Chapter 31 Vascularized Tendon Transfers for Reconstruction
A Tendon Vascularity and Gliding, Island and Free Vascularized Transfers
Outline
Potenza introduced the essential roles of adhesions for tendon healing in early 1960s.1 Since then, investigators sought to lessen functional impact of adhesion formation and optimize the sliding capacities of the tendon. Few attempts were made to understand why tendons need vascular or pseudo-vascular connections. Indeed, some researchers tended to minimize the role of vascularization. The efforts have led to less research aimed at providing vascular supplies to the tendon for many years because it seemed pointless to carry out research on this subject if tendons are very slightly vascularized and receive sufficient nourishment from the synovial fluid.2
Different avascular tendon grafts, such as the palmaris longus, the plantaris, and others, have been used thus far.3 The two-stage techniques became popular to treat the cases with serious scar during secondary repairs or reconstruction. Two-stage tenoplasty by James Hunter4 and Paneva Holevitch5 needs to be completed with multiple operations. In these operations, a silicone rod capable of recreating the conditions of a synovial sheath is inserted first. A tendon is then grafted or transferred in a second stage. Despite all the precautions, functional results were sometimes mediocre because, apart from Hunter’s series, which found 80% good results, other teams only approached 50% success rates. A good skin condition is required before surgery. The two-stage technique necessitates at least 6 months, thus discouraging patients and surgeons owing to the time factor and to the difficulty in obtaining good functional result. Moreover, in the mid to long term, the outcome becomes even less satisfactory sometimes, with some fingers in flexion deformities.
In the staged tendon reconstruction, the tendon is not vascularized and placed in a more or less sclerotic recipient site. It is not always possible to achieve both healing and gliding of the repaired tendon at the same time. Past investigations demonstrated that the tendon is a vascularized structure and has vascularity distributing both inside and outside the tendon, as well as having a very specific lymphatic drainage system.6–9 The current techniques remain completely alien to these biological realities and ignore, or at least exclude, necessity of vascular supplies to the grafted tendon during surgical reconstruction. Therefore, better knowledge of the intricate physiology of the tendon and the conditions favoring optimum function are necessary to obtain better outcome of the surgery.
For many decades, terms such as hierarchical tissue distribution, stratification, and virtual space between structures have been taken for granted, yet the real basis for them needs to be questioned. Their scientific underpinnings were limited to the notion of virtual space or the existence of loose connective tissue, but their biomechanical foundations were more than vague. In the past 50 years, research focused on the microscopic findings of these structures while the global concept of the structures has not been addressed. As we have examined these tissues more closely, new hypotheses and findings have emerged concerning the organization of the subcutaneous tissues and led to new concepts and to new surgical procedures.10
Anatomical Studies and Surgical Basis
Microvideo Observations of Tendon Structures in Zones 3, 4, and 5
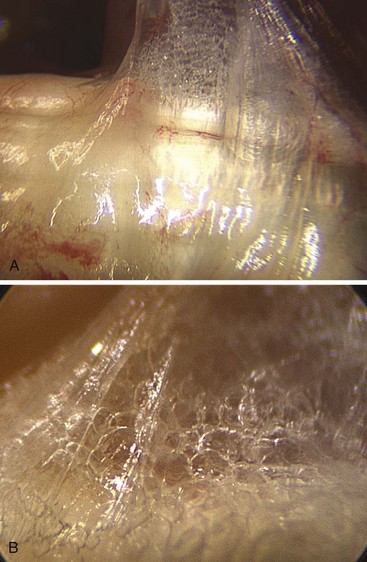
This gliding or sliding tissue structure, traditionally called “connective,” “areolar,” or “loose tissue and paratenon around the tendons,” has for long been considered to be packing tissue that fills spaces. In fact, this tissue plays a mechanical role, allowing movements between the structures it connects, preserving mobility and independence between organs, in particular, between tendons and skin. This tissue is important for the nutrition of the structures embedded in it and acts as a frame for blood and lymph vessels (Figure 31[A]-1).
Mechanically, it diminishes friction while facilitating deformability and adaptability. The fibrous tissue, known as the paratendon, surrounds the tendon. It is composed of multidirectional filaments, intertwining and creating partitions that enclose microvacuolar shapes (Figure 31[A]-2). We term this the Multimicrovacuolar Collagenous Dynamic Absorbing System (MVCAS) to emphasize its function. This system is situated between the tendon and its neighboring tissue and favors optimal sliding. The tendon is able to travel far and fast without any hindrance, and without inducing movement in any other neighboring tissue, thus accounting for the absence of any dynamic repercussions of movement on the skin surface. When the flexor tendon moves, its movement is barely discernible in the palm.
