Fig. 71.1
Semi-allogenic model of spontaneous in vivo creation of the donor-recipient chimeric cells (DRCC). Primary and secondary chimeras will be created in vivo by bone marrow transplantation across the MHC barrier between LBN (RT1n+l) (semi-allogenic model) and Lewis (RT1l) recipients. Creation of the primary chimeric animals will be performed by transplantation of 70 × 106 BMC harvested from the LBN (RT1n+l) rat femurs and tibias. Isolated BMC will be transplanted directly to the bone of the naïve Lewis (RT1l) rat recipients. The primary chimeric animals will serve as a source of the DRCC of MHC-mismatched phenotypes. Chimeric cells will be harvested from the BM compartment of the primary chimeras and will be purified using mAb specific for the RT1n MHC class I by magnetic-activated cell sorting (MACS) technique. Freshly isolated chimeric cells from the primary chimera donors will be delivered via intraosseous injection to the naïve Lewis recipients (secondary chimera) at the time of VCA transplant coming from the LBN donor. Both, the primary and the secondary chimeric animals will be additionally treated with a seven day protocol of combined αβ-TCR mAb (250 μg/day) and CsA (16 mg/kg/day) therapy.* seven day protocol of combined αβ-TCR mAb (250 μg/day) and CsA (16 mg/kg/day) therapy
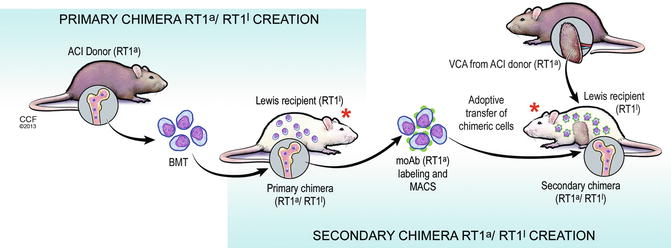
Fig. 71.2
Fully-allogenic model of spontaneous in vivo creation of the donor-recipient chimeric cells (DRCC). Primary and secondary chimeras will be created in vivo by bone marrow transplantation across the MHC barrier between ACI(RT1a) donors (fully allogenic model) and Lewis (RT1l) recipients. Creation of the primary chimeric animals will be performed by transplantation of 70 × 106 BMC harvested from the ACI (RT1a) rat femurs and tibias. Isolated BMC will be transplanted directly in the bone of the naïve Lewis (RT1l) rat recipients. The primary chimeric animals will serve as a source of the DRCC of MHC-mismatched phenotypes. Chimeric cells will be harvested from the BM compartment of the primary chimeras and will be purified using mAb specific for the RT1a MHC class I by magnetic-activated cell sorting (MACS) technique. Freshly isolated chimeric cells from the primary chimera donors will be delivered via intraosseous injection to the naïve Lewis recipients (secondary chimera) at the time of VCA transplant coming from ACI donor (ACI donor). Both the primary and the secondary chimeric animals will be additionally treated with a seven day protocol of combined αβ-TCR mAb (250 μg/day) and CsA (16 mg/kg/day) therapy. * seven day protocol of combined αβ-TCR mAb (250 μg/day) and CsA (16 mg/kg/day) therapy
The first application of chimerism as an approach for tolerance induction was reported in literature in the early 1950s [3, 4]. Billingham et al. [3] reported the induction of transplantation tolerance by injection of the donor hematopoietic cells into the neonatal mice within the first 24 hours after birth [5]. These experiments showed that it was possible to engraft splenic leukocytes or bone marrow cells (BMC) into the newborn mice recipients, which were immunologically immature and unable to reject [6]. Moreover, the recipient mice carrying donor leukocytes were only able to accept a skin graft from the leukocyte donor strain indicating that leukocyte chimerism was supporting tolerance [6]. In 1984, Ildstadt and Sachs published the results of their study about tolerance induction through mixed chimerism [7]. Sachs’s experimental design included a total body irradiation of the recipient mice and its reconstitution by a mixture of the T cell depleted bone marrow of the recipient with the bone marrow of the donor [5]. As the results