.
Cellular Additive
As I stated before [5, 29], current literature promotes two explanations for the contribution of BMSCs to nerve regeneration. The first explanation is the production of cytokines and growth factors by the BMSCs, which will positively impact neural cell survival [10, 16, 19–23, 32–34]. The second explanation is the differentiation of BMSCs into neural lineages including neurons, astrocytes, oligodendrocytes, microglia and, most important in this context, Schwann cells [16, 24, 25].
A similar finding has been published by Tohill et al. who found mesenchymal cells differentiating into a Schwann cell-like phenotype [35] and later demonstrated that differentiated mesenchymal stem cells can have the same characteristics as Schwann cells in rats [36, 37].
Recently, our group published the first meta-analysis evaluating animal-related studies in which the effect of different stem cells was investigated related to nerve reconstruction [38]. The main purpose for his project was that the evidence for beneficial effect undoubtedly is getting stronger, but high-level clinical studies investigating this specific effect for peripheral nerve reconstruction still have to be executed. Hence this meta-analysis was designed to discuss literature investigating the different stem cell lines (i.e. adipose, bone-marrow and other). The comparison of these luminal additives resulted in an overall positive effect. Clearly the use of BMSCs is far more accepted compared to other stem cell lines, regarding number of studies and experience. Nevertheless the use of stem cells, as a luminal additive, is a promising addendum within the arsenal of tools available for nerve regeneration.
Main Conclusion and Future Perspective
The ongoing effort to innovate and explore new and better conduits to replace the autologous nerve graft for bridging large gaps has always been stimulated by the limitations of the nerve autograft [29]. The results of our first combined project were promising and showed a significant improvement in the regenerative potential of the venous conduit, when using BMSCs as additive [39]. However, taking the results of our second study in consideration, we can conclude that the nerve autograft clearly outperformed the vein-muscle graft, even though the cellular additive (i.e. BMSCs) gave some perspective in an improved regeneration tendency [29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








