18 Tissue graft, tissue repair, and regeneration
Synopsis
 Tissue grafting is an important technique for tissue repair in reconstructive plastic surgery.
Tissue grafting is an important technique for tissue repair in reconstructive plastic surgery.
 More importantly, with the advancement of scientific research and newly developed biotechnologies as well as their applications in plastic surgery, the conventional tissue-grafting approach is likely to be switched to a tissue regeneration strategy using stem cell therapy and tissue engineering, and thus to avoid undesired appearance or functional disturbance at the graft donor site.
More importantly, with the advancement of scientific research and newly developed biotechnologies as well as their applications in plastic surgery, the conventional tissue-grafting approach is likely to be switched to a tissue regeneration strategy using stem cell therapy and tissue engineering, and thus to avoid undesired appearance or functional disturbance at the graft donor site.
 This chapter introduces the history and surgical techniques for tissue harvesting and repair of dermal graft, fat graft, fascial graft, tendon graft, and skeletal muscle graft as well as composite graft.
This chapter introduces the history and surgical techniques for tissue harvesting and repair of dermal graft, fat graft, fascial graft, tendon graft, and skeletal muscle graft as well as composite graft.
 Also included are recent developments in tissue graft research and their clinical applications, such as adipose-derived stem cells, acellular tissue graft, and engineered tissue graft fabrication.
Also included are recent developments in tissue graft research and their clinical applications, such as adipose-derived stem cells, acellular tissue graft, and engineered tissue graft fabrication.
Introduction
Plastic surgery is defined as a specialized branch of surgery concerned with the repair of deformities and the correction of functional deficits.1 In this specialty, preparation and transfer of tissue graft for tissue reconstruction and repair are among the most important surgical techniques to achieve the goal of deformity repair and deficit correction. The surgical technique to create a tissue graft such as skin tube or forehead pedicle flap can be traced back to as early as 1597 and 1794.1 In today’s practice, many conventional tissue grafts are still used as clinical routine procedures for tissue repair and reconstruction, for example, dermal, fat, fascial and tendon grafts or muscle flap or composite graft consisting of more than two types of tissues. In modern plastic surgery practice over the past 200 years, an obvious drawback of tissue grafts, i.e., tissue damage on donor site, has been a problem troubling both physicians and patients and has yet to be overcome. Fortunately, the rapid development of biotechnology in the past 20 years has also shed light on this puzzle. In particular, the development of tissue engineering and stem cell biology makes it possible to generate autologous tissue graft without causing donor site tissue damage.2
Dermal graft and repair
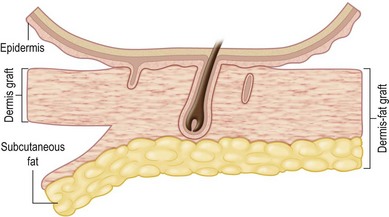
Dermal graft is defined as a graft that contains a deep layer of the papillary dermis and the entire reticular layer of the dermis along with a minimal amount of adherent subcutaneous fat and indigenous subepidermal extensions of the epithelial appendage (Fig. 18.1).3
History
