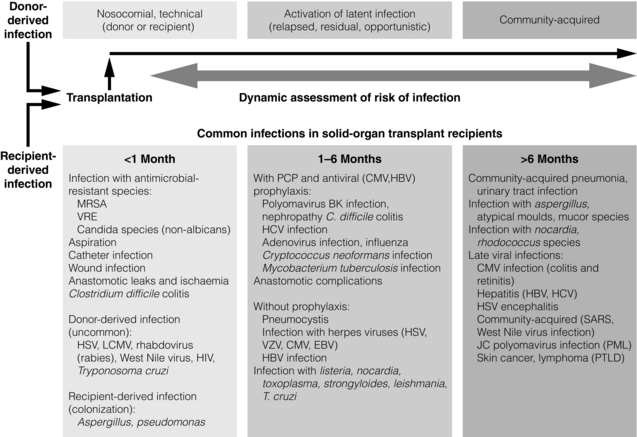
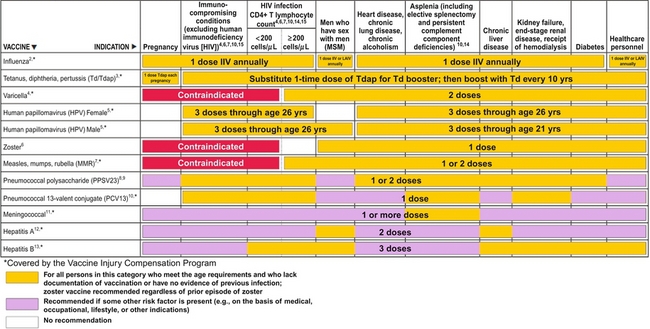
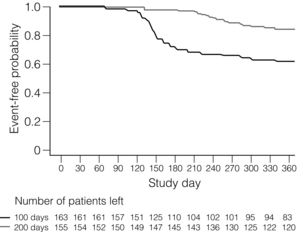
12 Infections are among the most common complications after transplantation, and greatly increase the morbidity and mortality of transplantation, while decreasing graft and patient survival. New infections occur from acquisition of infections in the hospital (i.e. nosocomial infections), from the organ transplant or blood product donor, or in the community. Reactivation of latent infections encompasses another significant number of infections. In general, the intensity of immunosuppression is considered highest for a year after solid-organ transplant, and for 2 years after hematopoietic stem cell transplant.1,2 Clinically focused guidelines on diagnosis, treatment and prevention of many infections after solid-organ transplants have recently been published by the Infectious Disease Community of Practice of the American Society of Transplantation.3 A timeline of infection after transplantation has been described (Fig. 12.1).1 In the first month after organ transplantation, infections tend to be related to the surgical procedure and hospital stay, and include wound infection, anastomotic leaks and ischaemia, aspiration pneumonia, catheter infection and Clostridium difficile colitis. In this population with frequent healthcare setting exposure, such infections are more likely to be due to resistant pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VRE) and non-albicans Candida. Donor-derived infections and recipient-derived infection, due to prior colonisation with agents such as Aspergillus or Pseudomonas, may present in this phase. Figure 12.1 Trends in the timings of infection after organ transplantation. Infections tend to occur in fairly predictable phases after solid-organ transplant. While many of the classic opportunistic infections occur in the first 6 months, during what is usually the period of most intense immunosuppression, the risk of such infection remains for the duration of time that the recipients are on immunosuppressive medications. The risk of infection is decreased by the use of prophylaxis, and augmented by the use of more potent immunosuppression (both in the induction and maintenance phases as well as during treatment of rejection), allograft rejection, concomitant infections, leucopenia, and technical surgical issues. HBV, hepatitis B virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; LCMV, lymphocytic choriomeningitis virus; MRSA, methicillin-resistant Staphylococcus aureus; PCP, Pneumocystis carinii pneumonia; PML, progressive multifocal leucoencephalopathy; PTLD, post-transplantation lymphoproliferative disorder; SARS, severe acute respiratory syndrome; VRE, vancomycin-resistant Enterococcus faecalis; VZV, varicella–zoster virus. Reproduced from Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–14. With permission from the Massachusetts Medical Society. Massachusetts Medical Society The stable and relatively healthy organ transplant recipient who is more than 6 months out from transplant tends to develop community acquired or routine infections, including urinary tract infections, upper respiratory infections and pneumonia, gastroenteritis and varicella zoster. Infections with unusual and opportunistic pathogens such as Aspergillus, unusual moulds, Nocardia and Rhodococcus are still seen. In the era of effective prophylaxis with valganciclovir, an increased risk of late cytomegalovirus (CMV; occurring more than 6 months after organ transplant) has been noted, primarily in the few months after prophylaxis has been stopped.4 Other late viral infections include polyomavirus infections (from BK, causing nephropathy primarily in renal transplant recipients, or JC, causing progressive multifocal leucoencephalopathy) and Epstein–Barr virus (EBV)-related post-transplant lymphoproliferative disease. Pre-transplant evaluation can help mitigate the risk of some infections, especially latent ones. Knowledge of serostatus for CMV, EBV, HBV and HCV can help optimise post-transplant management. Potential transplant recipients and donors are typically screened for latent tuberculosis, by history and sometimes by chest X-ray and either by skin testing or use of an interferon-gamma release assay-based blood test such as the T-SPOT.TB® or Quantiferon® TB Gold. Recipients from or in endemic regions should be screened for latent infections such as T. cruzi, Coccidioides and Strongyloides. Those seronegative for measles, mumps, rubella, hepatitis A and B, and varicella should undergo important pre-transplant vaccination, as some are with live viral vaccines that cannot be given after transplant when a recipient is significantly immunosuppressed,5 as shown in Table 12.1, leaving them potentially