showed, animals receiving a mixture of donor and recipient BMC became lymphohematopoietic chimeras without developing graft-versus-host disease (GVHD) and without complications due to T-cell depletion of the host bone marrow [5]. Moreover, long-term graft acceptance was achieved after transplantation of tail skin grafts onto the lateral thorax of the irradiated mice. Despite toxicity of total body irradiation, this study proved that mixed chimerism could lead to the development of long-term tolerance following skin graft transplantation [5, 7]. A new approach to study chimerism and tolerance induction was based on the use of T-cell depleting monoclonal antibodies, which are less toxic and more appropriate for clinical settings. Studies on mice [8], dogs [9–11], and monkeys [12, 13] showed that the infusion of donor BMC combined with depletion of the recipient T-cells prolonged allograft survival without the need for toxic and chronic immunosuppression. The first attempt to use donor BMC in a clinical scenario was performed by Monaco et al. in kidney transplant patients [14]. The use of BMC was performed as a supportive cellular therapy in solid organ transplants. The early results showed decreased level of kidney graft rejection and decreasing level of donor responsiveness [15]. In 1997, the first randomized trial was performed in liver transplant patients and supported by peri-operative BMC transplant showing significantly better results, thus favoring the protocol of multiple donor bone marrow infusions. In a study by Scandling et al., irradiated kidney transplant patients received 1 × 106 CD3+ T cells and 8 × 106 CD34+ enriched hematopoietic cells/kg under immunosuppressive protocol [16]. This regimen established mixed chimerism and tolerance towards kidney allograft to such an extent that all immunosuppressive medications were discontinued at six months after transplant [15, 17].
Clinical studies confirmed that the essential condition required for development of donor chimerism is the migration of passenger leukocytes. Confirmation of leukocyte migration and widespread distribution was reported in kidney transplants where the presence of donor-derived cells was confirmed in the skin, lymph nodes peripheral blood, and bone marrow compartment of the recipient [18]. Scientific efforts and experimental protocols of Billingham, Ilstadt, Sachs, and many other investigators studying tolerance induction in animal models opened a new era in the field of solid organ and VCA transplantation.
Chimerism Based Strategies for Tolerance Induction
Since confirmation of the crucial role of passenger leukocytes by Starzl in his pioneering work, the quest for discovering immunomodulatory properties of chimerism and its correlation with tolerance induction in solid organs and VCA transplants is still being investigated [19, 20]. Over the past decade, the Siemionow laboratory has been actively involved in the development of tolerance-inducting strategies by introducing new VCA models and performing basic science research on new tolerance inducing immunosuppressive protocols and cell-based therapies.
Siemionow’s team designed a variety of VCA experimental models and evaluated the effects of different tolerance inducing protocols on the development of donor specific chimerism and VCA transplant survival (Table 71.1). Using the single tissue (skin) vascularized allotransplantation model, the Siemionow team showed that donor-origin cells, which are present in the graft, are migrating to the recipient’s lymphoid compartments leading to chimerism induction. Furthermore, these studies confirmed the importance of immunosuppressive protocol adjustments for chimerism induction and maintenance.