Autologous dermal graft was originally developed in Germany and was first reported in 1913 by Loewe to serve as a substitute of fascia for hernia repair and serve as a tendon graft to repair severed tendons. In 1914, Rehn reported the clinical application to augment soft-tissue depression of nasal tip, ear, and check. Later on, it was used in 1920 by Lexer for cheek augmentation; in 1929, again by Lexer for repair of hernia and dura mater, bone fracture fixation, arthroplasty of the knee, hip, and elbow, in 1932 by Straastsma for correction of saddle-nose deformity, and further used to reinforce ligament after 1939.3
The technique was adopted in North America in the 1940s. Applications include treatment of ankylosis of mandible and repair of stenotic bronchial tubes by Gebauer in 1950, repair of diaphragmatic defect by Metheny, Lundmark, and Morcom in 1952, treatment of unstable scar by Hynes in 1954, protection of carotid artery by Corso and Gerold in 1961 and 1963, soft-tissue augmentation by Johnson in 1961, and replacement of articular disc of temporomandibular joint by Georgiade in 1962, and repair of dura mater defect by Guiot, Rougerie, and Tessier in 1967.3
Surgical technique3
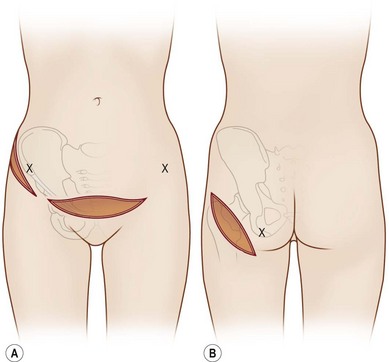
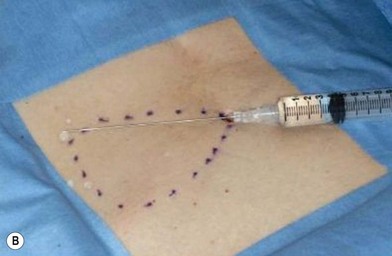
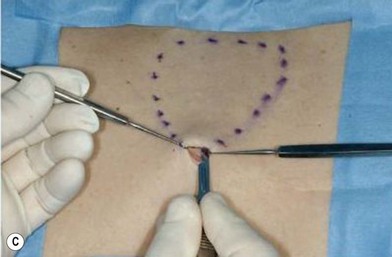
The general requirements for creating a dermal graft and successful application include selection of an inconspicuous donor site, good preparation of a recipient bed to insure it is free of infection and scar tissue, meticulous hemostasis, and adequate immobilization. The dermal graft can be harvested from the groin, gluteal fold, lateral gluteal region, submammary region in females, or lower abdomen. Due to natural contraction, a 25% excess in surface area should be considered when harvesting the graft and an ellipse shape is desired in order to close the wound in a linear fashion. Conventionally, the epidermis is removed by Reese dermatome.3 The epidermis can also be removed by Dispase treatment to maintain the whole structure of the dermis.4
The fate of implanted dermal graft
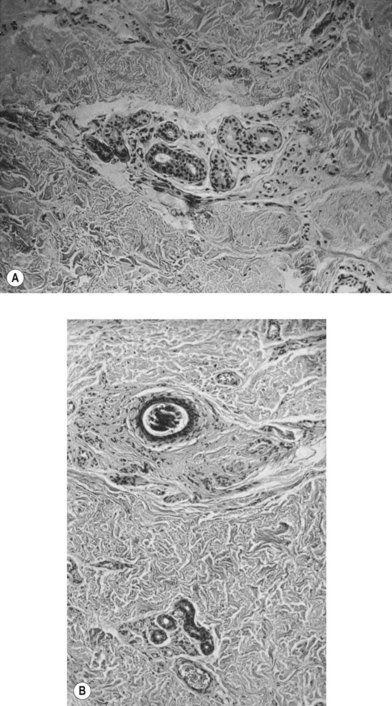
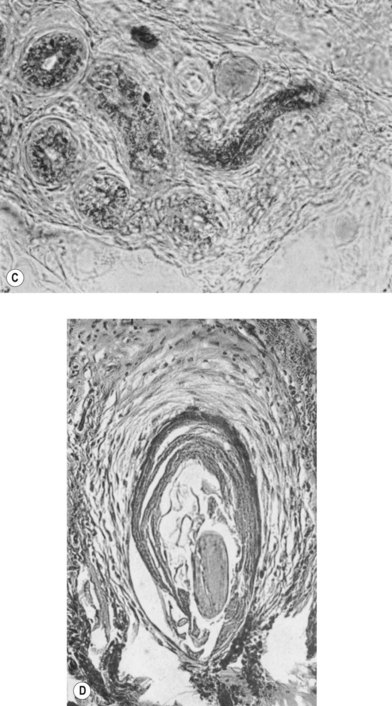
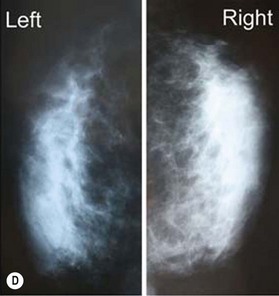
According to histological study, dermal graft along with skin appendage can survive well in subcutaneous environment but the appendage structure may disintegrate after 5 years. However, these structures tend to form dilated excretory duct and epidermoid cyst, eventually resulting in considerable fibrosis around them (Fig. 18.2).3
Clinical application for tissue repair and recent development
As a conventional technique, autologous dermal graft remains widely used in clinical treatments. Lemma et al. reported the repair of hernia of abdominal wall with autologous dermal graft.5 Maguina et al. reported its use in breast reconstruction and treatment of breast implant malposition.6 Karacaoglu reported the application in augmentation mastopexy in 21 patients with 20.6-month follow-up and the procedure was found to be cost-effective and could improve breast appearance.7 It has also been applied to repair parotid surgery wound, which can limit the common complication of depression deformity or neck scarring.8 Samson et al. have applied autologous dermal graft to the repair of large infected abdominal wall hernias in obese patients with success.9
The disadvantage of this traditional technique is the need to harvest a full-thickness skin graft, which is limited by the availability of the donor site skin and can cause scarring. In addition, buried skin appendage can also lead to cyst formation, as illustrated in Figure 18.2. One of the approaches to overcome this problem is the development of allogenic acellular dermal graft, and thus there will be no limitation in the graft supply. Additionally, the allogenic acellular graft will not cause immune response due to the elimination of immunogenic cells.
One important application of Alloderm is for abdominal wall reconstruction, which has been tested for its efficacy in both experimental study and clinical trials with success,10–19 including the repair of ventral hernia or contaminated and high-risk abdominal wall defect. It has been observed that implanted Alloderm could be fast revascularized and gradually remodeled into autologous tissue. In 2005, Butler et al. reported the multiple applications of Alloderm for pelvic, chest, and abdominal wall reconstruction, demonstrating its great potential in tissue repair.17 The other applications include gingival augmentation,20 facial soft-tissue reconstruction,21 and burn treatment.22
Future
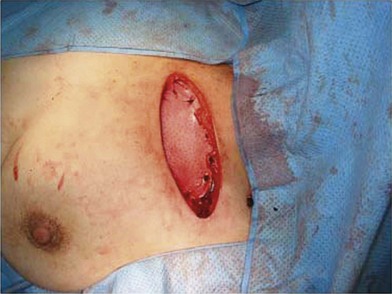
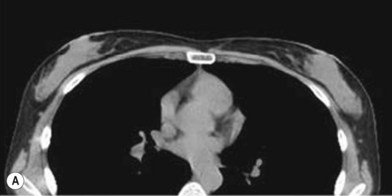
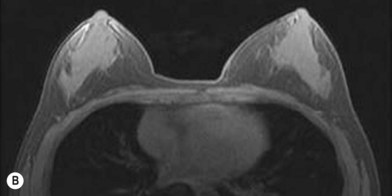
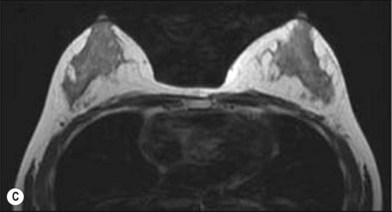
One direction will be the use of xenogenic acellular dermal matrix (ADM) as a replacement when concerning the source of donated human skin. Mirzabeigi et al. reported the use of porcine ADM (Permacol) to repair chest wall defects resulting from desmoid tumor resection (Fig. 18.3).23 However, an apparent immunogenic response was observed in xenogenic ADM compared to allogenic ADM during a clinical trial in burn treatment.24 It has been found that amino-terminal propeptide is the most immunogenic part of type I procollagen.25 In the future, transgenic pigs that are genetically modified to produce human type I procollagen might be an ideal source for generating less immunogenic ADM given the fact that such a genetic technique is already available for expressing some human molecule in pigs,26 and given the available technique that can provide pathogen-free animals.