permanently vulnerable to potentially life-threatening infection(s). Table 12.1 Vaccines that might be indicated for adults, based on medical and other indications1—United States, 2012 Available online, www.cdc.gov/vaccines/schedules/downloads/adult/mmwr-adult-schedule.pdf, accessed December 17, 2012. The importance of donor-derived infections has been increasingly recognised in recent times. Such infection occurs in up to 1% of deceased donor organ transplants.6 While transmission of some infections is expected, such as CMV and EBV, others have been a surprise to clinicians caring for patients. Such unanticipated donor-derived infections range from viruses such as rabies, lymphocytic choriomeningitis and West Nile virus, to bacteria including bacteraemias and tuberculosis, fungi including cryptococcosis and histoplasmosis, and parasites including Trypanosoma cruzi (causing Chagas’ disease) and Strongyloides.6 Enhanced appreciation of donor-derived infections has resulted in better screening and diagnosis. The specific immunosuppressive agents used can potentially alter the risk of certain infections. For example, ciclosporin may have some anti-hepatitis C properties, and might be preferable to using tacrolimus in infected patients. Ciclosporin has been shown in vitro and in animals to have anti-schistosomal properties, especially with Schistosoma mansoni; this effect has never been confirmed in humans, but could affect risk of disease.7 Although data have been somewhat conflicting, the mammalian target of rapamycin (mTOR) inhibitors (sirolimus, everolimus) may have an impact on various viral infections, including human herpes virus 8 (HHV-8) and risk of developing Kaposi’s sarcoma,8 as well as the risk of developing EBV-mediated post-transplant lymphoproliferative disorder (PTLD) and active CMV. Whether there are mechanistic explanations, or this just reflects the net potency of the immunosuppression used, remains to be determined. Transplant clinicians should be aware of the long-term impact of the use of anti-T-cell cytolytic induction therapies such as alemtuzumab, thymoglobulin, OKT3 and others. While these may decrease the risk of rejection, they often result in a prolonged period of lymphopenia, often lasting many months to over a year or two,9,10 which may render the host more vulnerable to infection. When such therapies are used to treat organ rejection, clinicians should remember to restart prophylaxis therapies (i.e. antiviral, antibacterial, antifungal and antiparasitic) and reinitiate local screening protocols for BK, CMV and EBV viruses, and other infections. Numerous other types of viruses cause disease in transplant recipients. Respiratory viruses such as influenza, respiratory syncitial virus (RSV), adenovirus, parainfluenza and human metapneumovirus are common and may present more subtly or with fulminate disease. Hepatitis viruses (primarily B and C) are common causes for liver transplantation and can be common complications after transplant, predominantly as reactivation of latent infections; in addition, the primarily zoonotic hepatitis E has been reported as an emerging pathogen that may cause chronic hepatitis in transplant recipients.11 Most adults have latent infection with the polyoma viruses BK and JC, which can reactivate in the setting of immunosuppression, causing primarily kidney and brain disease, respectively. While BK is predominantly a pathogen in kidney transplant recipients, it can cause disease in other transplant recipients. Risk of BK reactivation relates directly to the intensity of the immunosuppression; early diagnosis of BK replication and subsequent reduction in the immunosuppressive regimen largely abrogates the risk of BK nephropathy, which generally has poor outcomes in kidney transplant recipients, with high rates of graft loss. JC virus causes progressive multifocal leucoencephalopathy, which is often mortal but fortunately fairly rare. A multicentre, retrospective cohort study of progressive multifocal leucoencephalopathy after solid-organ transplant found a median time to development of first symptoms of 27 months, with a median survival of 6.4 months, with a rough incidence of 0.1%.12 Numerous other viruses have been shown to cause disease in transplant recipients, including parvovirus B19, West Nile virus, lymphocytic choriomeningitis virus and others. Hundreds of patients with human immodeficiency virus (HIV) infection have undergone organ transplant, primarily kidney but also liver and other organs. In a large multicentre trial between November 2003 and June 2009, a total of 150 HIV-positive patients underwent kidney transplantation: patient survival rates at 1 and 3 years were 94.6 ± 2.0% and 88.2 ± 3.8%, respectively, while the corresponding graft survival rates were 90.4% and 73.7%.13 