Table 71.1
Experimental VCA models for chimerism and tolerance induction developed and tested in Siemionow laboratory
Model | Immunosupressive/immunodepletive therapy | Supportive cellular therapy | Study conclusion | References |
|---|---|---|---|---|
Total abdominal wall transplantation (LBN to Lewis rat) | CsA tapered from 16 to 2 mg/kg/day over 4 week period | – | Single tissue component (skin) under adequate immunosupressive therapy is capable of long-term chimerism induction and extends allograft survival | [21] |
Vascularized and non-vascularized skin allograft transplantation (LBN to Lewis rat) | CsA tapered from 16 to 2 mg/kg/day over 4 week period | – | Development of donor derived chimerism is affected by the allograft size and vascularization | [22] |
Limb semi-allogenic transplant (LBN to Lewis rat) | Comparison between fluocinolone acetonide (50 mg/ml) applied topically, cyclosporine A 4 mg/kg/day, combined systemic cyclosporine with topical fluocinolone acetonide | Vascularized bone marrow component of the graft | Extended allograft survival was accomplished by combination of low-dose CsA and topical steroids | [23] |
Comparison between ALS only (0.4 mL/kg), CsA only (16 mg/kg), and a combination of CsA and ALS, conditioning was administered 12 h before surgery at three different intervals (7, 14, and 21 days) | Vascularized bone marrow component of the graft | Correlation of donor specific hematopoietic chimerism with transplant tolerance | [24] | |
Limb allogenic transplant (BN to Lewis rat) | CsA and ALS were administered 2 h before surgery and continued for 21 days. Tapered CsA-16 mg/kg/day – 1st week, 8 mg/kg/day – 2nd week, 4 mg/kg/day – 3rd week. ALS – 0.4 ml/day for the 1st week, every other day for the 2nd week, and twice weekly during the 3rd week | Vascularized bone marrow component of the graft | Extended survival of the allogenic limb transplant associated with transient chimerism | [25] |
Face/scalp semi-allogenic (LBN to Lewis rat) | CsA 16 mg/kg/day of – 24 h after transplantation; tapered to 2 mg/kg/day over 4 week period and maintained at that level thereafter | – | Confirmation of the feasibility of the model with >170 days survival on maintenance immunosuppression | [26] |
Limb semi-allogenic transplant (LBN to Lewis rat) | 35-day course of 250 μg/kg/day anti-αβTCR antibody and 16 mg/kg/day CsA | Vascularized bone marrow component of the graft | Confirmation of induction of donor-specific tolerance to rat hindlimb allografts (graft survival over XX) | [27] |
Skin allograft (LBN to Lewis rat) | 35-day course of 250 μg/kg/day anti-αβTCR antibody and 16 mg/kg/day CsA | “Crude” bone marrow transplantation | Combination of anti-αβTCR antibody and CsA extended skin allograft survival up to 65 days | [28] |
Limb semi-allogenic transplant (LBN to Lewis rat) | 21, 7 and 5-day course of 250 μg/kg/day anti-αβTCR antibody and 16 mg/kg/day CsA | Vascularized bone marrow component of the graft | Clinical tolerance and immunocompetence were confirmed by skin grafting in vivo and MLR in vitro. High level of donor chimerism in the peripheral blood of long-term survivors was detected | [29] |
Limb allogenic transplant (BN to Lewis rat) | 7-day protocol of 250 μg/kg/day anti-αβTCR antibody and 16 mg/kg/day CsA | Vascularized bone marrow component of the graft | Seven days combined therapy of anti-αβTCR and CsA induced tolerance in rat hind-limb allografts. Tolerance was directly associated with stable, donor-specific chimerism | [30] |
Hemiface transplantation (LBN to Lewis rat and ACI to Lewis rat) | CsA 16 mg/kg/day, tapered to 2 mg/kg/day and maintained at that level thereafter | – | Survival in 100 % of LBN graft up to 400 days and ACI up to 330 days. Low dose immunosuppression facilitates engraftment of donor-derived cells into the lymphoid organs (lymph nodes and spleen) and supports chimerism induction | [31] |
Hemiface/calvaria transplantation (LBN to Lewis rat) | CsA 16 mg/kg/day tapered to 2 mg/kg per day over 4 week period and maintained thereafter | Vascularized bone marrow component of graft | New reconstructive graft model with survival up to 220 days. Development of donor chimerism – predominantly in the B-cell population | [32] |
Maxilla transplantation (LBN to Lewis rat) | CsA 16 mg/kg/day tapered to 2 mg/kg per day over 3 week period and maintained thereafter | Vascularized bone marrow component of graft | Maxilla graft survival up to 105 days. High level of donor-specific chimerism for T-cell and B-cell lineages was maintained in the peripheral blood | [33] |
Hemiface/mandible/tongue transplantation (LBN to Lewis rat) | CsA 16 mg/kg/day tapered to 2 mg/kg per day over 4 week period and maintained thereafter | Vascularized bone marrow component of graft | Long-term allograft survival (up to 385 days) correlated with development and maintenance of donor-specific chimerism in lymphoid organs and BM compartment | |
Composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation model (LBN to Lewis rat) | CsA 16 mg/kg/day tapered to 2 mg/kg/day within 4 weeks and maintained thereafter | Vascularized bone marrow component of graft | Long-term allograft survival correlated with development and maintenance of donor-specific chimerism | [36] |
Single tissue component models provided the experience and knowledge to progress to more surgically and immunologically advanced multi-tissue models. In 2000, the Siemionow team was the first to perform the full composite face/scalp allograft transplantation in the rat. The introduction of multi-tissue transplant models containing bone marrow components such as limb, calvaria, maxilla, or hemiface/mandible/tongue transplantation, confirmed that addition of the bone marrow component in combination with adjusted immunosuppressive protocol significantly increased allografts survival (400–700 days). High chimerism level in these experimental designs encouraged Siemionow’s team to further evaluate the immunomodulatory role of BMC transplantation and its potential application as a supportive therapy facilitating allograft acceptance and long-term survival.