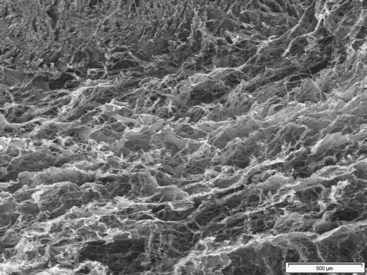
In the light of above new information obtained, the time has come to confirm some anatomical truths about this tissue. The notion of multilayered sliding between completely anatomically separate tissues—sliding thanks to what many believe to be an elastic process—is likely to be revised in the light of these observations. Continuous matter and microvacuolar framework should be noted between the tendon and surrounding structures.11,12 Scanning electron microscopy demolished the existence of different superimposed layers because they were never observed. Furthermore, the elementary laws of mechanics and rheology presented the problem in terms of global dynamics where continuous matter composed of millions of vacuoles, each measuring a few microns to a few millimeters in size, is organized in dispersed branching fractal patterns (Figure 31[A]-3). The sides of the vacuoles, which are intertwined, are composed of collagen fibers. This approach to tendon physiology also supposes a completely different way of perceiving the problem of reconstruction. These observations demonstrating the real histological continuity between the paratenon, the common carpal sheath, and the flexor tendons illustrate the perfect vascularization of this functional ensemble. The observations introduce a new concept: the sliding unit, composed of the tendon and its surrounding sheaths. Further functional detail of this sliding unit includes (1) a tendon only has optimal functional value when it is surrounded by its original sliding sheath and its vascular heritage; (2) a tendon is adherent only when it is artificially separated from its own sliding sheath, or when the harmony between the tendon and the sheath has been interrupted; and (3) a tendon is only one of the elements involved in the transmission of force through the sliding unit.
Basic Principles for Our Methods of Tendon Reconstruction
The idea is to transfer en bloc a digital flexion unit composed of the flexor tendon with the sheaths from zones 3, 4, and 5 to zones 1 and 2 at a single stage. The author uses this technique for the reconstruction of finger flexor tendons with Boyes grade 3 and 4 presentations.13,14 These patients include those with restricted passive motion in the proximal interphalangeal (PIP) or distal interphalangeal (DIP) joints even after a period of elastic traction (grade 3) and those with all possible complicating factors such as major soft-tissue damage, joint stiffness, poor vascularization, and trophic changes (grade 4).
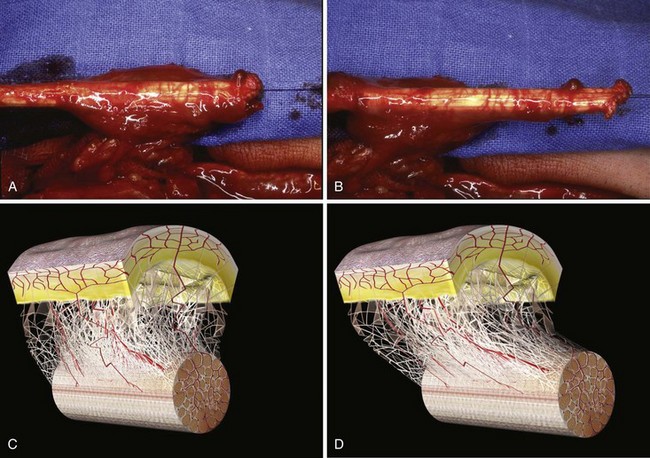
The theoretical bases for this technique are that the tendon can be conceived as vascularized and that the tendon is an element of a highly complex sliding and functional unit in association with its surrounding sheaths (Figure 31[A]-4).
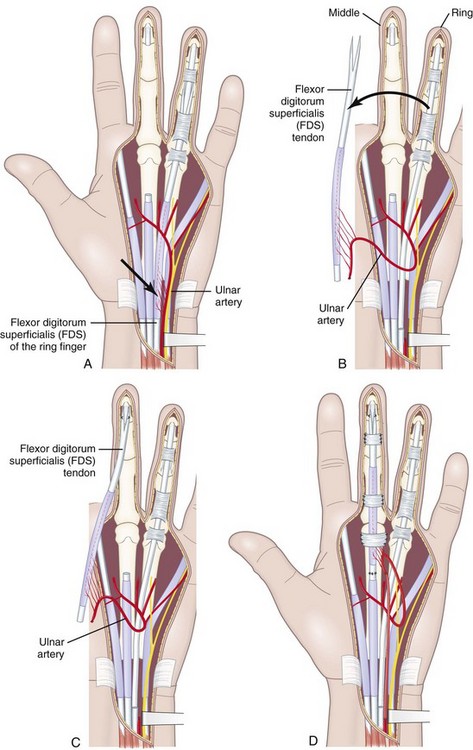
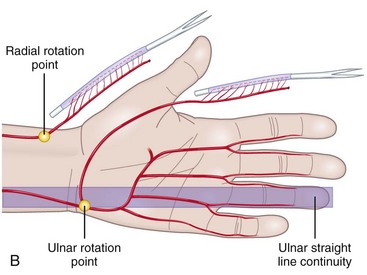
In developing this technique, the author sought to answer following basic questions: (1) Which sliding structures should be used to replace zone 1 and 2 tendons? The mesotendon and its vascular branches provide good vascularization of the flexor tendon and the sliding carpal sheath both extrinsically and intrinsically. The structure thus transferred is a real sliding structure which already exists naturally in zones 3, 4, and 5. (2) How will the replacement flexion structure be vascularized? Vascularization is ensured by a preretinacular mesotendon with branches arising from the ulnar artery. At the inferior third of the wrist, just before the flexor retinaculum or the annular ligament, the latter has two or three branches of around 1 mm in diameter. These branches pass through the common carpal sheath toward the superficial flexor tendons, especially those of the middle, the ring, and the little fingers, through a fine transparent mesotendon acting as a mesentery connecting the tendons. This vascular supply to the flexor system and the common carpal sheath is distal to the tendon–muscle junction, thus allowing harvesting of retrograde island transfers of purely tendinous structures without muscles. (3) How will the sliding unit be positioned in zone 2? For reversed vascularized tendon transfer, only the ulnar artery–based pedicle is suitable owing to its distally based palmar point of rotation and to its branch transmission at the level of the tendon. Since there are many clinical circumstances and forms of tissue destruction, this surgical technique has been developed over time to include a wide range of variants (Figure 31[A]-5).