Fat graft and repair
Fat graft is defined as a graft containing fat cells along with stromal tissues. Fat graft can be transplanted by the means of free fat transfer, or transfer with dermal tissue in the form of dermis fat graft or microvascular transfer of fat graft using microsurgery.3
History
In 1893, Neuber made the first attempt at fat graft transfer for the clinical treatment of facial deformity. In the early 1900s, fat graft transfer was used to establish a normal contour of hemifacial atrophy or enlargement of small breast by Lexer or used to fill the orbit by Barraquer, López, Lauber, Marx, and Key.3 The general requirements for fat graft include selection of a concealed donor site, proper preparation and gentle handling of the graft, secure placement of the graft into the recipient site with meticulous hemostasis and prevention of infection. It is also important to make an overcorrection due to absorption of the graft. The ideal donor sites include the groin, lateral gluteal area, and gluteal fold because an adequate amount of fat tissue can be harvested and also because of the availability of dermis to form a dermis fat graft (Fig. 18.4).3
Clinical application for tissue repair and recent developments
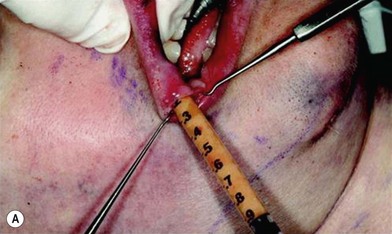
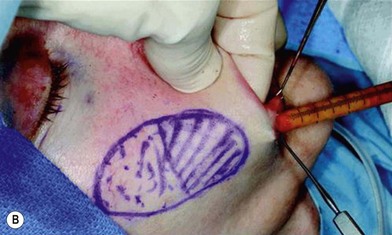
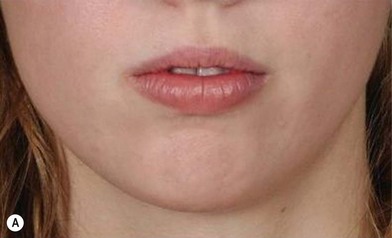
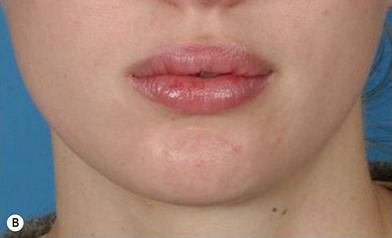
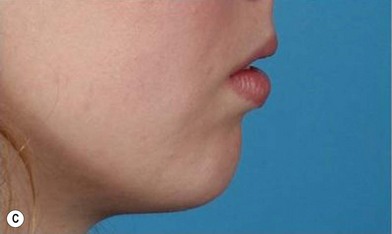
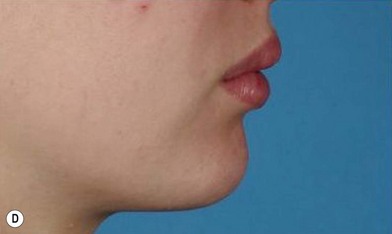
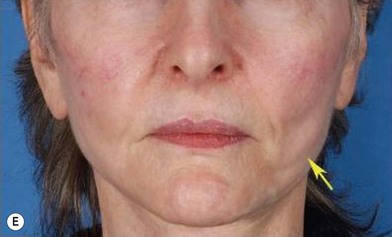
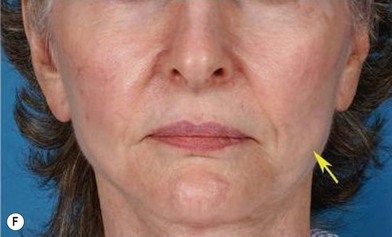
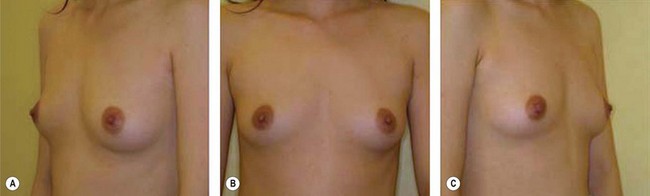
The use of fat injection as a way of transplanting free fat graft was established soon after the introduction of liposuction. Free fat graft has been widely used for various types of tissue repair.27 Breast cosmetic surgery or reconstruction is the most important application. The indications include treatment of micromastia, correction of postaugmentation deformity (with and without removal of implant), treatment of tuberous breasts and Poland’s syndrome, surgical repair of postlumpectomy deformity and postmastectomy deformity, treatment of deficits or tissue damage caused by conservative or surgical reconstruction, and nipple reconstruction.28–34 Facial augmentation and correction of defects are also important indications, including rhinoplasty with lipoinjection,35 facial contouring,36,37 and facial tissue augmentation.38 Guyuron and Majzoub developed a core fat graft technique for lip augmentation and correction of malar and buccal deficiency.39 Figures 18.5–18.7 demonstrate the procedure and the results for lip and buccal augmentation with fat transfer.39 In addition, free fat transplantation has been applied to gluteal augmentation and repair of contour deformities,40,41 hand rejuvenation,42 and penile enlargement and aesthetic improvement.43,44
To harvest free fat graft, minimal invasiveness and high tissue viability are the general principle. It is thus suggested to use 3–4-mm blunt cannula or similar needle and utilize minimal amounts of suction force to avoid mechanical damage. In addition, cell damage caused by centrifugation force and air exposure should be avoided.27 In regard to complications, in addition to infection, seroma, hematoma formation, graft volume loss via reabsorption and necrosis are the primary causes of poor results.27 Tissue or cell damage during harvest is also one of the reasons. The other factor is poor vascularization of implanted fat graft.45
