Chapter 50 Ultrasonic liposuction
• Be familiar with the UAL endpoint (loss of tissue resistance against the ultrasonic probe).
• Planning incision placement that provides direct linear access to the treatment area is of paramount importance to avoid placing torque on the ultrasonic probes.
• Use the generator energy setting and ultrasonic probe combination that will efficiently emulsify fat in a given area while delivering the lowest amount of ultrasound energy to the tissues.
• Aspirate harvested by means of third generation UAL contains viable fat cells and is highly suitable for fat grafting.
• Even dispersion of the wetting solution throughout the tissues is essential in UAL. Infusion volumes greater than the 1 : 1 ratio suggested for superwet SAL are frequently required for efficient UAL induced cavitation.
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
Historical Perspective
Over 300 000 liposuction surgeries were reported in the United States last year, making it the most common esthetic surgical procedure performed in 2011.1 Currently only 20% of these cases are ultrasound-assisted liposuctions (UAL) and this percentage has remained relatively unchanged during the past 9 years in spite of the recent advances in the safety and efficacy of the third generation ultrasonic liposuction devices.
Ultrasonic energy has been in use in dental and surgical instrumentation since the late 1950s. However, it was first applied to the field of body contouring surgery in the late 1980s, first by Scuderi2 and afterwards popularized by Zocchi,3,4 who introduced the technique to the United States in 1993.5 Approximately 2 years later the UAL task force was created in response to an increase in American interest in this new liposuction technology.6 The task force was composed of representatives from the American Society of Plastic and Reconstructive Surgeons, American Society for Aesthetic Plastic Surgery, Lipoplasty Society of North America, Aesthetic Surgery Education and Research Foundation and Plastic Surgery Educational Foundation, who collaborated with the Food and Drug Administration as well as the manufacturers of the UAL devices. The mission of the task force was to evaluate the safety and efficacy of UAL in body contouring, establish appropriate indications for its use and design the educational protocols necessary to train plastic surgeons in the United States in the use of UAL. By 1997 the safety and efficacy of UAL had been established by the task force and attention was then focused on surgical training. By 1997 comprehensive teaching courses in UAL that included practical, hands-on training, were offered under the direction and guidance of the UAL task force. Over 2000 plastic surgeons were trained in UAL techniques during the first two years that the courses were offered.7
The original UAL device was manufactured in Italy by the SMEI Company. It is often referred to as the first-generation UAL device and it involved the use of solid probes with a frequency of 20 kHz. It was a relatively slow device taking 10 to 12 minutes to obtain 250 to 300 ml of fat reduction.8
In the mid 1990s the second generation UAL devices were developed and sold in the United States. The two devices in this category consisted of the Mentor Contour Genesis (Mentor Corp., Santa Barbara, CA) (Fig. 50.1), which operated at a frequency of 27 kHz and the Lysonix 2000 (Lysonix Inc., Carpinteria,CA) (Fig. 50.2) with an operating frequency of 22.5 kHz. In 1999, Chang and Commons9 published their experience comparing these two UAL devices in over 200 patients over a 2 year period and found both systems to be effective.
FIG 50.1, 50.2 Appears ![]() ONLINE ONLY
ONLINE ONLY

FIG. 50.1 Mentor Contour Genesis.
Courtesy of Mentor Corp., Santa Barbara, CA, reproduced with permission.
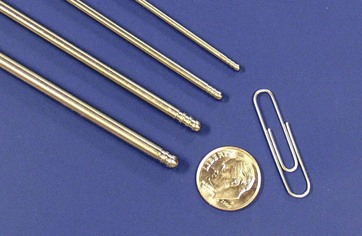
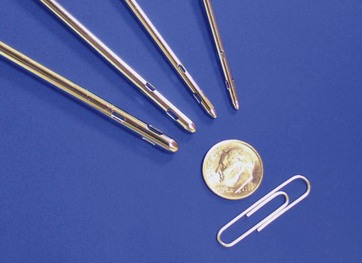
In 2001, a third generation UAL device was introduced. The VASER system (Sound Surgical Technologies, Louisville, CO) was named for vibration amplification of sound energy at resonance. This new device design diminished the amount of ultrasound energy delivered to the tissues, which had been implicated as the cause of many of the complications reported with the earlier UAL devices.10 The VASER system (Fig. 50.3) operates at a frequency of 36 kHz and is capable of emulsifying fat rapidly while delivering lower amounts of energy to the tissues. The system uses a smaller diameter solid probe design with grooves proximal to the tip (Fig. 50.4). These probes can significantly diminish the energy delivered to the tissues, particularly when coupled with the “VASER mode” pulsed energy delivery system. VentX suction cannulae (Sound Surgical Technologies, Louisville, CO) are an integral component of the system (Fig. 50.5).
FIG. 50.3 Appears ![]() ONLINE ONLY
ONLINE ONLY
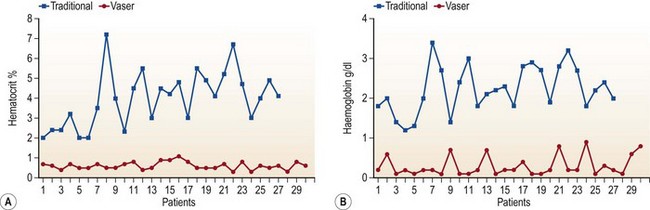
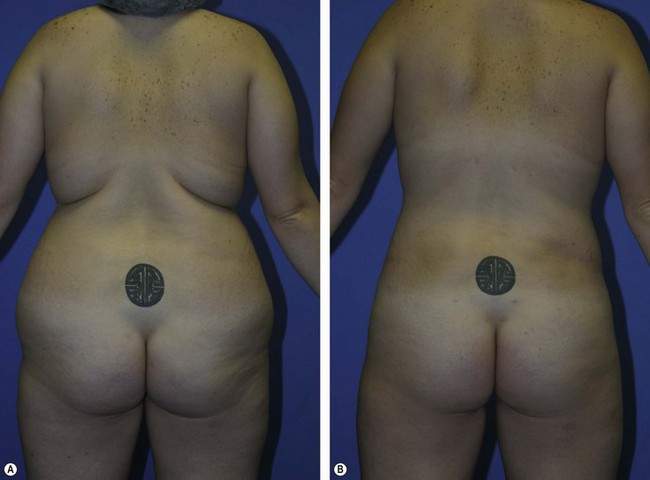
The first generation devices and the Mentor Contour Genesis are no longer on the market. The Lysonix device with second generation UAL technology currently remains available but, its use appears to be declining, leaving the third generation VASER as the most commonly used UAL technology at the present time. In 2002, Jewell et al11 published a multicentric, pilot clinical study using VASER-assisted lipoplasty (VAL) and reported good results without complications in 77 patients. The author12 reported significantly lower quantities of blood in VAL aspirate in comparison to SAL aspirate in 57 patients undergoing contouring of the posterior trunk (Fig. 50.6). A decrease in complications with third generation VAL when compared to early UAL technology was reported by de Souza Pinto.13 Currently, for these reasons, VAL appears to be supplanting UAL.
FIG 50.6 Appears ![]() ONLINE ONLY
ONLINE ONLY
Basic Physics of Ultrasonic Liposuction
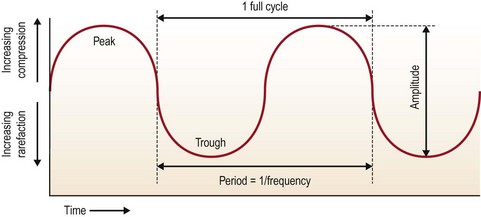
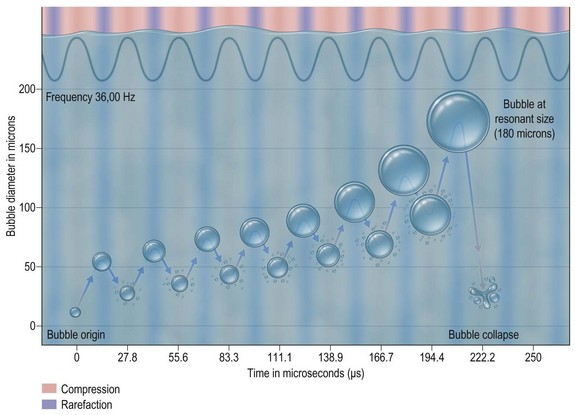
Sound is a vibratory form of mechanical energy which travels in waves, with peaks (compression) and troughs (rarefraction) (Fig. 50.7). Compression pushes tissue particles together and rarefraction pulls them apart. It is this alternating nature of sound waves that causes tissue particles to vibrate in place when exposed to ultrasound. Sound waves are measured by their frequency in Hertz (Hz). Ultrasound waves vibrate at frequencies higher than the sound detection limits of human hearing which is estimated to be about 18 kHz. Sound waves travel through media at speeds directly proportional to the density of the medium. For example, sound waves travel faster through bone and significantly slower through fat. Sound wave induced tissue fragmentation is inversely proportional to tissue density; thus fatty tissue fragments more readily in response to ultrasound energy than muscle or bone.