These outcomes fall somewhere in the national database between kidney transplant recipients who are 65 + years old and those reported for all kidney transplant recipients. Multivariate analysis showed that the risk of graft loss was increased among patients treated for rejection and those receiving antithymocyte globulin induction therapy, while living-donor transplants were protective. A higher than expected rejection rate was observed, with 1- and 3-year estimates of 31% and 41%, respectively. HIV infection remained well controlled, with stable CD4-positive T-cell counts and few HIV-associated complications. While some organ transplant centres use antiviral agents in certain cohorts of patients at risk for CMV (termed ‘universal prophylaxis’), others use ‘pre-emptive therapy’ where treatment is begun only when routine monitoring tests show evidence of active infection. Guidelines for optimal management of CMV after solid-organ transplantation have been published.4 CMV immunoglobulin and HBV and varicella hyperimmune globulins have been shown to be effective in preventing infection in certain settings, and repleting recipients with hypogammaglobulinaemia (often defined as < 400 IU/mL) with intravenous immunoglobulin can reduce their risk of infection.14 In addition, periodic monitoring has been shown to be helpful, as with CMV, EBV and BK viruses. The two methods of prevention each have their merits and disadvantages. While large, randomised trials have not been conducted, numerous studies suggest that universal prophylaxis may convey better outcomes compared with pre-emptive therapy, especially in the higher risk D +/R − population, as summarised in recent guidelines.4 Benefits of universal prophylaxis include lower drug cost, fewer opportunistic infections (including Kaposi’s sarcoma and PTLD), improved graft and patient survival, lower rates of rejection, easier logistics, and lower monitoring costs. Negatives include higher rates of late CMV, resistant virus, and higher drug costs and toxicities. Advantages of pre-emptive therapy include lower drug cost, reduced drug exposure, lower rates of late CMV (possibly due to enhanced immunological priming15), lower drug cost, reduced drug exposure and theoretically lower risk of resistant CMV due to lower rates of drug exposure. Disadvantages include lower rates of graft and patient survival, higher rates of opportunistic infections and more complex logistics (organising weekly testing for several months after transplant and managing results). Whether to initiate secondary chemoprophylaxis or viral monitoring after treatment of active CMV infection has not been well studied. Experts vary in their range of practice.4 Institutions should develop local protocols, based on previous clinical outcomes, use of cytolytic induction therapies, the net state of immunosuppression, type of organ(s) transplanted, costs, ability to do periodic testing and other factors. Perhaps some of the more compelling data come from a randomised clinical trial of oral ganciclovir prophylaxis (n = 74) versus pre-emptive therapy with intravenous ganciclovir (n = 74), where prophylaxis significantly increased long-term graft survival 4 years after transplant (92.2% vs. 78.3%; P = 0.0425).16 Patients with CMV donor seropositive/recipient seropositive (D +/R +) status had the lowest rate of graft loss following prophylaxis (0.0% vs. 26.8%; P = 0.0035), suggesting that perhaps the prophylaxis strategy can be tailored according to serostatus. Recent work demonstrated an unexpected and higher risk of antiviral resistance in those D +/R − renal recipients on pre-emptive therapy;17 prior work had shown an increased risk of resistance for those on universal prophylaxis, suggesting that perhaps both methods convey some risk of resistance. There has long been concern about the ‘direct’ and ‘indirect’ effects of CMV on transplant recipients. The direct effects, which are the manifestations of infection, are clinically obvious. The indirect effects are more insidious and may have negative impacts on both the organ graft outcome and the recipient, and include increased rates of bacterial, viral and fungal infections, more aggressive recurrent HCV after liver transplantation, higher rates of acute rejection and increased graft dysfunction and failure, chronic allograft nephropathy, vascular disease (coronary, aortic and transplant), cancer (especially PTLD) and diabetes.18 Costs are an important part of transplant care throughout the world, and may have a significant influence on choice of prevention method. Costs of serial testing (including personnel and laboratory monitoring costs) may be somewhat similar to the costs of medications (i.e. with ‘universal prophylaxis’) in some settings, although the net costs to the patient (i.e. co-payments