(1)
Department of Orthopaedics and Traumatology, Koc University School of Medicine, Istanbul, Turkey
Abstract
The rat hind-limb allograft is the most widely used animal model as composite tissue allografts for human hand transplantation researches. The monitorization of the composite tissue transplantation is essential to evaluate the transplantation-induced trauma, mechanisms of rejection, and the effectiveness of immunosuppressive protocols and the other treatment modalities. Because transplantation induced trauma begins with leukocyte-endothelial interactions in microvascular capillary structures, measurement of endothelial-cell function during the acute rejection period is important. Monitorization includes the evaluation microvascular permeability, capillary diameters, red blood cell velocities, leukocyte activation observations, functional capillary perfusion, and endothelial edema index.
Keywords
Composite tissue transplantationMonitoringMicrocirculationIntroduction
Hand transplantation has come a clinical reality on the base of long-running researches in microsurgical laboratories over the years. Because a functional primate model for hand transplantation did not exist, composite tissue allotransplantation has been introduced in 1970s. Composite tissue allografts consist of heterogeneous tissues derived from ectoderm and mesoderm, including skin, fat, muscles, nerves, lymph nodes, bone, cartilage, ligaments, and bone marrow. The rat hind-limb allograft is the most widely used animal model as composite tissue allografts for human hand transplantation researches [1–3]. Because composite tissue structure produces a highly antigenic stimulus (cartilage, ligaments, and fat present low antigenicity; bone, muscle, nerves, and vessels moderate antigenicity; skin high antigenicity), severe rejection process develops. Thus, different experimental models have been improved to monitor the transplantation-induced trauma, mechanisms of rejection, and the effectiveness of immunosuppressive protocols and the other treatment modalities [3–6].
Transplantation induced trauma begins with leukocyte-endothelial interactions in microvascular capillary structures. Endothelium has long been identified as a primary target of allograft rejection. The adherence of leukocytes to the endothelium in the microcirculation is considered to be an initial hallmark of an inflammatory process within allograft rejection. The vascular endothelium forms a barrier between the vascular lumen and the interstitial space. After damage of this barrier function caused by leukocytes accumulation on the surface of the endothelium, microvascular permeability begins to rise. Measurement of endothelial-cell function during the acute rejection period can be a more sensitive indicator of injury than examination of end-organ damage. Intravital microscopy has been extensively used to evaluate microvascular permeability and to attempt to quantify increased vessel permeability after induction of inflammation. Fluorescein conjugates were injected intravascularly and extravasation of the tracer was observed with fluorescein microscopy. With this technology, it has been possible to quantify the leakage of the fluorescein tracer in the microcirculation [7–10].
Experimental Model
The model is comprised of Lewis and/or Lewis Brown-Norway rats. The cremaster muscle of the donor animal with its supplying pubic-epigastric pedicle is prepared after extraction of the testes and spermatic cord (Fig. 31.1). The cremaster as an island-tube muscle flap is inserted into a subcutaneous tunnel in the antero-medial aspect of the thigh. After osteotomy of the femur, the iliac vessels are divided at a level just distal to the aortic bifurcation. In the recipient animal, the hind-limb is amputated at the middle third of the femur. In the beginning of the transplantation, the osteosynthesis is performed with an intramedullary pin and cerclage wire, and the muscle groups are approximated. Then, the iliac vessels of the donor and the recipient animals are anastomosed using 10/0 nylon suture materials. The skin is closed with 5/0 silk suture [4, 7, 11] (Fig. 31.2).
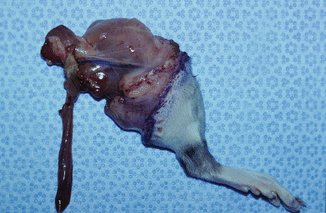
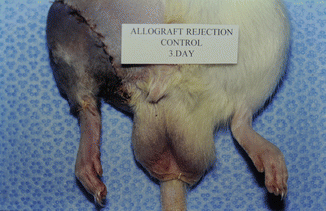

Fig. 31.1
Rat hind-limb with cremaster island-tube muscle flap model

Fig. 31.2
Transplanted animal 3 days after the procedure
Preparation of Cremaster Muscle for Observation in Monitorization
Depends on the experimental design, at the end of follow-up period of animals, rats are re-anesthetized with pentobarbital (50 mg/kg) intraperitoneally. Animal body temperature is maintained between 35 and 37 °C with a heating pad or heating lamp during surgery or evaluation under intravital microscopy [4, 7, 11].
Cremaster muscle tube-flap is carefully withdrawn from the subcutaneous tunnel of the limb and opened along its front wall using thermal cautery. A round flap is created with an axial pattern of main feeding vessels suitable for microcirculatory observations. The animal is secured in a specially designed Plexiglas tissue bath, and the cremaster muscle is spread out and fixed with 5/0 silk sutures over a cover glass in the bottom of the bath (Fig. 31.3). The muscle is kept moist with Ringer’s solution sealed with oxygen impermeable plastic film [4, 7, 11].
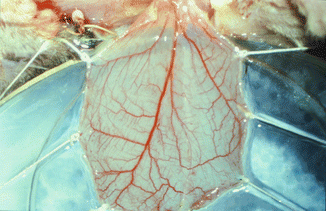

Fig. 31.3
Rounded cremaster muscle flap with an axial pattern of main feeding vessels over the Plexiglas tissue bath
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