Chimerism as a Base for Developing Chimeric Cell Therapy: Creation of Primary and Secondary Chimeric Animals
Several studies have shown that transplants containing bone component are more efficacious in chimerism induction and maintenance compared to vascularized skin allografts [36, 37]. Donor derived bone component due to the presence of hematopoietic stem cells creates a perfect source of donor-derived cells for chimerism establishment and preservation in the recipient. In the case of solid organs or VCA, such as face or abdominal wall transplants where there is no bone component, BMC infusion could be an alternative supporting chimerism induction, thereby increasing allograft survival. Billingham et al. demonstrated in their study using neonatal mice that transplantation of hematopoietic cells can induce tolerance of the recipient to the skin allograft via chimerism development [3]. Several animal and clinical case studies confirmed the beneficial effect of bone marrow derived therapies for survival of the solid organ allografts [38–40].
To assess the role of hematopoietic cells in chimerism and tolerance induction, the Siemionow group designed a series of experiments in which chimeric animals were created by an adoptive transfer of allogenic (ACI RT1a) or semi-allogenic (RT1l+n) BMC to Lewis rat recipients (RT1l). On the day of transplantation, animals received a non-myeloablative seven day immunosuppressive protocol of anti- αβTCR/CsA. Chimerism in both allogenic and semi-allogenic models was successfully developed. Interestingly, Siemionow group was able to detect MHC antigen’s characteristic for both ACI (RT1a) donor and Lewis (RT1l) recipient in the allogenic model and LBN (RT1l+n) donor and Lewis (RT1l) recipient in the semi-allogenic model in the bone marrow compartment of the Lewis recipient cells. To further investigate the properties of these in vivo created donor-recipient RT1a/RT1l and RT1l+n /RT1l chimeric cells, the Siemionow group harvested BMC from the primary chimera animals. Next donor-recipient chimeric cells (DRCC) were isolated using specific monoclonal MHC RT1a and RT1n antibodies via magnetic-activated cell sorting (MACS) method. Isolated donor-recipient (RT1a/RT1l) and (RT1l+n /RT1l) chimeric cells were used as a supportive therapy for VCA transplantation of allogenic (RT1a) or semi-allogenic (RT1l+n) skin graft to Lewis recipients (RT1l). Thereby, the secondary chimeric animals were created (Figs. 71.1 and 71.2). VCA survival in these animals was up to 365 days post-transplant as compared to 84 days in animals receiving no supportive cellular therapy (manuscript in preparation). These results showed that in vivo created DRCC carry pro-tolerogenic properties, which significantly improve VCA survival and could be a breakthrough in tolerance induction treatment.
Evaluation of in vivo created DRCC in a more complex hemiface transplantation model (article in press) confirmed that chimeric cell therapy successfully induces chimerism and increased survival of fully MHC mismatched hemiface allografts.
Mechanism of Chimeric Cell Creation In Vivo
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