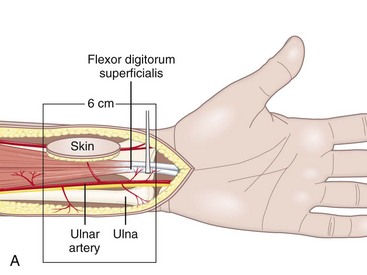
Anatomical Features of the Ulnar Artery–Based Pedicle Flap
In the distal forearm, the ulnar artery runs parallel to the nerve between the flexor carpi ulnaris (FCU) tendon laterally and the flexor digitorum superficialis (FDS) tendons of the fourth and fifth fingers medially. Covered only by skin, subcutaneous fat, and the superficial fascia, this artery is relatively superficial in the lower and middle compartments. However, in the upper compartment, the cutaneovascular relationships are less clear and the raising of a reverse island flap is thus more problematic. Cutaneous vascularization is ensured by a series of two to four small pedicles linked to the main pedicle through the fascia. The vessels are about 1 cm long and 1 to 3 mm in diameter. Since the small pedicles lie 15 to 25 mm apart, each flap usually contains at least two pedicles of good quality. In all our patients, the anatomic presentation was constant, both topographically and with regard to vascular distribution (Figure 31[A]-6). The anterior compartment of the forearm is drained by two venous systems, the venae comitantes of the ulnar artery and the superficial system, whose veins are of larger diameter. Both systems have abundant anastomotic networks, which make it easy to raise both free and pedicle flaps.
Operative Procedures
Vascularized FDS Tendon Graft Based on the Ulnar Artery: Essential Procedures
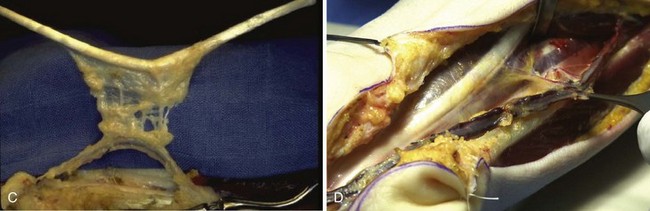
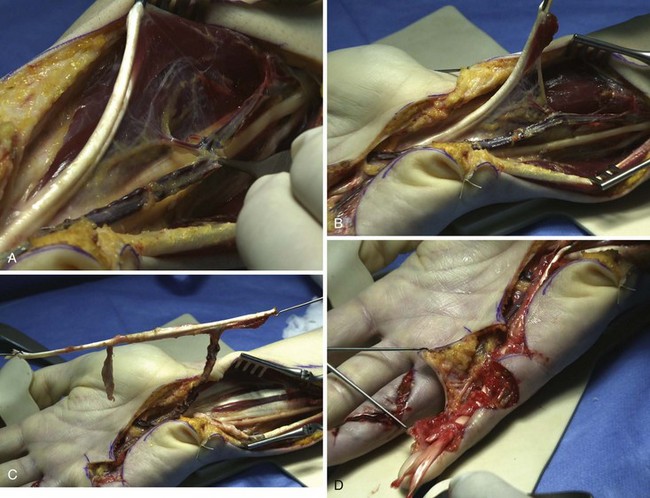
A mesotendinous structure composed of the FDS tendon of the ring finger is then raised with the carpal sheath and its vascular connections from the ulnar artery (Figure 31[A]-7). These connections, usually comprising two or three small branches on the anterolateral side and measuring on average 0.2 to 0.5 mm in diameter, are found just proximal to the proximal edge of the flexor retinaculum. At the level of the A1 pulley, the FDS tendon is transected proximal to the decussation after forceful traction on the tendon, avoiding laceration of the vinculum longum of the flexor digitorum profundus (FDP) tendon. The ulnar artery is then ligated proximally. All the other branches of the ulnar artery to the deep arch division are ligated to obtain a rotation point at the level of the deep branch. The MVCAS surrounding the tendon is kept in place, thus ensuring a real vascular connective link around the tendon. A composite mesotendinous unit of 20 cm long is then raised.