FIG 50.7 Appears ![]() ONLINE ONLY
ONLINE ONLY
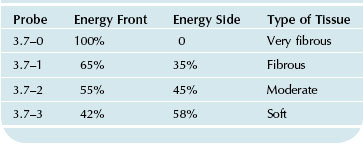
The UAL hand piece is designed to convert electrical energy into vibratory energy. Current UAL devices achieve this by means of piezoelectric crystals contained within the hand piece which expand and contract when exposed to alternating electrical current, thus producing vibrations. These vibrations are passed from the hand piece to the attached ultrasonic probe, which in turn vibrates in resonance with the hand piece. This longitudinal back and forth vibration occurs at the tip where third generation ultrasonic probes have an excursion of approximately 100 µm. Advances in ultrasonic probe design currently allow the third generation UAL devices to fragment fatty tissue at twice the efficiency rate of the earlier generation devices while decreasing the energy delivered to the tissues to approximately one half.14 In addition to the generator energy setting, the type of ultrasonic probe used determines how the energy is delivered to the tissues (Table 50.1). Selecting the proper combination for a given anatomical area allows the surgeon to apply the lowest amount of energy necessary to emulsify fat.
Ultrasound has three basic mechanisms by which it interacts with tissue: (1) thermal, (2) mechanical, and (3) cavitation. The thermal effect stems from the fact that rapidly vibrating ultrasonic probes generate heat. Although some thermal energy is passed on to the tissues, the net amount is relatively modest when there is good tissue dispersion of infiltrating solution, appropriate energy settings and proper movement of the ultrasonic probe within the tissues. Cimino15 has reported on the tissue thermal effects of ultrasonic devices. The mechanical effect is created when the rapidly vibrating tip of the ultrasonic probe contacts tissue. Although this effect has been well described by Cimino and Bond,16 it appears to play a minor role in fat emulsification when using the third generation devices.
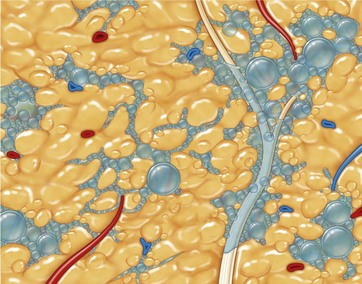
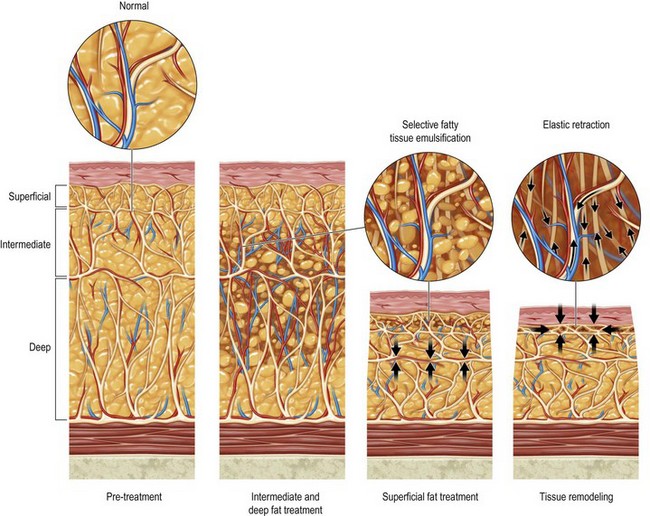
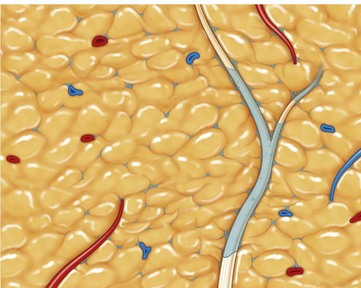
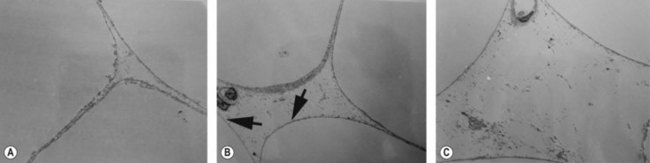
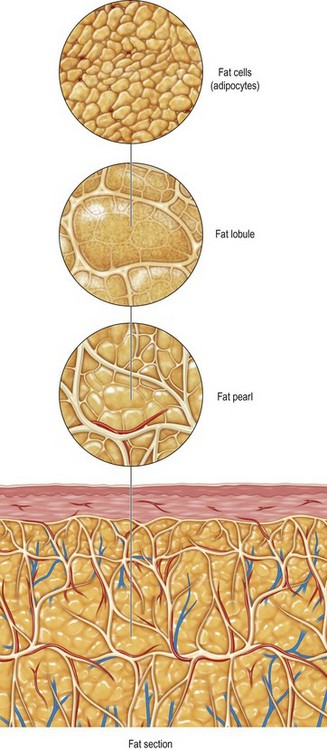