There are several reports on improving vascularization and survival of transplanted fat by incorporating vascular endothelial growth factor (VEGF) gene-transfected adipose stem cells.46 Potentially, other angiogenic growth factors like basic fibroblast growth factor (bFGF) and platelet-derived growth factor can be integrated into the fat grafts to support their survival by enhancing in vivo angiogenesis at the transplantation site.47 Circulating endothelial progenitor cells (EPC) are considered an important factor for promoting tissue vascularization, which can be recruited to an ischemic region by locally released factors.48 Therefore, mixing EPCs with fat graft will also potentially enhance vascularization and tissue survival.
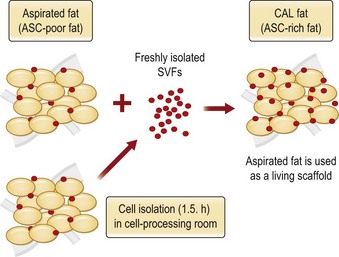
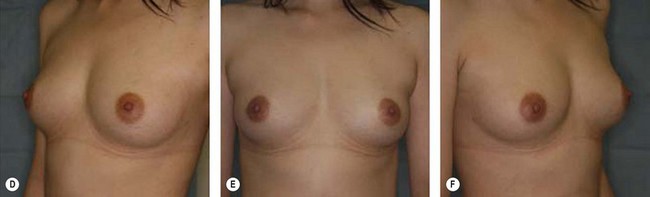
Although the translation of experimental discovery to clinical application remains at an early stage, Yoshimura et al. reported a practical approach for cosmetic breast augmentation called cell-assisted lipotransfer (CAL), which has benefited from the basic research of stem cell biology.28 In the reported study, adipose-derived stem/stromal cells (ASCs) were isolated from stromal vascular fraction and then mixed with lipoaspirated fat to form ASC-rich fat (Fig. 18.8). Because ASCs are much more angiogenic and potent than mature adipocytes and lipoaspirated fat can serve as the scaffold to support the growth and function of seeded ASCs, such an ASC-rich fat graft will have a better survival rate than a regular free fat graft due to better angiogenesis after implantation. The result of clinical application demonstrated good tissue survival (Fig. 18.9) with soft breast and natural contour and breast volume could be stabilized 2–3 months after transplantation. More importantly, a 4–8-cm increase in breast circumference was observed in this CAL, trial, in contrast to only 2–3-cm increase in conventional procedure (Fig. 18.10).28 The potential mechanisms of CAL method may include ASCs differentiating into adipocytes and endothelial cells, promoting angiogenesis and graft survival, releasing angiogenic growth factors in response to hypoxia and other conditions, and contributing to the turnover of implanted adipose tissue.28
In addition to free fat graft, dermis fat graft and microvascular fat graft are also the methods for fat tissue transplantation. Many applications have been reported for this type of grafting, including facial reconstruction and correction of forehead depression scar,49,50 orbital reconstruction,51 and nerve or tendon covering.52 However, volume loss remains a concern for this type of graft, as revealed either by experimental study53 or by the study of transplanted human dermis fat graft.54 One of the approaches to overcome volume loss is microvascular transfer of fat graft such as free omental fat flap or de-epithelialized dermis fat graft for various tissue repairs, which will be described in other chapters.
Future
Application of ASCs and fat tissue engineering are apparently important directions towards fat tissue repair and regeneration. As shown in Figure 18.11, ASCs are located at the stromal vascular fraction and can be concentrated by centrifugation.55 As previously described, mixing isolated ASCs with lipoaspirated fat graft can better maintain tissue survival via several mechanisms.27 To master this process, Cytori Therapeutics developed a special facility called Celution system, which allows for separating and collecting ASCs from freshly harvested fat tissue in about 1 hour in the operating room and enables surgeons to mix ASCs with fat tissue for breast augmentation or other soft-tissue repair (information derived from Cytori website: www.cytori.com). Therefore, the concept of ASC-assisted fat tissue transplantation proved by experimental study and clinical trials is likely to be translated into a therapeutic procedure.56
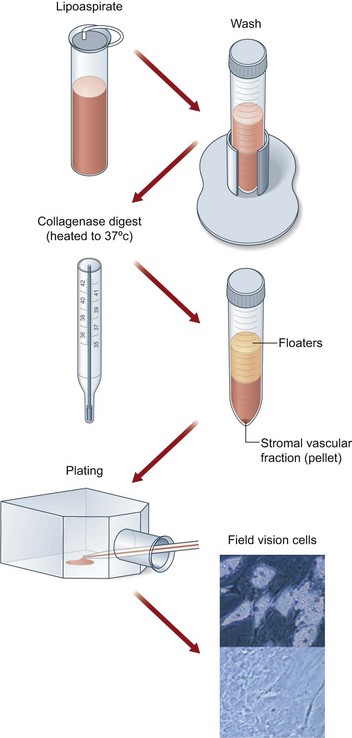
Fig. 18.11 Processing of lipoaspirate and isolation of adipose-derived stem cells.
(Reprinted from Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260.)
Another important approach, adipose tissue engineering, may represent a more suitable alternative for adipose tissue regeneration via de novo adipogenesis. In particular, to correct a large adipose defect, adult stem cells like ASCs or adipose progenitor cells can be seeded on a three-dimensional scaffold and implanted in vivo for adipogenesis. The advantage of this approach is the possibility of incorporating angiogenic growth factors like VEGF or bFGF into the scaffold or mixing EPCs or endothelial cells along with ASCs. In addition, implantation of adult stem cells is likely to be induced for angiogenesis and/or vascularization during the de novo adipogenic process, and eventually develop a fully vascularized fat tissue to ensure correct tissue volume and function. In contrast to mature adipocytes, implanted ASCs or adipose progenitor cells also provide a means of self-repair or regeneration via their self-renewal function.57
Fascial graft and repair
History
Facial graft without vascular supply was first reported by McArthur in 1901 and 1904, using the strips of the external oblique aponeurosis as biologic sutures to repair inguinal hernias. Following this, Kirshner in 1909 and Busch in 1913 also reported the use of the slings of autogenous fascia latae to correct a facial nerve palsy deformity. This technique was further refined and popularized by Blair in 1926 and became a well-established surgical procedure which continues to be employed today.3
Surgical technique and tissue repair application
Fascia is characterized by its unique dense collagen structure, which can provide great mechanical strength. As an example, the average tensile strength of fascia latae is approximately 7000 lb per square inch or 10.73 lb per quarter-inch strip.3,58,59 Because of this unique character, fascial graft is usually employed to repair a defective tissue that requires mechanical strength to carry out its normal function. These indications include repair of facial palsy,60 paralytic lagophthalmos,61 congenital unilateral lower lip palsy,62 reconstruction of ruptured Achilles tendon,63 promoting watertight dural closure to prevent cerebrospinal fluid leakage postsurgery,64 assisting closure of abdominal wall after multiple organ transplantation,65 and reconstruction of the palatal aponeurosis.66
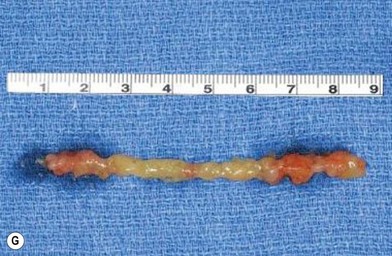
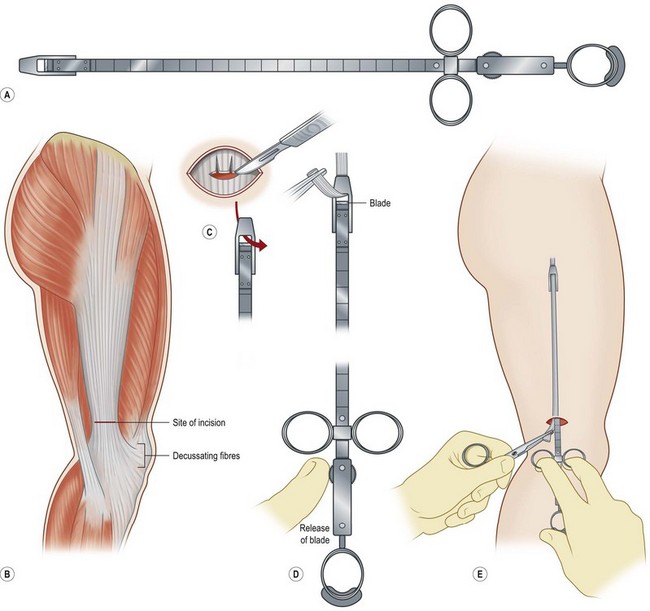
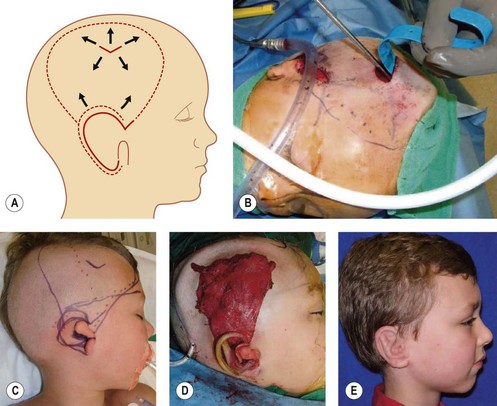
Fascia latae is the most common donor site for nonvascularized fascia. Generally, a strip 10–15 mm wide can be harvested; removing a further wider fascia strip is likely to make muscle herniate through the fascia defect. Figure 18.12 demonstrates the procedure of harvesting a strip of fascia latae by a refined version of a fascia tripper developed by Wilson and Castroviejo.3 For vascularized fascial graft, the temporoparietal fascial flap is the common type of fascia free flap; it is ultrathin and leaves a minimal donor site defect.3 In recent years, the endoscope has been used to harvest temporoparietal fascial flap with fascular pedicle. This fascial flap can either be used in local repair or in distant areas as a free flap. Figure 18.13 demonstrates the process of harvesting a temporoparietal fascial flap and its application in auricular reconstruction.67
Future
It remains difficult to find a biological tissue to replace fascia graft fully due to fascia’s unique tissue structure and its great mechanical strength. One potential approach is the fabrication and application of tissue-engineered fascia. Hung et al. reported the formation of tissue-engineered neofascia in an animal study which may potentially be applied for reconstructive pelvic surgery.68 Fann et al. reported a model of tissue-engineered ventral hernia repair employing novel aligned collagen tube and autologous skeletal muscle satellite cells, which could form a “living repair,” and the established approach may potentially be applied for clinical therapy.69 In addition, decellularized fascia may be a potential strategy.
Tendon graft and repair
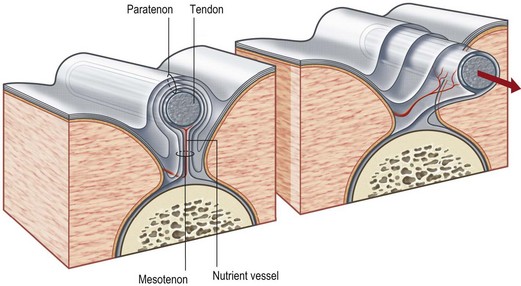
Tendon is a white fibrous cord tissue that connects a muscle to a bone to transmit the contracting force for the movement of extremities. It may lie in a paratenon, a loose areolar tissue surrounding tendon, or it may pass through a tendon sheath, a tube of condensed fibrous tissue surrounding tendon. The anatomy of tendon as well as its related structures is shown in Figure 18.14.70