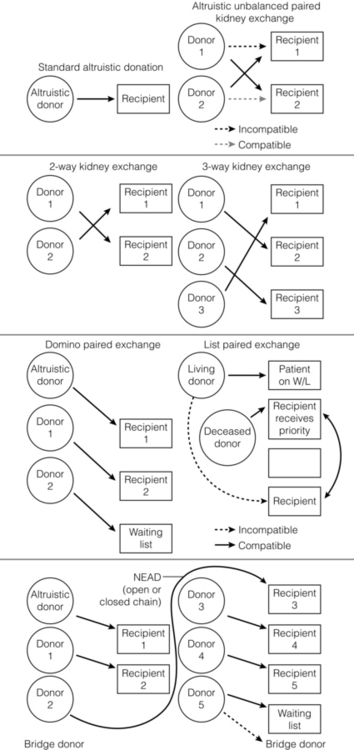
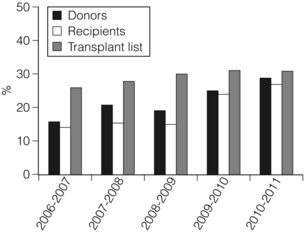
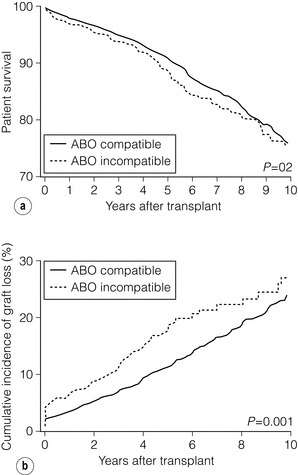
7 Renal transplantation is firmly established as the optimal form of renal replacement therapy. Despite significant improvements in dialysis, transplantation remains superior, both in terms of survival and quality of life.1 In fact, very few patients will not benefit from transplantation.2 And herein lies our problem, as nearly every patient who starts dialysis aspires to receive a renal transplant. An escalating demand for transplantation has been evident over the years, patients joining the transplant lists in higher numbers than ever before. The demographic trend to an ageing population in many countries has also led to an increase in the age of patients requiring transplantation. Currently in the UK, one-third of the waiting list patients are aged 60 or over and 25% of the transplants are performed in this age group (Fig. 7.1). A similar trend has been seen in the USA, where older patients represent the fastest growing group on the list. Figure 7.1 The prevalence of patients aged > 60 years old on the transplant waiting list and among transplant recipients in the UK (prevalence amongst donors shown for comparison). Unfortunately, the rise in demand means that not everybody will become a transplant candidate. Rationing policies (overt or covert) lead to a significant number of patients, particularly of older age, never joining the list. Despite the fact that some of the reasons are clear (comorbidity burden), there remains a wide practice variation in the evaluation of transplant candidacy,3 which inevitably leads to a well-documented inequity in access. Similarly, those patients who do join the waiting list require a more careful assessment to ensure their suitability for a transplant, in the current context of longer waiting times. Sadly, the increase in waiting time has been associated with an increase in waiting list mortality, which ranges from around 3% per 100 waiting-list years for young patients to around 9% for patients aged 65 or over (US data). These two intertwined factors have led to the inevitable question that perhaps the current demand may be artificially too high. Several studies have shown that some groups of patients (e.g. the elderly) will spend a disproportionate amount of time on the list and the chance of transplantation reduces dramatically with increasing waiting times. Furthermore, all patients over 60 years of age currently joining the waiting list have a 50% risk of dying on the list, without a kidney transplant.4 In an ideal world, these patients may well benefit from transplantation but, in reality, their hopes may be inappropriately raised by a listing decision.5 Altruistic donation (non-directed donation) has seen a surge following the reported success of spousal and unrelated living donor transplantation with ‘read across’ to stranger donation.6 This has introduced a whole new level of complexity and ethical dilemmas in the evaluation of the living donor. However, beyond the ethical challenges (motivation, nature of altruism, emotional benefit derived from donation), it is widely accepted that there are benefits in the practice of altruistic donation, as donors gain self-esteem and the society sees an increase in the donor pool. Altruistic donor kidneys are allocated to a matched recipient on the waiting list and this can be carried out by use of one of a number of models of allocation: donor-centric, recipient-centric and socio-centric allocation.7,8 In the donor-centric setting, the kidney is allocated to the recipient likely to have the best outcome, whilst in the recipient-centric scenario, the kidney is allocated to the patient with the greatest need. The socio-centric model treats the kidney as a national resource and is allocated as per national algorithm as for any deceased donor kidney. Kidney paired donation was initially proposed in 19869 in order to allow two incompatible donor–recipient pairs to exchange kidneys to allow two compatible transplants to take place. Donor–recipient incompatibility accounts for approximately 35–50% of pairs being declined transplantation,10,11 and represents a significant obstacle for further expansion of living donation. In order to increase the donor pool and the number of potential matches, several variations of paired donation have been developed. Paired donation may now include a three-way (or higher number) of exchanges, which although logistically more challenging make the finding of a matched pair easier. Another protocol involves the deceased donor waiting list (list paired donation), whereby the incompatible living donor donates to a recipient on the list and in return, their incompatible recipient acquires priority on the waiting list for a deceased donor.12 An alternative paired exchange protocol (altruistic unbalanced paired kidney exchange) includes compatible pairs in the match run.13,14 These pairs may participate in exchange schemes for medical reasons (finding a younger donor or a better match) or altruistic reasons (helping another incompatible pair). Although ethically complex, this approach could lead to a 10% increase in the number of transplants in a paired programme. Altruistic donation has been combined with the paired exchange programmes in order to increase the flexibility of the paired schemes and increase the potential donor pool for match runs. In a domino paired donation, the altruistic kidney donor initiates a chain of matches, by donating the kidney to the first recipient. The recipient’s incompatible donor then donates to the next compatible recipient, whose donor donates to the national waiting list, creating a domino effect.7 This scheme could be extended to include multiple centres and several incompatible pairs before finally transplanting a recipient on the deceased donor list.15 An alternative arrangement is the non-simultaneous extended altruistic donor (NEAD) chain.16 This consists of domino paired donation segments, except that the last donor in the first segment (‘bridge donor’) is asked to donate later and trigger another segment of the chain. The NEAD chain is a significant departure from the requirement of simultaneous donation in the paired exchange, but so far no chains have been broken due to a donor’s unwillingness to donate, once their incompatible recipient has been transplanted. This new approach raises the prospect of multiple transplants with shorter waiting times, but does require an entirely new level of logistics and cooperation, all within a delicate ethical environment. Paired exchange and altruistic donation options are summarised in Fig. 7.2. The graft loss was higher in the ABOi patients in the first 14 days post-transplant, with no significant difference beyond this time-point (Fig. 7.3a), indicating that a rapid increase in the isohaemagglutin in titres in the immediate postoperative period is likely to lead to graft loss. Interestingly, this study showed no difference in outcome between donor A1 (considered high-risk) and A2 (considered low-risk transplant) subtypes. Throughout the study, patient survival was comparable (Fig. 7.3b). Figure 7.3 Patient survival (a) and graft loss (b) after live-donor kidney transplantation, comparing ABO-incompatible (ABOi) recipients (dashed line) with ABO-compatible (ABOc)-matched controls (solid line). Reproduced from Montgomery JR, Berger JC, Warren DS et al. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation 2012; 93:603–9. With permission from Wolters Kluwer Health. The optimal protocol for immunomodulation is yet to be agreed. What is now generally accepted is that heavy immunosuppressive regimens and splenectomy are not required, as there is no increase in the risk of antibody-mediated rejection and graft loss.19 The removal/reduction of circulating antibodies is an essential part of the current protocols and the general aim is to achieve anti-ABO titres < 16. This can be achieved via plasmapheresis (PP) or double-filtration plasmapheresis. The latter has a plasma separator for the first filter and a plasma fractionator for the second filter, thus reducing the requirements for fluid replacement. An alternative approach is the use of immunoadsorption, using columns with synthetic ABO antigens. This method has a high capacity for antibody removal but is rather costly.20 Plasmapheresis may be required post-transplant to prevent the rebound of anti-A or anti-B titres until accommodation occurs. The number of post-transplant treatments is guided by the initial antibody titres (Table 7.1) and the risk of antibody-mediated rejection (AMR). Table 7.1 Indicative guide for the number of plasmapheresis treatments according to the initial antibody titre Treatment plans are individualised and the number of sessions is detailed after the transplant date is set. A suggested template includes PP performed every other day (with pre- and post-determination of antibody level), immunoglobulin administration with each session of PP and starting tacrolimus and mycophenolate mofetil (MMF) after the first PP session. Induction therapy and standard triple therapy (tacrolimus/MMF/steroids) is continued post- transplant. An alternative is immunoadsorption, intravenous immunoglobulin (IVIG), anti-CD20 and conventional immunosuppression, which achieves comparable results.20 One of the main concerns with these approaches is the risk of AMR. This requires careful post-transplant monitoring and prompt treatment. No optimal AMR treatment has emerged, but PP with IVIG and anti-CD20 appears to be favoured.21 Using a similar philosophy, transplantation in HLA-incompatible patients has become increasingly successful. The degree of sensitisation is determined by various methods (see Chapter 4). The single-antigen beads test is the most sensitive analysis and therefore detects the lowest strength antibody, which is usually most easily treated. At the other end of the spectrum, a positive cytotoxic crossmatch (least sensitive assay) identifies the highest antibody strength, which is most difficult to desensitise. Three approaches to desensitisation have been described. High-dose IVIG (2 g/kg) is effective for patients receiving living or deceased donor kidneys but requires infusion over a 4-month period to ensure good results.22 Plasmapheresis with low dose IVIG (with or without rituximab (anti-CD20)) leads to a more consistent desensitisation and lower humoral rejection rates, but is suitable only for living donor recipients.23 Finally, a combination of IVIG and rituximab treatment over a 4-week period appears to be effective in desensitising patients and allowing them to receive a living donor or even a deceased donor transplant.24 The decrease in DBD organs has been replaced by a resurgence of donation after cardiac death (DCD) and currently, in the UK, one-third of all kidney transplants are from DCD donors. This increase is an under-representation of the upsurge in real activity, as even in the most dynamic DCD programmes, only approximately 20–25% of referrals proceed to actual donation.25 The utilisation rate of DCD is highest in the UK (93%) compared with all other European countries.26 The recent revival of category II DCD donation is largely due to the success of the Spanish programmes.27 The fundamental difference between the current Spanish programmes and the earlier category II DCD programmes (such as the ones in Newcastle and Leicester in the UK) is the use of normothermic regional perfusion (NRP) to optimise the function of abdominal organs. The complex ethical questions facing category II DCD donation have been debated widely,28 but it is important to reiterate that an open and sensitive discussion, within the legal framework and moral environment of each individual country, is likely to resolve many of these issues and facilitate such programmes. The benefit is likely to be substantial.
Recent trends in kidney transplantation
Demand inflation or supply recession?
Innovations in living donation
Incompatible transplantation
ABO-incompatible transplantation
ABO antibody titre
Pre-transplant treatments
Post-transplant treatments
< 16
2
2
16
2
2
32
3
3
64
4
3
128
5–6
3
256
7–8
4
512
9–10
4
1024
10–12
4
> 1024
> 15
5
HLA-incompatible transplantation
Trends in deceased kidney donation
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree