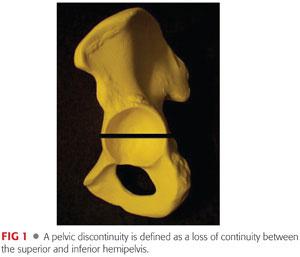
Pelvic discontinuity occurs when there is loss of continuity between the superior and inferior aspects of the pelvis as a result of disruption of both the anterior and posterior columns.
ANATOMY
 See FIG 1.
See FIG 1.
PATHOGENESIS
 Present in 0.9% of revision total hip arthroplasty procedures1
Present in 0.9% of revision total hip arthroplasty procedures1
 Bone loss leading to a pelvic discontinuity can be the result of osteolysis secondary to polyethylene wear particles in combination with migration of the acetabular component.
Bone loss leading to a pelvic discontinuity can be the result of osteolysis secondary to polyethylene wear particles in combination with migration of the acetabular component.
 Other causes include trauma, stress fractures, infection, iatrogenic fractures, and aggressive reaming in either primary or revision surgery.
Other causes include trauma, stress fractures, infection, iatrogenic fractures, and aggressive reaming in either primary or revision surgery.
 Risk factors include female sex, rheumatoid arthritis, previous radiation exposure, and massive pelvic bone loss.
Risk factors include female sex, rheumatoid arthritis, previous radiation exposure, and massive pelvic bone loss.
NATURAL HISTORY
 A pelvic discontinuity leads to motion between the superior and inferior hemipelvis. Pelvic discontinuities rarely heal without surgical intervention.1,12
A pelvic discontinuity leads to motion between the superior and inferior hemipelvis. Pelvic discontinuities rarely heal without surgical intervention.1,12
PATIENT HISTORY AND PHYSICAL FINDINGS
 History should include all prior hip operations performed; reason for revision; and details of the surgery, outcomes, and complications.
History should include all prior hip operations performed; reason for revision; and details of the surgery, outcomes, and complications.
 It is particularly important to obtain information about prior surgeries, with particular attention paid to risk factors for infection such as prolonged wound drainage.
It is particularly important to obtain information about prior surgeries, with particular attention paid to risk factors for infection such as prolonged wound drainage.
 The patient should be queried regarding a history of trauma.
The patient should be queried regarding a history of trauma.
 Obtaining prior operative reports is paramount; information including the approach used, surgical findings, and details of the prosthesis in situ should be sought.
Obtaining prior operative reports is paramount; information including the approach used, surgical findings, and details of the prosthesis in situ should be sought.
 Pain can often be quite significant, especially in acute discontinuities, and usually is located in the groin but may also present in the buttock or thigh.
Pain can often be quite significant, especially in acute discontinuities, and usually is located in the groin but may also present in the buttock or thigh.
 A history of unexplained fevers and rest pain should alert the physician to the possibility of infection.
A history of unexplained fevers and rest pain should alert the physician to the possibility of infection.
 The neurovascular status of the extremity should be assessed and documented. Patients with vascular compromise should be referred to vascular specialists for evaluation.
The neurovascular status of the extremity should be assessed and documented. Patients with vascular compromise should be referred to vascular specialists for evaluation.
 Abductor function should be assessed. Nonfunction of the abductors may occur from disruption of the abductors; a shortened lever arm secondary to superior migration; and/or medialization of the acetabular component, superior gluteal nerve injury, or pain inhibition.
Abductor function should be assessed. Nonfunction of the abductors may occur from disruption of the abductors; a shortened lever arm secondary to superior migration; and/or medialization of the acetabular component, superior gluteal nerve injury, or pain inhibition.
 Leg lengths should be measured and recorded. The patient should be counseled regarding postoperative leg length inequality and reasonable expectations.
Leg lengths should be measured and recorded. The patient should be counseled regarding postoperative leg length inequality and reasonable expectations.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Pelvic discontinuity can be identified on plain radiographs; however, it can be unrecognized preoperatively and one must maintain an index of suspicion with certain bone loss patterns. Paprosky et al13 noted, in a series of 147 patients with discontinuity, that 11% were diagnosed at operation and not detected by radiographs.
Pelvic discontinuity can be identified on plain radiographs; however, it can be unrecognized preoperatively and one must maintain an index of suspicion with certain bone loss patterns. Paprosky et al13 noted, in a series of 147 patients with discontinuity, that 11% were diagnosed at operation and not detected by radiographs.
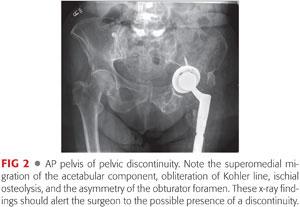
 Anteroposterior (AP) pelvis (FIG 2) radiographic findings suggestive of pelvic discontinuity include the following:
Anteroposterior (AP) pelvis (FIG 2) radiographic findings suggestive of pelvic discontinuity include the following:
A visible fracture line or bone defect that includes both columns of the acetabulum
A medial shift or rotation of the inferior hemipelvis in relation to the superior hemipelvis
Asymmetry of the obturator foramen in a well-centered radiograph
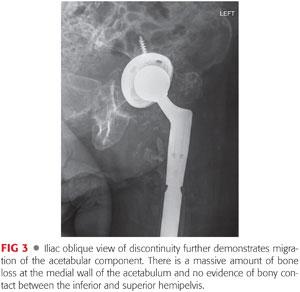
 Iliac oblique (FIG 3) and false-profile views of Lequesne should also be obtained to increase sensitivity for the detection of discontinuity as opposed to a single AP view of the pelvis.24
Iliac oblique (FIG 3) and false-profile views of Lequesne should also be obtained to increase sensitivity for the detection of discontinuity as opposed to a single AP view of the pelvis.24
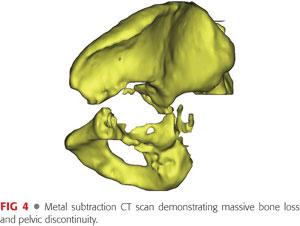
 Computed tomography (CT) studies with a metal artifact suppression technique can be useful in confirming the presence of a discontinuity, further defining the extent of bone loss, aiding in preoperative planning, and for the design of custom implants, if needed (FIG 4).
Computed tomography (CT) studies with a metal artifact suppression technique can be useful in confirming the presence of a discontinuity, further defining the extent of bone loss, aiding in preoperative planning, and for the design of custom implants, if needed (FIG 4).
 For components that have migrated medial to Kohler line, contrast CT can define the proximity of the intrapelvic contents to the implant.
For components that have migrated medial to Kohler line, contrast CT can define the proximity of the intrapelvic contents to the implant.
 Patients should be screened for infection with serology (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]). If either ESR or CRP is significantly elevated, image-guided aspiration should be performed to obtain synovial white blood cell count, differential, and culture.
Patients should be screened for infection with serology (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]). If either ESR or CRP is significantly elevated, image-guided aspiration should be performed to obtain synovial white blood cell count, differential, and culture.
DIFFERENTIAL DIAGNOSIS
 Pelvic discontinuity can occur from aseptic loosening, osteolysis as a result of particulate wear, iatrogenic damage, trauma, infection, or tumor.
Pelvic discontinuity can occur from aseptic loosening, osteolysis as a result of particulate wear, iatrogenic damage, trauma, infection, or tumor.
NONOPERATIVE MANAGEMENT
 Can be used in cases of massive bone loss, resulting in a defect that cannot be reconstructed
Can be used in cases of massive bone loss, resulting in a defect that cannot be reconstructed
 Also useful in cases where there is an unacceptable surgical risk
Also useful in cases where there is an unacceptable surgical risk
 Conservative management includes walking aids and/or wheelchairs for mobility, shoe modifications for leg length discrepancy, and analgesia medications.
Conservative management includes walking aids and/or wheelchairs for mobility, shoe modifications for leg length discrepancy, and analgesia medications.
SURGICAL MANAGEMENT
 The goals of surgical management of a pelvic discontinuity are as follows:
The goals of surgical management of a pelvic discontinuity are as follows:
Stabilization of the hemipelvis
Achievement of stable acetabular component fixation
Restoration of hip biomechanics
Appropriate soft tissue balancing
Leg length optimization
Preoperative Planning
 Treatment and prognosis differs depending on the type and degree of bone loss and the length of time the discontinuity has been present.
Treatment and prognosis differs depending on the type and degree of bone loss and the length of time the discontinuity has been present.
Acute (healing potential)
Chronic (reduced healing potential)
 An AP of the pelvis can be used to define the extent of the majority of the bony defects. There are four factors that help classify acetabular bone loss:
An AP of the pelvis can be used to define the extent of the majority of the bony defects. There are four factors that help classify acetabular bone loss:
Presence and degree of superior migration of the hip center
Presence and degree of ischial osteolysis
Presence of “teardrop” osteolysis
Position of the acetabular component in relation to Kohler line
 These factors allow the surgeon to anticipate the degree and location of bone loss as well as plan the reconstruction.
These factors allow the surgeon to anticipate the degree and location of bone loss as well as plan the reconstruction.
 The Paprosky classification is one of the more commonly used classification systems and is useful to define bone loss and guide subsequent reconstruction.13
The Paprosky classification is one of the more commonly used classification systems and is useful to define bone loss and guide subsequent reconstruction.13
 The Paprosky classification does not have a specific pelvic discontinuity subclassification; however, the majority of pelvic discontinuities are Paprosky type IIIB, in which radiographs demonstrate extensive ischial osteolysis and obliteration of the teardrop, more than 3 cm of superomedial migration of the acetabular component, and a break in Kohler line. There is typically less than 50% of host bone available for osseointegration.
The Paprosky classification does not have a specific pelvic discontinuity subclassification; however, the majority of pelvic discontinuities are Paprosky type IIIB, in which radiographs demonstrate extensive ischial osteolysis and obliteration of the teardrop, more than 3 cm of superomedial migration of the acetabular component, and a break in Kohler line. There is typically less than 50% of host bone available for osseointegration.
 Several surgical techniques for dealing with pelvic discontinuity have been reported. The technique of choice is based on the degree of bone loss, the chronicity of the discontinuity, and cost.
Several surgical techniques for dealing with pelvic discontinuity have been reported. The technique of choice is based on the degree of bone loss, the chronicity of the discontinuity, and cost.
 Intrapelvic hardware may require a retroperitoneal approach to extract the hardware and avoid iatrogenic damage to vital structures.
Intrapelvic hardware may require a retroperitoneal approach to extract the hardware and avoid iatrogenic damage to vital structures.
 Surgical techniques include the following:
Surgical techniques include the following:
Posterior column plating and a cementless acetabular component with or without porous metal augments/structural bone graft
Acetabular distraction
The use of an off-the-shelf reconstruction cage with or without porous metal augments/structural bone allograft with or without posterior column plating
The use of a triflange prosthesis (custom)
The use of cup–cage constructs
Positioning
 The patient should be positioned according to surgeon preference and in accordance with standard surgical principles.
The patient should be positioned according to surgeon preference and in accordance with standard surgical principles.
 Draping should allow for extensile exposure, allowing for the possible need for a retroperitoneal approach in case of a vascular complication.
Draping should allow for extensile exposure, allowing for the possible need for a retroperitoneal approach in case of a vascular complication.
Approach
 The approach is chosen based on the surgeon’s preference but may also be dictated by the pattern of the bone loss and reconstructive plan.
The approach is chosen based on the surgeon’s preference but may also be dictated by the pattern of the bone loss and reconstructive plan.
 The acetabulum, including the ilium, ischium, and pubis, can be adequately exposed through the following approaches:
The acetabulum, including the ilium, ischium, and pubis, can be adequately exposed through the following approaches:
Posterior
Direct lateral (Hardinge)
Transtrochanteric
 A posterior approach may facilitate exposure of the posterior column for plating.
A posterior approach may facilitate exposure of the posterior column for plating.
 A trochanteric slide or extended trochanteric osteotomy may be helpful to maneuver the femur out of the way.
A trochanteric slide or extended trochanteric osteotomy may be helpful to maneuver the femur out of the way.
TECHNIQUES
 Posterior Column Plating and a Cementless Acetabular Component with or without Porous Metal Augments and/or Structural Allograft
Posterior Column Plating and a Cementless Acetabular Component with or without Porous Metal Augments and/or Structural Allograft
Plating may be more useful in acute as opposed to chronic discontinuity.
Plating relies on the ability of the bone to heal, but in the case of a chronic discontinuity with a large amount of bone loss and an unfavorable biologic environment, nonunion is a common outcome.1
Open reduction and internal fixation of the posterior column is performed primarily.
Acetabular reamers are placed in position to restore the hip center. The reamer/trial should achieve two points of fixation (anterior to posterior, anteroinferior to posterosuperior, or anterosuperior to posteroinferior).
Porous metal augments or structural allograft may be used to restore a rim to enhance press-fit fixation. The location and orientation of the augments depends on the pattern of the bone loss. The available size and shape of porous metal augments allows for significant flexibility. The augments may be applied either before or after the acetabular component is implanted. To reduce fretting between the augments and the acetabular component, it is recommended that the interface between the two be fixed with bone cement.
Particulate bone graft may then be placed in any remaining crevices or defects.
Screws are recommended to supplement acetabular component fixation.
 Acetabular Distraction
Acetabular Distraction
Acetabular distraction is an option in the presence of chronic pelvic discontinuity. In chronic discontinuity, there is often both poor bone stock and an inadequate biologic environment for bone healing, leading to high failure rates of internal fixation and cage techniques.7,14,16
The principle of acetabular distraction depends on the elastic nature of the pelvis. The discontinuity is distracted and the elasticity of the pelvis provides some intrinsic stability of the component that is wedged between the superior and inferior hemipelvis. Osseointegration then provides long-term fixation of the component.16
All fibrous tissue is removed from the acetabulum to reveal bleeding, viable bone.
Hemispherical reamers are placed in the acetabulum in a position to restore the native hip center. The reamers are sequentially increased in size until contact is made between the superior and inferior hemipelvis. It is important to avoid aggressive reaming as the goal is not to remove bone but achieve two-point fixation. This provides for initial stability of the component and stabilization of the discontinuity.
Porous metal augments or structural allograft may be helpful to fill any large cavitary defects or augment initial stability of the acetabular component.
Particulate bone graft may be used to fill cavitary defects as well.
It is recommended to use bone cement to fix the interface of the porous metal augment and the acetabular component to avoid fretting.
A porous metal acetabular component 6 to 8 mm larger than the last reamer is chosen and impacted into place by positioning the inferior aspect of the component against the ischium. The implant is then rotated into proper position while keeping the inferior aspect of the component secured against the ischium. The oversized implant generates a distracting force against the elasticity of the pelvis and creates initial stability (TECH FIG 1).
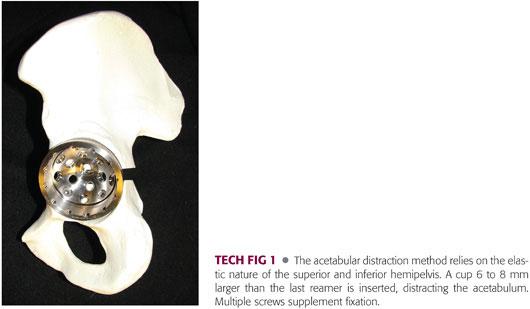
Supplemental fixation with multiple screws is recommended.
A polyethylene component can be cemented into the shell. Cementation allows the screws to function as a fixed-angle device, aiding the stability of the construct.
 Off-the-Shelf Reconstruction Cages with or without Porous Metal Augments/Structural Bone Allograft with or without Posterior Column Plating
Off-the-Shelf Reconstruction Cages with or without Porous Metal Augments/Structural Bone Allograft with or without Posterior Column Plating
Cages are relatively affordable and easily available.
These are large metallic cups that have malleable flanges with screw holes for fixation in the ilium and ischium (TECH FIG 2A).
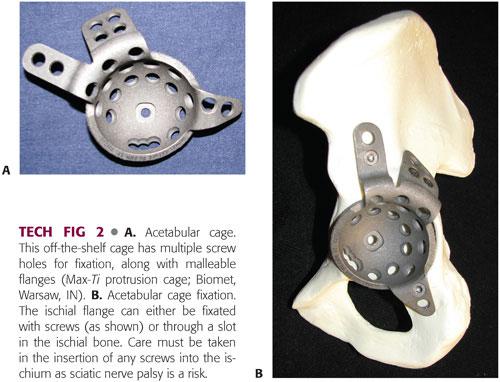
Cages are often used in conjunction with bone allograft or posterior plating and serve to mechanically protect the construct while incorporation of the allograft and/or healing of the discontinuity occurs.
The cages can also serve as internal fixation devices, especially in acute discontinuity.
Potential disadvantages include the following:
Limited or no osseointegration potential
Malleable flanges predispose the construct to fatigue failure.
Predetermined shapes and sizes do not readily conform to the host bone, creating increased areas of strain and additional intraoperative work.
The acetabulum must be exposed to allow visualization of the ilium, ischium, inferior cotyloid fossa, and remainder of the columns.
Care must be taken when exposing the ilium so as not to endanger the superior gluteal nerve and artery. The deep branch of the superior gluteal artery and superior gluteal nerve traverse deep to the gluteus medius, roughly 4 to 6 cm superior to the acetabular rim.
All fibrous tissue should be removed from the acetabulum.
If structural allograft is required, it should be prepared at this time.
The remaining ilium, which provides support to the allograft, is identified.
Acetabular reamers are used to size the extent of the cavity and help identify the amount and position of potentially supportive bone.
The structural allograft is prepared to provide support for the superior aspect of the cage.
The allograft is secured to the ilium using multiple large fragment screws with washers.
A porous metal augment can be used in the same fashion as allograft.
An appropriately sized cage is chosen to fit the reconstructed acetabulum and bridge the ilium and ischium, thereby protecting the allograft/augment.
The flanges can be bent so as to ensure maximum bone contact. Care should be taken to minimize the number of times that the flange is bent as excessive bending results in metal fatigue and leads to early failure.
The ischial flange can be fixated either by the use of a slot in the ischium or with a screw. Slotted fixation is safer as there is an increased rate of screw fracture associated with migration of the cage and sciatic nerve palsy.2,7
To create the slot for the ischial flange, the ischium needs to be exposed. Care must be taken to protect the sciatic nerve during this maneuver due to its close proximity.
A drill is used to place a hole posteroinferiorly in the optimum direction for the flange. This hole is widened with a small osteotome to allow the inferior flange to slot into the bone.
Ischial fixation is paramount to avoid cage failure.
The ilial flange is secured with multiple bicortical screws. Care must be taken to avoid intrapelvic penetration of drills or screws. Dome screws in the acetabular portion of the cage are recommended. There may be limited bone available for screw fixation (TECH FIG 2B).
A polyethylene liner can then be cemented into the cage. Care must be taken to achieve correct inclination and version.
 Custom Triflange Component
Custom Triflange Component
The custom triflange component is most often used in type IIIB defects with an associated pelvic discontinuity.
The advantages of the custom triflange over the noncustom cage are as follows:
Potential improved conformity with host bone
Greater construct rigidity and less chance of fatigue failure
Porous ongrowth surface to allow for osseointegration (some noncustom cages have limited coating as well)
The disadvantages of the triflange include the following:
Limited availability; requirement for advanced imaging, manufacture time, and expense
Inability to modify the cage intraoperatively
The goal of acetabular reconstruction with the triflange cage is to achieve initial stable fixation through intimate contact between structural host bone and the ilial, ischial, and pubic flanges augmented with multiple screws.
The design of the triflange component is a customized process based on a metal subtraction, implant-specific CT scan sequence (TECH FIG 3A).
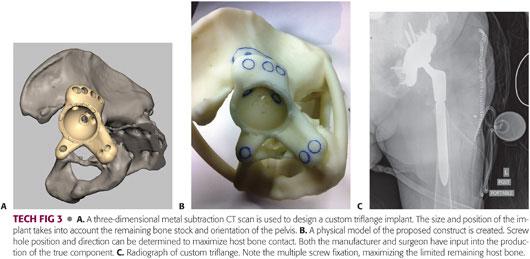
A three-dimensional computer generated one-to-one physical model of the patient’s hemipelvis is created (TECH FIG 3B).
From the remaining landmarks (ilium, obturator foramen, and pubic ramus), the patient’s hip center, cup orientation, and flange geometry are determined. A minor amount of host bone may need to be removed to allow the implant to seat appropriately. This is detailed on the model.
Screw hole positioning in the cup and flanges is determined and is available in either standard or locking configurations.
The exposure of the acetabulum is similar to that previously mentioned. All fibrous tissue in the remaining acetabular bone is removed. The iliac wing and ischium need to be adequately visualized to ensure the flanges are seated. As mentioned, a small amount of host bone may need to be removed to allow the implant to seat appropriately.
Bone allograft can be placed at this point.
The ischial flange is placed on the surface of the ischium and secured with screws. Care must be taken to avoid iatrogenic damage to the sciatic nerve.
It is recommended that the first ilial screw be nonlocking, pulling the flange into intimate contact with the host bone, helping reduce the discontinuity, and rotating the inferior half of the hemipelvis into the correct orientation. Pubic screws are then placed (TECH FIG 3C).
 Cup–Cage Construct
Cup–Cage Construct
The rationale for the cup–cage construct is to use the ilioischial cage to provide initial stability to a pelvic discontinuity and to protect a porous metal acetabular component from mechanical forces, allowing for biologic stabilization from both the superior and inferior aspects of the hemipelvis into the porous metal component. This biologic fixation gives the construct its long-term stability.
Exposure and confirmation of the discontinuity are the same as for the previous techniques.
Exposure of the ilium should be performed with care to avoid damage to the superior gluteal nerve and artery.
The acetabulum is reamed sequentially until two-point contact is made and bleeding bone is seen (usually anterosuperior and posteroinferior). This is similar to the technique described in the posterior column plating and cementless acetabular component section earlier. However, this technique is only used in cases with more extensive bone loss.
Particulate bone graft is packed into the defects and reverse reamed. Care should be taken to ensure that the pelvis is not breached.
The acetabulum is sized for the appropriate porous metal acetabular component. It is preferred to use a nonmodular porous metal component to allow for the cup portion of the off-the-shelf cage to fit inside the porous metal acetabular component. The cage then spans the defect from ilium to ischium.
The porous metal component is impacted and supplemental fixation is achieved with screws. If necessary, new drill holes can be made in the porous metal cup adjacent to bone (TECH FIG 4A).

If press-fit cannot be achieved due to the severity of the bone loss, or if a large portion of the superior cup is uncovered, a porous metal augment may be placed to supplement fixation and act as a buttress.
The cage is then placed in position, ensuring bony contact with both the ilial and ischial flanges using the techniques described earlier. Additionally, screws are used to transfix the cage through the porous metal cup (TECH FIG 4B).
The polyethylene component is cemented into the cage. The cement needs to be pressurized so that any gaps between the cage and the trabecular metal acetabular component are eliminated. This reduces micromotion between the hemispherical parts of the cage and the acetabular component.
PEARLS AND PITFALLS | |
Preoperative planning is invaluable. |
|
Adequate exposure is paramount. |
|
The fibrous interface must be removed from the acetabulum. |
|
It is critical to obtain rigid fixation. |
|
Spatial awareness is essential. |
|
Plan for the worst. |
|
POSTOPERATIVE CARE
 Depending on the construct, weight bearing may be restricted for 6 to 12 weeks. Gradual increase in weight bearing is then permitted. The degree and duration of weight bearing is individual according to bone defect, bone quality, and surgeon preference.
Depending on the construct, weight bearing may be restricted for 6 to 12 weeks. Gradual increase in weight bearing is then permitted. The degree and duration of weight bearing is individual according to bone defect, bone quality, and surgeon preference.
 We recommend hip precautions during the acute postoperative period secondary to the large surgical dissection usually required. Hip precautions are specific to approach.
We recommend hip precautions during the acute postoperative period secondary to the large surgical dissection usually required. Hip precautions are specific to approach.
 Patients are followed at routine intervals after surgery. The authors prefer follow-up at 6 weeks, 3 months, 6 months, and annually.
Patients are followed at routine intervals after surgery. The authors prefer follow-up at 6 weeks, 3 months, 6 months, and annually.
OUTCOMES
 There is no perfect solution for to the difficult problem of a pelvic discontinuity, especially in the situation where there is a large bone stock deficiency. Each discontinuity has its own “personality,” and no one solution can be used for all types. The outcomes of surgical treatment of pelvic discontinuity include the following:
There is no perfect solution for to the difficult problem of a pelvic discontinuity, especially in the situation where there is a large bone stock deficiency. Each discontinuity has its own “personality,” and no one solution can be used for all types. The outcomes of surgical treatment of pelvic discontinuity include the following:
A high rate of complications
Early and late implant failure
Failure to consistently restore bone stock
 Compression plating of the posterior column with 3.5-mm reconstruction plates, supplemented by porous metal shell in the acute fracture setting, has seen relatively positive results, with survivorship at 18 to 36 months of up to 100%.15,18,20 This suggests that, in acute discontinuity, the bone does indeed possess fracture healing potential.
Compression plating of the posterior column with 3.5-mm reconstruction plates, supplemented by porous metal shell in the acute fracture setting, has seen relatively positive results, with survivorship at 18 to 36 months of up to 100%.15,18,20 This suggests that, in acute discontinuity, the bone does indeed possess fracture healing potential.
 Chronic pelvic discontinuities have traditionally had high failure rates (14% to 50%), particularly in the presence of Paprosky type IIIB bone loss.1,5,7,9,14 This failure is largely believed to occur due to nonunion.
Chronic pelvic discontinuities have traditionally had high failure rates (14% to 50%), particularly in the presence of Paprosky type IIIB bone loss.1,5,7,9,14 This failure is largely believed to occur due to nonunion.
 Noncustom off-the-shelf acetabular cages are designed to bridge the defect. They are often used in conjunction with large structural allografts to help restore bone stock. Osseointegration potential is often limited to none, and failure due to micromotion and fatigue occur. Failure rates of 50% to 60% have been reported due to mechanical loosening or fatigue failure of the flange.7,16,25 This usually occurs in the first 18 months after implantation and is evident on radiographs. Typically, the failure pattern is loosening of the ischial screws and disengagement of the ischial flange. The addition of posterior column plates or structural allograft does not seem to improve the results. In a series by Goodman et al7 of 10 pelvic discontinuities fixed with bulk allograft and a support cage, 5 were unsuccessful. Three of these became loose, two continued to have a pelvic dissociation, two flanges fractured, and three patients experienced hip instability.
Noncustom off-the-shelf acetabular cages are designed to bridge the defect. They are often used in conjunction with large structural allografts to help restore bone stock. Osseointegration potential is often limited to none, and failure due to micromotion and fatigue occur. Failure rates of 50% to 60% have been reported due to mechanical loosening or fatigue failure of the flange.7,16,25 This usually occurs in the first 18 months after implantation and is evident on radiographs. Typically, the failure pattern is loosening of the ischial screws and disengagement of the ischial flange. The addition of posterior column plates or structural allograft does not seem to improve the results. In a series by Goodman et al7 of 10 pelvic discontinuities fixed with bulk allograft and a support cage, 5 were unsuccessful. Three of these became loose, two continued to have a pelvic dissociation, two flanges fractured, and three patients experienced hip instability.
 The relatively poor results of using noncustom cages and the lack of biologic ingrowth into the supporting structures led to the use of custom triflange components. Their increased mechanical stability and porous ongrowth surface make them an attractive option. There are reports of success with this technique.3,4,22 Taunton et al22 described a large series of 57 patients treated with a custom triflange. At a minimum follow-up of 2 years, only 1 of the 57 (1.7%) had signs of loosening and 81% had radiographic evidence of stable components with a healed pelvic discontinuity. At an average of 10 years follow-up, DeBoer et al4 demonstrated healing of the discontinuity in 18 of 20 hips. These results are encouraging. However, expense and the time to create the models for implants are drawbacks to routine use of these constructs.
The relatively poor results of using noncustom cages and the lack of biologic ingrowth into the supporting structures led to the use of custom triflange components. Their increased mechanical stability and porous ongrowth surface make them an attractive option. There are reports of success with this technique.3,4,22 Taunton et al22 described a large series of 57 patients treated with a custom triflange. At a minimum follow-up of 2 years, only 1 of the 57 (1.7%) had signs of loosening and 81% had radiographic evidence of stable components with a healed pelvic discontinuity. At an average of 10 years follow-up, DeBoer et al4 demonstrated healing of the discontinuity in 18 of 20 hips. These results are encouraging. However, expense and the time to create the models for implants are drawbacks to routine use of these constructs.
 Failure of allograft and autograft bone grafting techniques have driven surgeons to find other materials, such as porous metal, that can span the discontinuity and provide internal fixation to the superior and inferior hemipelvic fragments. Surgeons have used porous metal to span the superior and inferior aspects of the pelvis (cup–cage construct or distraction) with some success.4,10,15,17,19,22
Failure of allograft and autograft bone grafting techniques have driven surgeons to find other materials, such as porous metal, that can span the discontinuity and provide internal fixation to the superior and inferior hemipelvic fragments. Surgeons have used porous metal to span the superior and inferior aspects of the pelvis (cup–cage construct or distraction) with some success.4,10,15,17,19,22
 The cup–cage technique, which was first described by Hanssen and Lewallen,8 does not try to restore bone stock with bone but porous metal. The cage is seen as a temporary fixation measure, whereas the trabecular metal cup achieves union with both the superior and inferior aspects of the pelvis, giving it long-term stability. Midterm results have been generally positive. Kosashvili et al10 reported no component migration in 88.5% of their 26 cases at a mean follow-up of 44 months. Rogers et al15 reported an 86.3% survivorship rate at 8 years in nine patients.
The cup–cage technique, which was first described by Hanssen and Lewallen,8 does not try to restore bone stock with bone but porous metal. The cage is seen as a temporary fixation measure, whereas the trabecular metal cup achieves union with both the superior and inferior aspects of the pelvis, giving it long-term stability. Midterm results have been generally positive. Kosashvili et al10 reported no component migration in 88.5% of their 26 cases at a mean follow-up of 44 months. Rogers et al15 reported an 86.3% survivorship rate at 8 years in nine patients.
 In the presence of a pelvic discontinuity, achieving initial stability of a trabecular metal cup can be difficult because of the relative motion between the two segments of the acetabulum and the degree of bone loss. An alternative technique, which has gained recent favor, is acetabular distraction, first described by Sporer et al17 In their original series, 19 of 20 patients were radiologically stable at an average of 4 years follow-up and 17 of these were reported to be pain free. Four of the original 20 did have some early component migration that later became stable and asymptomatic. The majority in this series (13) were hips with a Paprosky type IIIB bone defect.
In the presence of a pelvic discontinuity, achieving initial stability of a trabecular metal cup can be difficult because of the relative motion between the two segments of the acetabulum and the degree of bone loss. An alternative technique, which has gained recent favor, is acetabular distraction, first described by Sporer et al17 In their original series, 19 of 20 patients were radiologically stable at an average of 4 years follow-up and 17 of these were reported to be pain free. Four of the original 20 did have some early component migration that later became stable and asymptomatic. The majority in this series (13) were hips with a Paprosky type IIIB bone defect.
COMPLICATIONS
 High complication rates have been reported for reconstruction for pelvic discontinuity, ranging from 25% to 80%.3,5,7 The most frequent complications are dislocation, infection, nerve injury, and loss of fixation or implant failure.
High complication rates have been reported for reconstruction for pelvic discontinuity, ranging from 25% to 80%.3,5,7 The most frequent complications are dislocation, infection, nerve injury, and loss of fixation or implant failure.
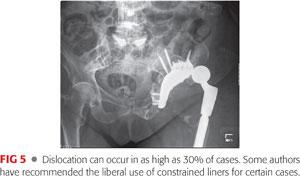
 Dislocation may occur in up to 15% to 30% of cases (FIG 5).3,4,6,22,23 Secondary to these high rates, some authors have reported the use of constrained liners in all revisions with an associated pelvic discontinuity.6 The potential drawback of constrained liners in this setting is increased strain on the construct and perhaps an increased chance of nonunion and failure.
Dislocation may occur in up to 15% to 30% of cases (FIG 5).3,4,6,22,23 Secondary to these high rates, some authors have reported the use of constrained liners in all revisions with an associated pelvic discontinuity.6 The potential drawback of constrained liners in this setting is increased strain on the construct and perhaps an increased chance of nonunion and failure.
 Others have proposed a more selective use of constrained liners, such as in cases with severe abductor insufficiency or extensive soft tissue scarring and damage, a deficient proximal femur, nonunion of the greater trochanter, or history of recurrent dislocation.3,23
Others have proposed a more selective use of constrained liners, such as in cases with severe abductor insufficiency or extensive soft tissue scarring and damage, a deficient proximal femur, nonunion of the greater trochanter, or history of recurrent dislocation.3,23
 Neurologic injury is a concern, with injury to either the superior gluteal nerve or the sciatic nerve.
Neurologic injury is a concern, with injury to either the superior gluteal nerve or the sciatic nerve.
 Superior gluteal nerve injury can be the result of surgical dissection or by placement of the component. This may be an etiology of postoperative Trendelenburg gait, poor abductor function, and instability of the hip.
Superior gluteal nerve injury can be the result of surgical dissection or by placement of the component. This may be an etiology of postoperative Trendelenburg gait, poor abductor function, and instability of the hip.
 Sciatic nerve injury most commonly occurs during exposure of the ischium for fixation of the inferior flange of a cage.
Sciatic nerve injury most commonly occurs during exposure of the ischium for fixation of the inferior flange of a cage.
 Reported rates of infection associated with pelvic discontinuity reconstruction range from 6% to 10%.1,7,11,14,19,21
Reported rates of infection associated with pelvic discontinuity reconstruction range from 6% to 10%.1,7,11,14,19,21
REFERENCES
1. Berry DJ, Lewallan DG, Hanssen AD, et al. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am 1999;81:1692–1702.
2. Chahal J, McCarthy T, Safir O, et al. Late presentation of sciatic neuropathy after failure of acetabular reconstruction rings in revision hip arthroplasty: a report of two cases. Curr Orthop Pract 2008;19:688–690.
3. Christie MJ, Barrington SA, Brinson MF. Bridging massive acetabular defects with the triflanged cup: 2 to 9 years result. Clin Orthop Relat Res 2001;393:216–227.
4. DeBoer DK, Christie MJ, Brinson MF, et al. Revision total hip arthroplasty for pelvic discontinuity. J Bone Joint Surg Am 2007;89:835–840.
5. Eggli S, Muller C, Ganz R. Revision surgery in pelvic discontinuity. Clin Orthop Relat Res 2002;398:136–145.
6. Garbuz D, Morsi E, Mohamed N, et al. Classification and reconstruction in revision acetabular arthroplasty with bone stock deficiency. Clin Orthop Relat Res 1996;(324):98–107.
7. Goodman S, Sastamoinen H, Shasha N. Complications of ilio-ischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty 2004;19:436–446.
8. Hanssen AD, Lewallen DG. Modular acetabular augments: composite void fillers. Orthopedics 2005;28:971–971.
9. Holt GE, Dennis DA. Use of custom triflanged acetabular components in revision total hip arthroplasty. Clin Orthop Relat Res 2004;429:209–214.
10. Kosashvili Y, Backstein D, Safie O, et al. Acetabular revision using an anti-protrusion (ilio-ischial) cage and trabecular metal acetabular component for severe acetabular bone loss associated with pelvis discontinuity. J Bone Joint Surg Br 2009;91:870–876.
11. Lietman SA, Bhawnani K. The partial pelvic replacement cup in severe acetabular defects. Orthopedics 2001;24(12):1131–1135.
12. Moed BR, McMichael JC. Outcomes of posterior wall fractures of the acetabulum. J Bone Joint Surg Am 2007;89:1170–1176.
13. Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994;9:33–44.
14. Paprosky WG, Sporer S, O’Rourke MR. The treatment of pelvic discontinuity with acetabular cages. Clin Orthop Relat Res 2006;453:183–187.
15. Rogers BA, Whittingham-Jones PM, Mitchell PA, et al. The reconstruction of periprosthetic pelvic discontinuity. J Arthroplasty 2012;27:1499–1506.
16. Sembrano JN, Cheng EY. Acetabular cage survival and analysis of factors related to failure. Clin Orthop Relat Res 2008;466:1657–1665.
17. Sporer SM, Bottros JJ, Hulst JB, et al. Acetabular distraction. Clin Orthop Relat Res 2012;470:3156–3163.
18. Sporer SM, O’Rourke M, Paprosky WG. The treatment of pelvic discontinuity during acetabular revision. J Arthroplasty 2005;20(4)(suppl 2):79–84.
19. Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty 2006;21:87–90.
20. Springer BD, Berry DJ, Cabanela ME, et al. Early postoperative transverse pelvic fracture: a new complication related to revision arthroplasty with an uncemented cup. J Bone Joint Surg Am 2005;87(12):2626–2631.
21. Stiehl JB, Saluja R, Diener T. Reconstruction of major column defects and pelvic discontinuity in revision total hip arthroplasty. J Arthroplasty 2000;15:849–857.
22. Taunton MJ, Fehring TK, Edward P, et al. Pelvic discontinuity treated with custom triflange component: a reliable option. Clin Orthop Relat Res 2012;470:428–434.
23. Udomkiat P, Dorr LD, Won YY. Technical factors for success with metal ring acetabular reconstruction. J Arthroplasty 2001;16:961–969.
24. Wendt MC, Adler MA, Trousdale RT, et al. Effectiveness of false profile radiographs in detection of pelvic discontinuity. J Arthroplasty 2012;27:1408–1412.
25. Winter E, Piert M, Volkmann R, et al. Allogeneic cancellous bone graft and a Burch-Schneider ring for acetabular reconstruction in revision hip arthroplasty. J Bone Joint Surg Am 2001;83:862–867.
< div class='tao-gold-member'>