for medication) or to the transplant programme or healthcare system may be different. Nonetheless, it is important to remember to focus on long-term outcomes and overall cost and benefit to the patient and to the programme. In one recent study, the incidence of CMV infection in seropositive kidney transplant recipients was 4.1% and 55.5% within the first year after transplant while under universal prophylaxis and pre-emptive therapy, respectively.19 Universal prophylaxis incurred $1464 more in direct cost compared with pre-emptive therapy, while saving $7309 in indirect cost, and resulted in a net gain of 0.209 in quality-adjusted life-years per patient over a 10-year period. Thus, universal prophylaxis resulted in a cost saving of $27 967 for 1 quality-adjusted life-year gained when compared with pre-emptive therapy, and the authors concluded that universal prophylaxis in CMV-seropositive kidney transplant patients is clinically effective and cost saving. Evidence from the Improved Protection Against CMV in Transplant (IMPACT) trial demonstrated that prolonged prophylaxis of 200 days with valganciclovir compared with 100 days significantly reduces the incidence of CMV in high-risk kidney transplant (D +/R −) recipients, as shown in Fig. 12.2.20 Subsequent researchers developed a cost-effectiveness model to evaluate prolonged prophylaxis of 200 days with valganciclovir in D +/R − kidney transplant recipients and its long-term economic impact from the US healthcare payer perspective.21 They found that for the 5-year time horizon, the incremental cost-effectiveness ratio of US $14 859/quality-adjusted life-year suggests that 200-day valganciclovir prophylaxis is cost-effective over the 100-day regimen considering a threshold of US $50 000 per quality-adjusted life-year. The 10-year analysis revealed the 200-day prophylaxis as cost saving with a 2380 quality-adjusted life-year gain (per 10 000 patients) and simultaneously lower costs. The authors concluded that prolonged prophylaxis with valganciclovir reduces the incidence of events associated with CMV infection in high-risk kidney transplant recipients and is a cost-effective strategy in CMV disease management. Another single-centre, retrospective study reached the same conclusion, that 6 months of prophylaxis in those who are CMV D +/R − was more cost-effective than 3 months, with an incremental cost of $34 362 and $16 215 per case of infection and disease avoided, respectively, and $8304 per one quality-adjusted life-year gained.22 Figure 12.2 From the IMPACT trial in D +/R − kidney transplant recipients, the Kaplan–Meier plot of time to cytomegalovirus disease up to month 12 post-transplant, comparing 100 versus 200 days of valganciclovir prophylaxis.20 Reproduced from Humar A, Lebranchu Y, Vincenti F et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 2010; 10(5):1228–37. With permission from John Wiley and Sons. EBV is mainly an issue for those who were seronegative before transplant and for those who are very potently immunosuppressed (lung, intestinal, composite tissue transplants). EBV replication increases the risk of PTLD, 90% of which are EBV mediated. An analysis of the SRTR National Registry Data in the United States found that the unadjusted hazard ratio (HR) for PTLD (if recipient EBV seronegative) was 5.005 for kidney transplant, 6.528 for heart transplant and 2.615 for liver transplant (P < 0.001 for all).23 Some centres screen periodically in the first year after transplant in EBV D +/R−, and reduce the immunosuppression with significant viraemia.24,25 In a recent series, 34 D +/R − adult renal transplant recipients at a single institution were prospectively monitored for EBV during the first year post-transplant.24 Twenty (60.6%) of the 34 recipients developed viraemia during the first year post-transplant, of which six were given rituximab and did not develop PTLD; of the six recipients who were not monitored, three (50%) developed PTLD and lost their grafts. It was concluded that recipients who were not monitored on the protocol were more likely to have PTLD and graft loss compared to those who were monitored (P = 0.008). There are no data to suggest that antiviral agents can prevent or decrease EBV viraemia; ganciclovir only works in the very small percentage of virus that is in the lytic phase. Belatacept, one of the newer immunosuppressive medications, is approved only for use in EBV-seropositive recipients, due to a higher risk of PTLD in seronegative recipients.26
Transplant infectious disease
Introduction and general concepts
Viruses: epidemiology, prophylaxis, diagnosis and treatment
Prophylaxis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Transplant infectious disease
Only gold members can continue reading. Log In or Register to continue