Cavitation is the tissue interaction most responsible for causing fat emulsification with the current UAL devices. When the wetting solution is dispersed within the tissues (Fig. 50.8), small microbubbles of approximately 5 to 10 µm are lodged within the fat tissue matrix (Fig. 50.9). Under the effects of ultrasound these microbubbles increase in size to 180 µm, at which point they implode and collapse (Fig. 50.10). This process separates fat cells within the fat tissue matrix (Fig. 50.11), which subsequently mix with the tumescent solution by means of acoustic streaming to create the emulsion (Fig. 50.12). Earlier theories regarding cavitation induced by UAL devices described an “implosion of the fat cell” with loss of cell membrane integrity and release of cellular contents. Current theories on UAL induced cavitation do not support the fat cell implosion theory. Cavitation occurs in the gas microbubbles not in the fat cells. Stable cavitation requires the gas containing microbubbles in the infiltrating solution. Fat cells do not contain gas, are not subject to ultrasonic induced cavitation and their cellular membranes are not disrupted during the cavitation process, as depicted in electron microscopy of third generation UAL aspirate (Fig. 50.13A, B, C). This explains why third generation UAL aspirate has been described as highly suitable for fat grafting,17 since it contains viable fat cells within the emulsion.18 Whereas fat harvested by SAL may contain fat lobules of approximately 50 or more cells (Fig. 50.14), the VAL harvested emulsion is comprised of smaller two to three viable fat cell clusters mixed with the tumescent solution. The loose nature of the fat matrix allows the microbubbles to easily intersperse within the fat cells for cavitation to occur, in contrast to the denser cellular structure of blood vessels, nerves and muscle tissue. This phenomenon forms the basis for the “tissue selectivity” of the third generation UAL devices (Fig. 50.15).
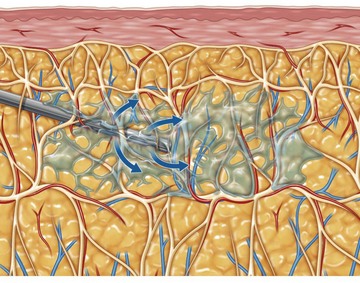
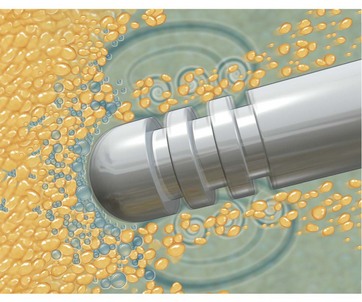
FIG. 50.8 The infusion cannula dispersing the wetting solution under pressure within the subcutaneous tissue.
FIG 50.9, 50.10, 50.13, 50.14 Appears ![]() ONLINE ONLY
ONLINE ONLY
Preoperative Preparation
Patient selection has always been of paramount importance in liposuction.19 During the early days of SAL, plastic surgeons soon learned that strict patient selection was essential in order to consistently achieve better esthetic results with fewer complications. This practice involved limiting the procedure to young, healthy patients with good skin tone, who were close to their ideal body weight and had well defined, localized areas of lipodystrophy.20 With the advent of wetting solutions, superficial liposuction techniques, UAL and currently VAL, plastic surgeons have been able to significantly expand the patient selection criteria for these procedures.21 Using third generation VAL, the author frequently performs high volume lipoplasty with acceptable results on healthy but significantly overweight patients with moderate skin tone and poorly defined fatty deposits (Fig. 50.16).
FIG 50.16 Appears ![]() ONLINE ONLY
ONLINE ONLY
Risk factors that could potentially lead to deep vein thrombosis (DVT) and pulmonary emboli (PE) should be carefully examined in these patients. Teimurian and Rogers22 reported an incidence of DVT of approximately 33/100 000 and PE of approximately 12/100 000 in a survey of over 75 000 major liposuctions. In a survey of members of the American Society for Aesthetic Plastic Surgery, Grazer and de Jong23 reported a mortality rate of 19/100 000 for patients undergoing liposuction, with approximately 25% of the deaths being the result of PE. In the author’s experience, the proper use of intermittent pneumatic compression boots coupled with early ambulation is associated with virtually zero DVT related complications in liposuction procedures. The protocol for patients at high risk for DVT during excisional body contouring procedures should include prophylactic doses of low molecular weight heparin (LMWH). Most surgeons do not use pharmacological DVT prophylaxis for routine liposuction procedures because of the complications related to excessive bleeding/bruising during the procedure.
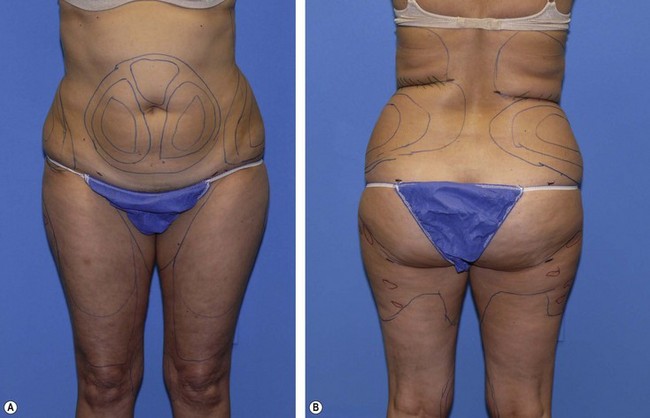
Preoperative markings are performed in the standing position. Waterproof markers of different colors are used to outline the specific areas planned for contouring, contour irregularities (marked in red) and incision sites [Fig 50.17]. The specific areas to be contoured will vary from patient to patient. However, there are five distinct anatomical areas that should be avoided in patients undergoing SAL or UAL. These areas, termed the “zones of adherence” (Fig. 50.18), include: the (1) gluteal crease, (2) lower lateral thigh area of the iliotibial tract, (3) posterior distal thigh above the popliteal crease, (4) mid-medial thigh area and (5) lateral gluteal depression area. Violation of these areas with either UAL probes or suction cannulae often leads to iatrogenic contour deformities. During the preoperative marking process, pay close attention to the placement of the access incisions. UAL requires a greater number of slightly longer access incisions to accommodate the skin protectors and avoid placing torque on the ultrasonic probe over curved anatomical areas. Whenever possible, the author prefers to mark the patient in the office the day before surgery, photograph the markings and review them with the patient. This procedure helps the patient have a better understanding of the surgical plan and avoids misunderstandings regarding the extent of the contouring or the placement of the incisions. Setting appropriate patient expectations preoperatively avoids having to manage them postoperatively.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree