Fig. 30.1
Dissection of composite osseomusculocutaneous thymus flap
Later, the xiphoid process was held by means of a clamp and suspended upwards, the peritoneal cavity was entered, and the diaphragm was observed. The diaphragm was opened bilaterally right under the sternum. Thus, the mediastinum was reached and the backside of the sternum and the internal mammary vessels were observed. Next, the costal arches on both sides were cut caudally to cranially by means of blunt and sharp scissors. Next, the sternum was held on the xiphoid and lifted upward (Fig. 30.2).
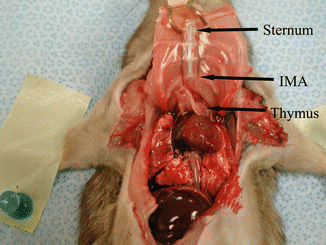

Fig. 30.2
Inner side of the flap, showing the thymus and internal mammarian artery (IMA)
After completely separating the costal arches, both clavicle joints were easily freed from the sternum. Thus, the sternum was separated en-block from the surrounding tissues. The thymus tissue covering the internal mammary arteries was also incorporated into the allograft. Later, the aorta and the superior vena cava were ligated just at the level entering and leaving the heart. Finally, the composite flap was harvested with the skin, pectoralis major, minor muscles, sternum, ribs and thymus based on the common carotid artery and external jugular vein (Fig. 30.3).
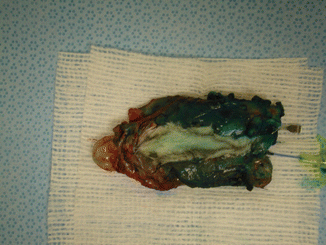

Fig. 30.3
Methylene blue injection showing the perfusion of the flap
Preparation of the Recipient and Transplantation
The inguinal region of the recipient rat was shaved and, following a lazy S incision, the femoral vessels were exposed. The femoral artery and vein were prepared for anastomoses. The allograft was transferred to the recipient site and a few stay sutures were used to fix the allograft. Arterial anastomosis was performed between the common carotid artery and the femoral artery; venous anastomosis was performed between the external jugular vein and the femoral vein. The vascular anastomoses were performed with 10/0 nylon sutures in an end-to end fashion (Fig. 30.4). The skin was closed with great care to ensure there was no kinking or stretching of the anastomosed vessels. After the transplantation, recipients received 10 ml of subcutaneous lactated Ringer’s solution to compensate for perioperative fluid loss. After allogenic transplantation, animals received cyclosporine A (CsA) monotherapy, tapered from 16 to 2 mg/kg/day over 21 days, and maintained at the same level thereafter.
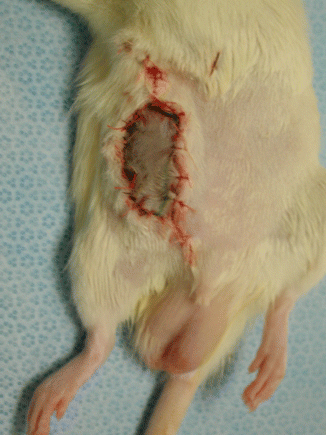

Fig. 30.4
Heterotopic transplantation of the flap to the inguinal region of the recipient
Flow Cytometric Analysis
At days 7, 21, 35, 63, and 100 days after the transplantation, 0.5 ml of blood was collected from the jugular vein. Two-color flow cytometry was used to evaluate the presence of donor-specific chimerism for MHC class I (RT1n) antigen in the peripheral blood of Lewis recipients on the aforementioned days. Donor chimerism was assessed with combinations of conjugated mouse anti-rat RT1n-FITC (for MHC class I donor cells RT1l+n, clone MCA156; Serotec, UK) with CD4-PE (clone OX-35), CD8a-PE (clone OX-8), CD45RA-PE (clone OX-33) and CD11b/c -PE (clone OX-42). After incubation, the samples were lysed and fixed with 1 % PFA solution.
Assessment of Bone Marrow Cells in the Sternum
The sternums of naïve LBN rat were harvested and bone marrow cells were washed with PBS. Then, the BMC suspension was passed through a nylon filter, mixed with ACK lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA, pH 7.3) for 5 min, centrifuged, washed and re-suspended in PBS. The count and viability of BMC were assessed using Trypan Blue exclusion.
Results
All allografts survived over 100 days and no vascular complications were observed. The bone component of the graft contained 5 × 106 of hematopoietic cells. Donor-specific chimerism was detected at day 7 post-transplant and was calculated as the sum of donor T cells, B cells and monocytes/macrophages/dendritic cells. At day 7 post-transplant, chimerism developed in the T-cell population and the mean level was 2.65 % for RT1n/CD4 and 1.0 % for RT1n/CD8. B-lymphocytes were present at 0.2 % for RT1n/CD45RA and monocytes/granulocytes/dendritic cells subsets were 0.55 % for RT1n/ CD11b/c. During the follow-up period, we observed a progressive increase in the T cell population and peak levels of donor-specific chimerism were observed 63 days after transplantation. The mean level of RT1n/CD4 and RT1n/CD8 donor T cells were 5.94 % and 1.83 %, respectively. However, the mean number of the donor-origin CD4 subset slightly decreased starting from day 100 post-transplant and was 5.11 % for RT1n/CD4. On the other hand, in terms of RT1n/CD8 cells, there were no significant changes compared to the results at day 63 post-transplant. At day 100 post-transplant, the chimerism of B-cells and RT1n/CD11b/c cells was 3.06 % and 1.75 %, respectively.
Discussion
Organ transplantation has become the most effective therapy for end-stage organ failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








